Polyphenolic Profile and Antioxidant Activity of Whole Grape Juices from Vitis labrusca and Brazilian Hybrid Grapes in Two Training Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of the Experimental Area
2.2. Elaboration of Whole Grape Juices
2.3. Colorimetric and Physicochemical Analysis
2.4. Analysis by UV-Visible Spectrophotometry and Profile of Phenolic Compounds
2.4.1. Total Contents of Phenolic Compounds and Monomeric Anthocyanins
2.4.2. Antioxidant Activity by UV-Visible Spectrophotometry
2.4.3. Phenolics Profile by Reverse-Phase High-Performance Liquid Chromatography (HPLC)
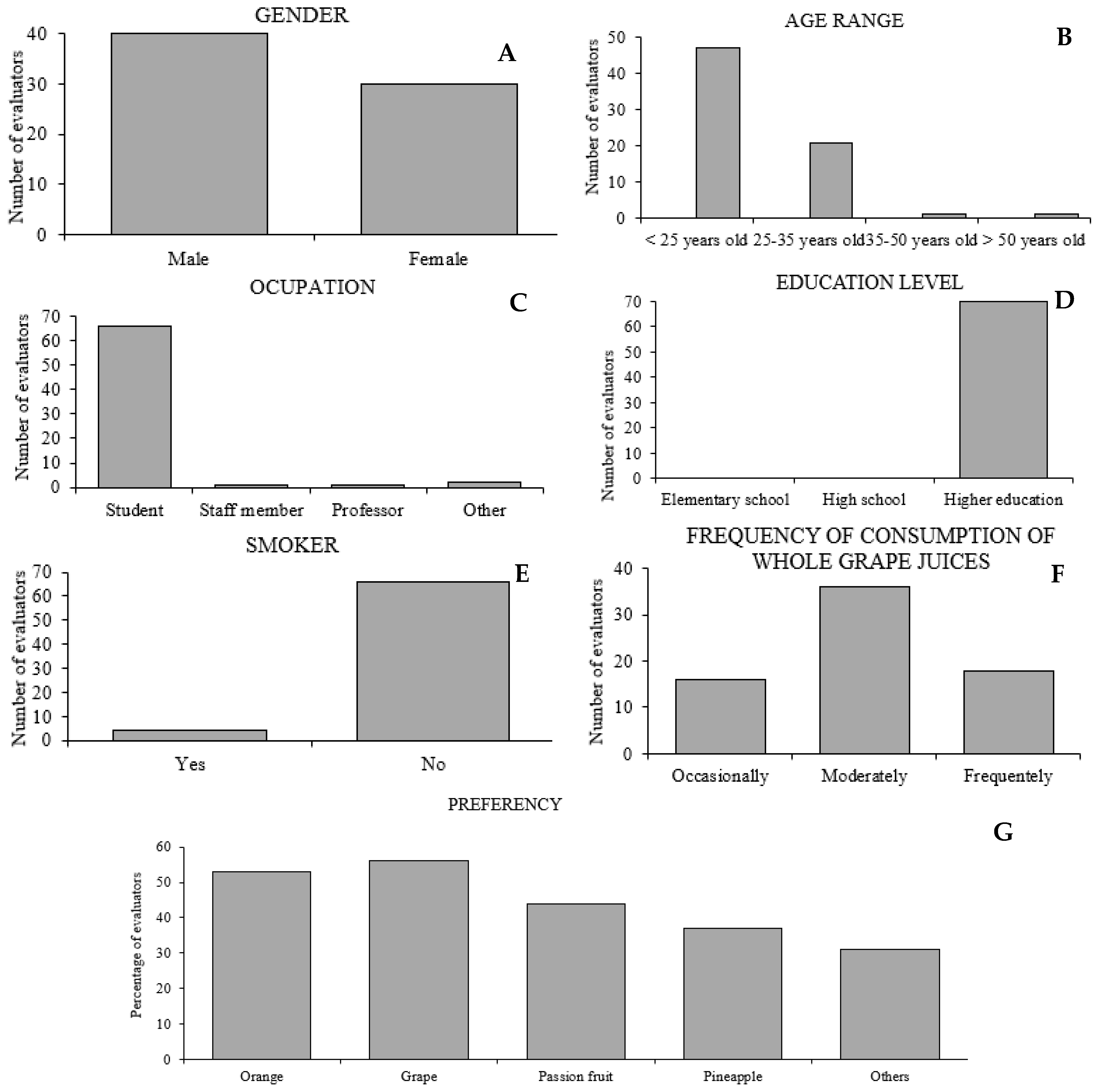
2.5. Sensory Analysis of Whole Grape Juices
2.6. Statistical Analysis
3. Results and Discussions
3.1. Colorimetric, Physicochemical, and Biochemical Analyses via UV-Visible Spectroscopy in Whole Grape Juices
3.2. Profile of Phenolic Compounds and Antioxidant Activity of Whole Grape Juices
3.3. Sensory Analysis
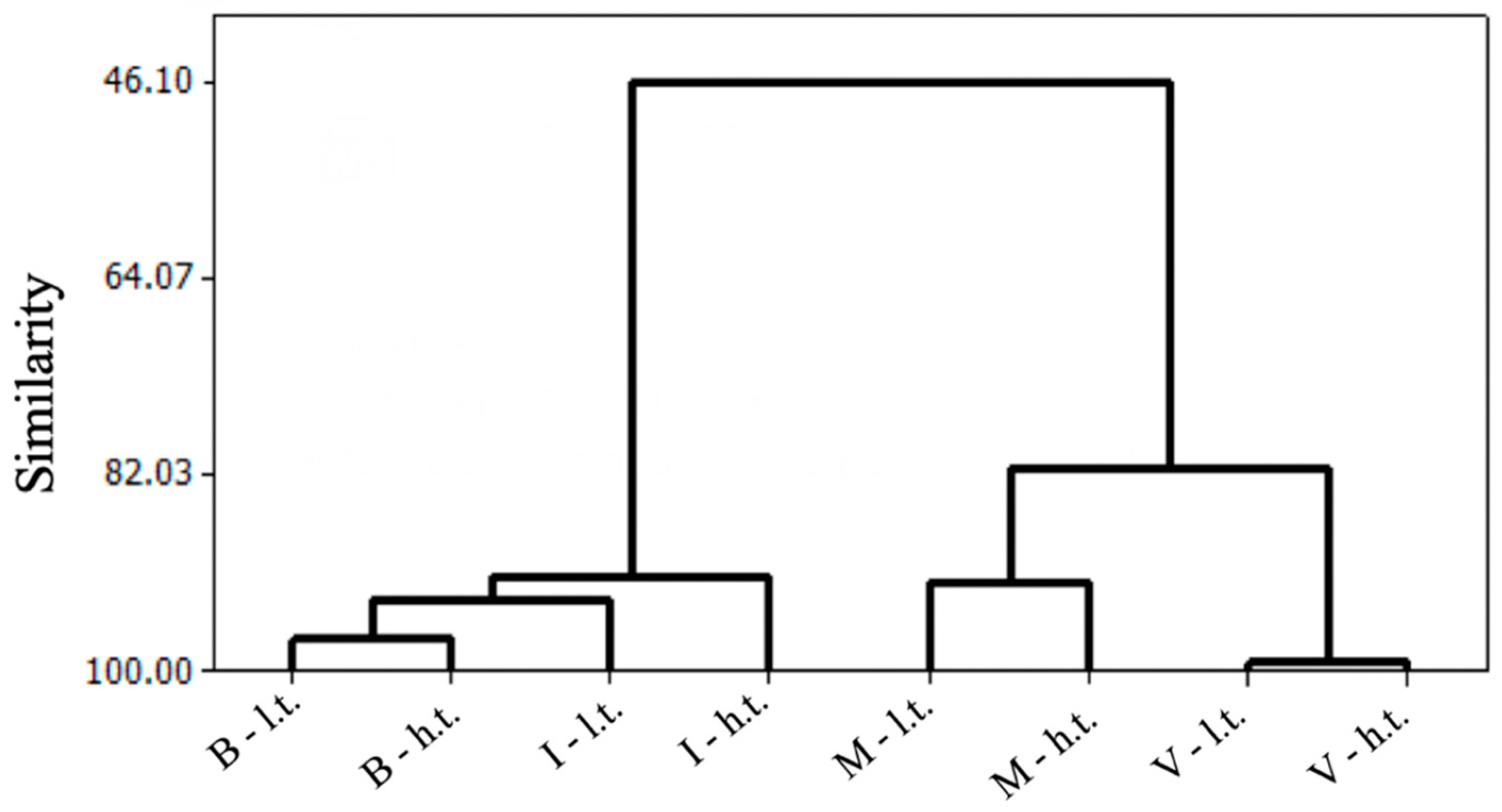
3.4. Principal Component Analysis of Colorimetric, Physicochemical, and Biochemical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Granato, D.; de Magalhães Carrapeiro, M.; Fogliano, V.; Van Ruth, S.M. Effects of geographical origin, varietal and farming system on the chemical composition and functional properties of purple grape juices: A review. Trends Food Sci. Technol. 2016, 52, 31–48. [Google Scholar] [CrossRef]
- Agrianual. Anuário da Agricultura Brasileira; Agrianual: São Paulo, Brazil, 2023; 464p. [Google Scholar]
- Lima, M.D.S.; Silani, I.D.S.V.; Toaldo, I.M.; Corrêa, L.C.; Biasoto, A.C.T.; Pereira, G.E.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic compounds, organic acids and antioxidant activity of grape juices produced from new Brazilian varieties planted in the Northeast Region of Brazil. Food Chem. 2014, 161, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Burin, V.M.; Ferreira-Lima, N.E.; Panceri, C.P.; Bordignon-Luiz, M.T. Bioactive compounds and antioxidant activity of Vitis vinifera and Vitis labrusca grapes: Evaluation of different extraction methods. Microchem. J. 2014, 114, 155–163. [Google Scholar] [CrossRef]
- Lago-Vanzela, E.S.; Procópio, D.P.; Fontes, E.A.F.; Ramos, A.M.; Stringheta, P.C.; da-Silva, R.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Aging of red wines made from hybrid grape cv. BRS Violeta: Effects of accelerated aging conditions on phenolic composition, color and antioxidant activity. Food Res. Int. 2014, 56, 182–189. [Google Scholar] [CrossRef]
- Domingues Neto, F.J.; Tecchio, M.A.; Junior, A.P.; Vedoato, B.T.F.; Lima, G.P.P.; Roberto, S.R. Effect of ABA on colour of berries, anthocyanin accumulation and total phenolic compounds of “Rubi” table grape (Vitis vinifera). Aust. J. Crop Sci. 2017, 11, 199–205. Available online: https://www.cropj.com/neto_11_2_2017_199_205.pdf (accessed on 15 September 2024). [CrossRef]
- Domingues Neto, F.J.; Borges, C.V.; Lima, G.P.P.; Pimentel Júnior, A.; Monteiro, G.C.; Figueira, R.; Venturini Filho, W.G.; Minatel, I.O.; Moura, M.F.; Tecchio, M.A. Improvement of biogenic amines in grape juice from Vitis labrusca and hybrid grapes grown in different training systems. J. Hortic. Sci. Biotechnol. 2023, 98, 223–232. [Google Scholar] [CrossRef]
- Gomez, H.A.G.; Marques, M.O.M.; Borges, C.V.; Minatel, I.O.; Monteiro, G.C.; Ritschel, P.S.; Zanus, M.C.; Diamante, M.S.; Kluge, R.A.; Lima, G.P.P. Biogenic Amines and the Antioxidant Capacity of Juice and Wine from Brazilian Hybrid Grapevines. Plant Foods Hum. Nutr. 2020, 75, 258–264. [Google Scholar] [CrossRef]
- Gomes, E.P.; Vanz Borges, C.; Monteiro, G.C.; Belin, M.A.F.; Minatel, I.O.; Pimentel Junior, A.; Tecchio, M.A.; Lima, G.P.P. Preharvest salicylic acid treatments improve phenolic compounds and biogenic amines in ‘Niagara Rosada’ table grape. Postharvest Biol. Technol. 2021, 176, 111505. [Google Scholar] [CrossRef]
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araújo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos. In Sistema Brasiileiro e Classificação e Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Rizzon, L.A. Metodologia para análise de vinho. Embrapa 2010, 120, 37–41. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/887323/1/Metodologiaanalisevinhotintoed012010.pdf (accessed on 15 September 2024).
- Zenebon, O.; Pascuet, N.S.; Tiglea, P. Métodos Físicos e Químicos Para Análise de Alimentos, 4th ed.; Instituto Adolfo Lutz: São Paulo, Brazil, 2008. Available online: http://www.ial.sp.gov.br/resources/editorinplace/ial/2016_3_19/analisedealimentosial_2008.pdf (accessed on 15 September 2024).
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi Junior, A. Colorometry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Giusti, M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-visible Spectroscopy. Handb. Food Anal. Chem. 2005, 2, 9–31. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Natividade, M.M.P.; Corrêa, L.C.; de Souza, S.V.C.; Pereira, G.E.; Lima, L.C.d.O. Simultaneous analysis of 25 phenolic compounds in grape juice for HPLC: Method validation and characterization of São Francisco Valley samples. Microchem. J. 2013, 110, 665–674. [Google Scholar] [CrossRef]
- Meilgaarde, M.; Civille, G.V.; Carr, B.T.; Civille, G.V. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 1999; 416p, Available online: https://www.taylorfrancis.com/books/mono/10.1201/9781003040729/sensory-evaluation-techniques-morten-meilgaard-gail-vance-civille-thomas-carr (accessed on 15 September 2024).
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Instrução Normativa n° 01, de 07 de janeiro de 2000. Regulam. Técnico Geral Para Fixação Padrões Identidade Qual. Para Polpa Fruta. Diário Of. República Fed. Bras. 2000, Volume 1, pp. 54–58. Available online: https://sogi8.sogi.com.br/Manager/texto/arquivo/exibir/arquivo?eyJ0eXAiOiJKV1QiLCJhbGciOiJIUzI1NiJ9AFFIjAvMTAwNi9TR19SZXF1aXNpdG9fTGVnYWxfVGV4dG8vMC8wL0RPQ1VNRU5UTyAxLnBkZi8wLzAiAFF-PrY0AgIRKZ-v7L2u54yTTXEsLtTom6nh_2Ohh3bv6A (accessed on 15 September 2024).
- Lima, M.S.; Dutra, M.d.C.P.; Toaldo, I.M.; Corrêa, L.C.; Pereira, G.E.; de Oliveira, D.; Bordignon-Luiz, M.T.; Ninow, J.L. Phenolic compounds, organic acids and antioxidant activity of grape juices produced in industrial scale by different processes of maceration. Food Chem. 2015, 188, 384–392. [Google Scholar] [CrossRef]
- Borges, R.S.; Prudêncio, S.H.; Roberto, S.R.; de Assis, A.M. Avaliação sensorial de suco de uva cv. Isabel em cortes com diferentes cultivares. Rev. Bras. Frutic. 2011, 33, 584–591. [Google Scholar] [CrossRef]
- Camargo, U.A.; Maia, J.D.G.; Ritschel, P.S. Técnico. In Comunicado Técnico 84; Embrapa: Brasília, Brazil, 2008; Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPUV/9543/1/cot084.pdf (accessed on 15 September 2024).
- Borges, D.S.R.; da Silva, G.A.; Roberto, S.R.; de Assis, A.M.; Yamamoto, L.Y. Phenolic compounds, favorable oxi-redox activity and juice color of “Concord” grapevine clones. Sci. Hortic. 2013, 161, 188–192. [Google Scholar] [CrossRef]
- Padilha, C.V.d.S.; Miskinis, G.A.; de Souza, M.E.A.O.; Pereira, G.E.; de Oliveira, D.; Bordignon-Luiz, M.T.; Lima, M.d.S. Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP-HPLC/DAD: Method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem. 2017, 228, 106–115. [Google Scholar] [CrossRef]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Gimenez-Martinez, R.; Navarro-Alarcon, M.; Rufián-Henares, J.A. Phenolic compounds and antioxidant activity of Spanish commercial grape juices. J. Food Compos. Anal. 2015, 38, 19–26. [Google Scholar] [CrossRef]
- Dávalos, A.; Bartolomé, B.; Gómez-Cordovés, C. Antioxidant properties of commercial grape juices and vinegars. Food Chem. 2005, 93, 325–330. [Google Scholar] [CrossRef]
- Nassur, R.C.M.R.; Pereira, G.E.; Alves, J.A.; Lima, L.C.O. Chemical characteristics of grape juices from different cultivar and rootstock combinations. Pesqui. Agropecuária Bras. 2014, 49, 540–545. [Google Scholar] [CrossRef]
- Silva, M.J.R.; Paiva, A.P.M.; de Souza, J.F.; Silva Padilha, C.V.; Basílio, L.S.P.; dos Santos Lima, M.; Pereira, G.E.; Corrêa, L.C.; Vianello, F.; Lima, G.P.P.; et al. Phytochemical profile of Brazilian grapes (Vitis labrusca and hybrids) grown on different rootstocks. PLoS ONE 2022, 17, e0275489. [Google Scholar] [CrossRef]
- Nixford, S.L.; Hermosín-Gutiérrez, I. Brazilian red wines made from the hybrid grape cultivar Isabel: Phenolic composition and antioxidant capacity. Anal. Chim. Acta 2010, 659, 208–215. [Google Scholar] [CrossRef]
- Silva, J.K.; Cazarin, C.B.B.; Correa, L.C.; Batista, Â.G.; Furlan, C.P.B.; Biasoto, A.C.T.; Pereira, G.E.; de Camargo, A.C.; Maróstica Junior, M.R. Bioactive compounds of juices from two Brazilian grape cultivars. J. Sci. Food Agric. 2016, 96, 1990–1996. [Google Scholar] [CrossRef]
- Leong, S.Y.; Burritt, D.J.; Oey, I. Evaluation of the anthocyanin release and health-promoting properties of Pinot Noir grape juices after pulsed electric fields. Food Chem. 2016, 196, 833–841. [Google Scholar] [CrossRef]
- Casassa, L.F.; Bolcato, E.A.; Sari, S.E. Chemical, chromatic, and sensory attributes of 6 red wines produced with prefermentative cold soak. Food Chem. 2015, 174, 110–118. [Google Scholar] [CrossRef]
- Decendit, A.; Mamani-Matsuda, M.; Aumont, V.; Waffo-Teguo, P.; Moynet, D.; Boniface, K.; Richard, E. Malvidin-3-O-β glucoside, major grape anthocyanin, inhibits human macrophage-derived inflammatory mediators and decreases clinical scores in arthritic rats. Biochem. Pharmacol. 2013, 86, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Mackert, J.D.; McIntosh, M.K. Combination of the anthocyanidins malvidin and peonidin attenuates lipopolysaccharide-mediated inflammatory gene expression in primary human adipocytes. Nutr. Res. 2016, 36, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Cimino, F.; Molonia, M.S.; Ferrari, D.; Saija, A.; Virgili, F.; Speciale, A. Cyanidin-3-O-glucoside ameliorates palmitate-induced insulin resistance by modulating IRS-1 phosphorylation and release of endothelial derived vasoactive factors. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar] [CrossRef]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006, 19, 396–404. [Google Scholar] [CrossRef]
- Iacopini, P.; Baldi, M.; Storchi, P.; Sebastini, L. Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: Content, in vitro antioxidant activity and interactions. J. Food Compos. Anal. 2008, 208, 589–598. [Google Scholar] [CrossRef]
- Basílio, L.S.P.; Vanz Borges, C.; Minatel, I.O.; Vargas, P.F.; Tecchio, M.A.; Vianello, F.; Lima, G.P.P. New beverage based on grapes and purple-fleshed sweet potatoes: Use of non-standard tubers. Food Biosci. 2022, 47, 101626. [Google Scholar] [CrossRef]
- Bagdas, D.; Gul, N.Y.; Topal, A.; Tas, S.; Ozyigit, M.O.; Cinkilic, N.; Gul, Z.; Etoz, B.C.; Ziyanok, S.; Inan, S.; et al. Pharmacologic overview of systemic chlorogenic acid therapy on experimental wound healing. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 1101–1116. [Google Scholar] [CrossRef]
- Pontes, P.R.B.; Santiago, S.S.; Szabo, T.N.; Toledo, L.P.; Gollücke, A.P.B. Sensory attributes and acceptance of commercial grape juices. Cienc. Tecnol. Aliment. 2010, 30, 313–318. [Google Scholar] [CrossRef][Green Version]

| Variables | Trellis | Varieties | CV (%) | |||
|---|---|---|---|---|---|---|
| ‘Bordô’ | ‘IAC 138-22 Máximo’ | ‘BRS Violeta’ | ‘Isabel’ | |||
| Soluble solids (°Brix) | Low | 15.96 ± 0.15 bB | 15.43 ± 0.15 bB | 17.83 ± 0.06 aA | 17.66 ± 0.06 aA | 2.39 |
| High | 16.76 ± 0.71 aAB | 16.33 ± 0.81 aB | 16.40 ± 0.36 bAB | 17.30 ± 0.001 aA | ||
| pH | Low | 3.61 ± 0.08 aB | 3.69 ± 0.07 aB | 3.78 ± 0.01 aA | 3.44 ± 0.01 aC | 1.02 |
| High | 3.51 ± 0.04 bB | 3.62 ± 0.01 aA | 3.63 ± 0.01 bA | 3.46 ± 0.01 aB | ||
| Titratable acidity (% tartaric acid) | Low | 0.67 ± 0.07 bB | 0.76 ± 0.06 aB | 0.62 ± 0.11 bB | 0.95 ± 0.06 aA | 8.95 |
| High | 0.84 ± 0.02 aAB | 0.63 ± 0.06 bB | 0.79 ± 0.04 aAB | 0.99 ± 0.08 aA | ||
| Reducing sugar (%) | Low | 10.91 ± 0.08 aC | 12.19 ± 1.06 bBC | 13.97 ± 1.67 aAB | 14.99 ± 1.41 aA | 7.68 |
| High | 12.24 ± 1.07 aB | 14.07 ± 0.60 aAB | 12.79 ± 1.08 aB | 16.26 ± 0.04 aA | ||
| 420 nm (yellow) | Low | 1.79 ± 0.42 bB | 1.33 ± 0.08 bBC | 3.50 ± 0.03 aA | 0.99 ± 0.001 aC | 13.36 |
| High | 2.37 ± 0.61 aB | 2.04 ± 0.22 aB | 3.33 ± 0.08 aA | 1.07 ± 0.02 aC | ||
| 520 nm (red) | Low | 3.81 ± 0.04 aA | 3.56 ± 0.02 bB | 3.64 ± 0.001 aB | 3.34 ± 0.01 bC | 1.83 |
| High | 3.81 ± 0.13 aA | 3.91 ± 0.07 aA | 3.45 ± 0.01 bB | 3.60 ± 0.09 aB | ||
| 620 nm (violet) | Low | 3.15 ± 0.07 aA | 2.75 ± 0.14 bB | 3.31 ± 0.12 aA | 1.82 ± 0.07 aC | 2.45 |
| High | 3.22 ± 0.07 aA | 3.00 ± 0.05 aB | 3.13 ± 0.06 bAB | 1.78 ± 0.04 aC | ||
| Color intensity (CI) | Low | 8.76 ± 0.46 bB | 7.65 ± 0.21 bC | 10.46 ± 0.14 aA | 6.17 ± 0.08 aD | 3.49 |
| High | 9.40 ± 0.56 aAB | 8.95 ± 0.10 aB | 9.92 ± 0.12 bA | 6.46 ± 0.11 aC | ||
| Hue | Low | 0.47 ± 0.10 bB | 0.37 ± 0.02 bB | 0.96 ± 0.01 aA | 0.29 ± 0.001 aB | 13.56 |
| High | 0.62 ± 0.17 aB | 0.52 ± 0.06 aB | 0.96 ± 0.03 aA | 0.29 ± 0.01 aC | ||
| FRAP (mM Fe/L) | Low | 27.73 ± 2.39 aB | 14.66 ± 3.46 bC | 55.06 ± 4.39 aA | 9.46 ± 1.45 aC | 9.67 |
| High | 31.56 ± 1.55 aB | 22.66 ± 3.66 aC | 49.46 ± 1.84 bA | 10.20 ± 0.52 aD | ||
| Variables | Varieties | CV (%) | |||
|---|---|---|---|---|---|
| ‘Bordô’ | ‘IAC 138-22 Máximo’ | ‘BRS Violeta’ | ‘Isabel’ | ||
| Lightness (L*) | 20.50 ± 0.30 ab | 20.54 ± 0.15 ab | 20.32 ± 0.05 b | 20.76 ± 0.23 a | 1.14 |
| Chroma (C*) | 1.10 ± 0.15 b | 1.35 b ± 0.24 | 1.26 ± 0.03 b | 2.13 ± 0.37 a | 13.81 |
| Hue | 22.45 ± 8.98 b | 20.94 ± 4.46 b | 43.73 ± 4.51 a | 18.80 ± 2.33 b | 19.81 |
| Anthocyanins (mg/L) | 829.99 ± 63.25 b | 893.13 ± 31.42 b | 1411.08 ± 77.30 a | 223.59 ± 22.73 c | 8.09 |
| Phen. comp. (mg/L) | 1768.81 ± 59.89 c | 2787.33 ± 35.67 b | 3357.36 ± 35.31 a | 1187.42 ± 38.57 d | 3.29 |
| DPPH (µg TEAC/mL) | 103.58 ± 8.38 b | 79.60 ± 5.81 c | 115.43 ± 5.52 a | 55.36 ± 1.39 d | 6.88 |
| ABTS (µg TEAC/mL) | 85.23 ± 0.82 b | 68.98 ± 1.30 c | 95.48 ± 0.88 a | 48.94 ± 1.63 d | 0.91 |
| Variables | Trellis | CV (%) | |||
| Low | High | ||||
| Lightness (L*) | 20.55 ± 0.19 a | 20.52 ± 0.31 a | 1.14 | ||
| Chroma (C*) | 1.56 ± 0.54 a | 1.36 ± 0.36 b | 13.81 | ||
| Hue | 24.31 ± 10.61 a | 28.65 ± 12.44 a | 19.81 | ||
| Anthocyanins (mg/L) | 805.44 ± 35.83 b | 873.45 ± 35.55 a | 8.09 | ||
| Phen. comp. (mg/L) | 2209.56 ± 47.85 b | 2340.90 ± 47.13 a | 3.29 | ||
| DPPH (µg TEAC/mL) | 87.67 ± 24.50 a | 89.31 ± 18.71 a | 6.88 | ||
| ABTS (µg TEAC/mL) | 75.56 ± 24.94 a | 73.75 ± 18.07 a | 0.91 | ||
| Compounds | Varieties | Trellis | CV (%) | |
|---|---|---|---|---|
| Anthocyanins | Low | High | ||
| Cyanidin-3,5-dig. | ‘Bordô’ | 3.68 ± 0.06 bA | 3.98 ± 0.01 bA | 4.06 |
| ‘IAC 138-22 Máximo’ | 1.95 ± 0.001 cA | 2.05 ± 0.02 bA | ||
| ‘BRS Violeta’ | 78.67 ± 2.03 aA | 76.48 ± 1.02 aB | ||
| ‘Isabel’ | - | - | ||
| Malvidin-3,5-dig. | ‘Bordô’ | 79.97 ± 4.40 aA | 80.89 ± 3.81 aA | 4.10 |
| ‘IAC 138-22 Máximo’ | 55.86 ± 1.67 bA | 54.99 ± 0.81 bA | ||
| ‘BRS Violeta’ | 60.36 ± 2.02 bA | 57.95 ± 0.65 bA | ||
| ‘Isabel’ | 5.13 ± 0.001 cA | 6.05 ± 0.12 cA | ||
| Delphinidin-3-O-g. | ‘Bordô’ | 3.48 ± 0.001 bcA | 4.07 ± 0.36 cA | 8.05 |
| ‘IAC 138-22 Máximo’ | 3.90 ± 0.07 bB | 7.80 ± 0.29 bA | ||
| ‘BRS Violeta’ | 21.40 ± 1.75 aA | 19.76 ± 0.25 aB | ||
| ‘Isabel’ | 2.28 ± 0.03 cA | 3.19 ± 0.04 cA | ||
| Cyanidin-3-O-g. | ‘Bordô’ | 0.10 ± 0.04 bB | 0.21 ± 0.03 cA | 15.07 |
| ‘IAC 138-22 Máximo’ | 0.09 ± 0.001 bB | 0.34 ± 0.07 bA | ||
| ‘BRS Violeta’ | - | - | ||
| ‘Isabel’ | 0.40 ± 0.001 aB | 0.50 ± 0.01 aA | ||
| Peonidin-3-O-g. | ‘Bordô’ | 0.26 ± 0.001 cB | 0.31 ± 0.001 cA | 2.64 |
| ‘IAC 138-22 Máximo’ | 0.52 ± 0.02 bA | 0.51 ± 0.01 bA | ||
| ‘BRS Violeta’ | 0.17 ± 0.01 dA | 0.16 ± 0.001 dA | ||
| ‘Isabel’ | 2.10 ± 0.001 aB | 2.22 ± 0.005 aA | ||
| Malvidin-3-O-g. | ‘Bordô’ | 1.75 ± 0.04 cA | 1.74 ± 0.12 cA | 7.77 |
| ‘IAC 138-22 Máximo’ | 8.78 ± 0.20 aB | 12.77 ± 1.02 aA | ||
| ‘BRS Violeta’ | 0.31 ± 0.01 dA | 0.30 ± 0.01 dA | ||
| ‘Isabel’ | 6.20 ± 0.02 bA | 6.33 ± 0.02 bA | ||
| Flavonol | ||||
| Rutin | ‘Bordô’ | 1.80 ± 0.06 dB | 2.95 ± 0.15 dA | 4.25 |
| ‘IAC 138-22 Máximo’ | 5.28 ± 0.14 aA | 5.35 ± 0.03 aA | ||
| ‘BRS Violeta’ | 3.65 ± 0.26 bA | 3.45 ± 0.23 cA | ||
| ‘Isabel’ | 2.52 ± 0.01 cB | 4.57 ± 0.08 bA | ||
| Estilbene | ||||
| Trans-resveratrol | ‘Bordô’ | 0.05 ± 0.001 aA | 0.05 ± 0.001 aA | 0.37 |
| ‘IAC 138-22 Máximo’ | 0.05 ± 0.001 aA | 0.05 ± 0.001 aA | ||
| ‘BRS Violeta’ | 0.05 ± 0.001 aA | 0.05 ± 0.001 aA | ||
| ‘Isabel’ | 0.04 ± 0.001 bA | 0.04 ± 0.001 bA | ||
| Phenolic acids | ||||
| Gallic acid | ‘Bordô’ | 3.02 ± 0.06 aA | 2.75 ± 0.08 aB | 3.55 |
| ‘IAC 138-22 Máximo’ | 1.40 ± 0.03 cA | 1.36 ± 0.06 cA | ||
| ‘BRS Violeta’ | 2.16 ± 0.04 bA | 2.16 ± 0.10 bA | ||
| ‘Isabel’ | 0.84 ± 0.02 dB | 1.29 ± 0.05 cA | ||
| 3-hydroxytyrosol acid | ‘Bordô’ | 0.14 ± 0.01 bB | 0.21 ± 0.01 bA | 5.38 |
| ‘IAC 138-22 Máximo’ | 0.06 ± 0.01 cA | 0.07± 0.01 cA | ||
| ‘BRS Violeta’ | 0.45 ± 0.01 aA | 0.39 ± 0.01 aB | ||
| ‘Isabel’ | 0.09 ± 0.01 cA | 0.08 ± 0.01 cA | ||
| Caffeic acid | ‘Bordô’ | 9.19 ± 0.23 bA | 7.86 ± 0.21 bB | 2.20 |
| ‘IAC 138-22 Máximo’ | 7.02 ± 0.19 bA | 6.28 ± 0.07 cB | ||
| ‘BRS Violeta’ | 9.82 ± 0.28 aA | 9.66 ± 0.03 aA | ||
| ‘Isabel’ | 6.55 ± 0.13 cB | 8.01 ± 0.15 bA | ||
| Chlorogenic acid | ‘Bordô’ | 10.13 ± 0.30 dA | 9.54 ± 0.29 dB | 2.59 |
| ‘IAC 138-22 Máximo’ | 10.88 ± 0.36 cA | 11.01 ± 0.20 cA | ||
| ‘BRS Violeta’ | 14.31 ± 0.30 aA | 14.17 ± 0.38 aA | ||
| ‘Isabel’ | 13.15 ± 0.22 bA | 13.06 ± 0.23 bA | ||
| p-coumaric acid | ‘Bordô’ | 0.28 ± 0.01 bA | 0.24 ± 0.001 bB | 6.74 |
| ‘IAC 138-22 Máximo’ | 0.07 ± 0.001 cA | 0.08 ± 0.001 cA | ||
| ‘BRS Violeta’ | 0.46 ± 0.001 aB | 0.52 ± 0.04 aA | ||
| ‘Isabel’ | 0.07 ± 0.001 cB | 0.10 ± 0.001 cA | ||
| trans-cinnamic acid | ‘Bordô’ | - | - | - |
| ‘IAC 138-22 Máximo’ | - | - | ||
| ‘BRS Violeta’ | - | - | ||
| ‘Isabel’ | - | - | ||
| trans-ferulic acid | ‘Bordô’ | 0.21 ± 0.01 bA | 0.22 ± 0.02 bA | 3.12 |
| ‘IAC 138-22 Máximo’ | 0.15 ± 0.001 cA | 0.15 ± 0.001 cA | ||
| ‘BRS Violeta’ | 0.63 ± 0.001 aA | 0.61 ± 0.001 aB | ||
| ‘Isabel’ | 0.14 ± 0.001 cA | 0.13 ± 0.001 dA | ||
| Flavan-3-ol | ||||
| Catechin | ‘Bordô’ | 4.57 ± 0.16 bA | 4.21 ± 0.13 cA | 3.65 |
| ‘IAC 138-22 Máximo’ | 3.67 ± 0.11 cB | 4.93 ± 0.03 cA | ||
| ‘BRS Violeta’ | 20.76 ± 0.79 aB | 21.84 ± 0.19 aA | ||
| ‘Isabel’ | 2.12 ± 0.03 dB | 8.68 ± 0.15 bA | ||
| Total | ‘Bordô’ | 118.78 ± 3.71 bA | 119.40 ± 4.04 bA | 2.80 |
| ‘IAC 138-22 Máximo’ | 99.74 ± 2.82 cB | 107.77 ± 0.34 cA | ||
| ‘BRS Violeta’ | 213.24 ± 7.46 aA | 207.55 ± 0.41 aA | ||
| ‘Isabel’ | 41.67 ± 0.36 dB | 54.29 ± 0.91 dA | ||
| Whole Grape Juices | Color | Aroma | Flavor | Texture | Overall Acceptance |
|---|---|---|---|---|---|
| ‘Bordô’ low | 296.21 ab | 361.28 a | 328.06 ab | 312.05 a | 337.22 a |
| ‘Bordô’ high | 309.47 a | 356.16 a | 335.74 ab | 325.16 a | 336.64 a |
| ‘IAC 138-22 Máximo’ low | 305.17 ab | 228.47 b | 282.21 bc | 293.04 a | 272.92 b |
| ‘IAC 138-22 Máximo’ high | 305.79 ab | 213.65 b | 258.34 c | 279.28 a | 248.51 b |
| ‘BRS Violeta’ low | 266.19 abc | 209.46 b | 174.84 d | 215.21 b | 176.11 c |
| ‘BRS Violeta’ high | 270.00 abc | 220.90 b | 174.64 d | 217.14 b | 189.54 c |
| ‘Isabel’ low | 253.19 bc | 325.49 a | 369.85 a | 307.75 a | 349.06 a |
| ‘Isabel’ high | 237.97 c | 328.59 a | 320.33 ab | 294.36 a | 334.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingues Neto, F.J.; Pimentel Junior, A.; Borges, C.V.; Rodrigues, J.D.; Figueira, R.; Moura, M.F.; Minatel, I.O.; Nunes, A.; Lima, G.P.P.; Tecchio, M.A. Polyphenolic Profile and Antioxidant Activity of Whole Grape Juices from Vitis labrusca and Brazilian Hybrid Grapes in Two Training Systems. Antioxidants 2024, 13, 1132. https://doi.org/10.3390/antiox13091132
Domingues Neto FJ, Pimentel Junior A, Borges CV, Rodrigues JD, Figueira R, Moura MF, Minatel IO, Nunes A, Lima GPP, Tecchio MA. Polyphenolic Profile and Antioxidant Activity of Whole Grape Juices from Vitis labrusca and Brazilian Hybrid Grapes in Two Training Systems. Antioxidants. 2024; 13(9):1132. https://doi.org/10.3390/antiox13091132
Chicago/Turabian StyleDomingues Neto, Francisco José, Adilson Pimentel Junior, Cristine Vanz Borges, João Domingos Rodrigues, Ricardo Figueira, Mara Fernandes Moura, Igor Otavio Minatel, Aline Nunes, Giuseppina Pace Pereira Lima, and Marco Antonio Tecchio. 2024. "Polyphenolic Profile and Antioxidant Activity of Whole Grape Juices from Vitis labrusca and Brazilian Hybrid Grapes in Two Training Systems" Antioxidants 13, no. 9: 1132. https://doi.org/10.3390/antiox13091132
APA StyleDomingues Neto, F. J., Pimentel Junior, A., Borges, C. V., Rodrigues, J. D., Figueira, R., Moura, M. F., Minatel, I. O., Nunes, A., Lima, G. P. P., & Tecchio, M. A. (2024). Polyphenolic Profile and Antioxidant Activity of Whole Grape Juices from Vitis labrusca and Brazilian Hybrid Grapes in Two Training Systems. Antioxidants, 13(9), 1132. https://doi.org/10.3390/antiox13091132






