Abstract
Plants have evolved complex mechanisms to cope with diverse abiotic stresses, with the phenylpropanoid pathway playing a central role in stress adaptation. This pathway produces an array of secondary metabolites, particularly polyphenols, which serve multiple functions in plant growth, development, regulating cellular processes, and stress responses. Recent advances in understanding the molecular mechanisms underlying phenylpropanoid metabolism have revealed complex regulatory networks involving MYB transcription factors as master regulators and their interactions with stress signaling pathways. This review summarizes our current understanding of polyphenol-mediated stress adaptations in plants, emphasizing the regulation and function of key phenylpropanoid pathway compounds. We discussed how various abiotic stresses, including heat and chilling stress, drought, salinity, light stress, UV radiation, nanoparticles stress, chemical stress, and heavy metal toxicity, modulate phenylpropanoid metabolism and trigger the accumulation of specific polyphenolic compounds. The antioxidant properties of these metabolites, including phenolic acids, flavonoids, anthocyanins, lignin, and polyphenols, and their roles in reactive oxygen species scavenging, neutralizing free radicals, membrane stabilization, and osmotic adjustment are discussed. Understanding these mechanisms and metabolic responses is crucial for developing stress-resilient crops and improving agricultural productivity under increasingly challenging environmental conditions. This review provides comprehensive insights into integrating phenylpropanoid metabolism with plant stress adaptation mechanisms, highlighting potential targets for enhancing crop stress tolerance through metabolic adjustment.
1. Introduction
Challenging ecological and atmospheric conditions pose various environmental challenges to plants during their growth and development, causing significant threats to global agricultural productivity and sustainability [1]. Plants have evolved diverse adaptive mechanisms to ensure their survival under adverse conditions, with the phenylpropanoid pathway and its derived bioactive molecules, particularly polyphenols, playing crucial roles in stress tolerance [2]. This metabolic pathway, extensively studied over the past two decades, produces essential bioactive compounds that facilitate plant adaptation and survival under unfavorable conditions [3]. The phenylpropanoid pathway begins with an amino acid phenylalanine, followed by a complex series of enzymatic reactions, ultimately yielding various classes of bioactive polyphenolic compounds including phenolic acids, flavonoids, anthocyanins, proanthocyanidins, lignin, stilbenes, and hydroxybenzoic acid derivatives [3,4]. These bioactive polyphenol compounds serve multiple functions in plants, from structural support to chemical defense, and, most notably, protection against various abiotic and biotic stresses [5]. Understanding the modulation of the phenylpropanoid pathway under stress conditions has become increasingly important for developing stress-resistant crops, sustainable production, and ensuring food security [3,4,5,6]. This pathway synthesizes more than 8000 different types of bioactive compounds [7,8], which are known for their antioxidant properties and have diverse roles in plant defense. Recent advances in metabolomics and molecular biology have revealed the intricate regulation of this pathway and its responsiveness to unfavorable environmental stimuli [9].
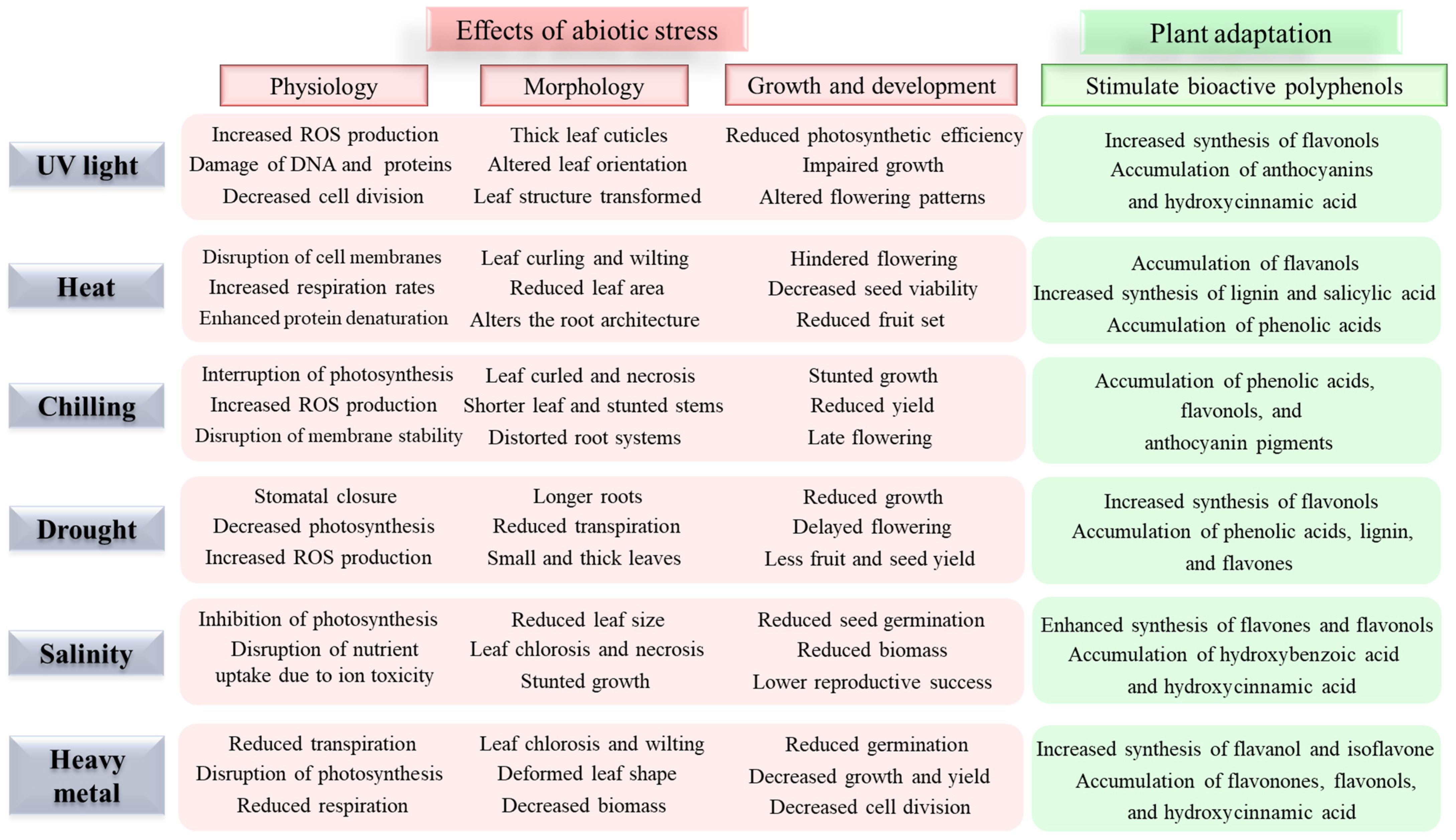
Abiotic stresses, including drought, heavy metals, salinity, extreme temperatures, UV light, and oxidative stress, significantly impact plant physiology and agricultural productivity [1,10]. These stresses trigger the production of reactive oxygen species (ROS), leading to oxidative damage of cellular components and impaired physiological functions (Figure 1). To cope with these stresses, plants actively accumulate specific bioactive polyphenolic compounds as part of their stress response mechanism [3,9]. The role of bioactive polyphenols in abiotic stress tolerance is multifaceted [5,6,11,12,13,14,15,16,17]. These compounds function as powerful antioxidants, protecting cellular components from ROS-induced oxidative damage [18,19]. Additionally, polyphenols contribute to membrane stability, osmolyte balance, neutralizing ROS, scavenging free radicals, and the signal transduction pathways involved in stress responses [1,4,5]. Recent studies have demonstrated that different classes of polyphenols exhibit varying levels of effectiveness against specific stresses, suggesting a complex and finely tuned defense mechanism [3,4,6,20].
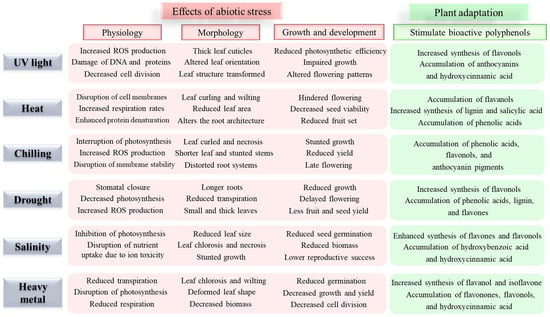
Figure 1.
Abiotic stresses affect the physiology, morphology, growth, and development of plants and the plants’ adaptive response by stimulating bioactive polyphenols [3,4,5,6].
The regulation of the phenylpropanoid pathway and polyphenol molecule accumulation exhibits significant variation across plant species and among different tissues within the same plant [3]. Environmental factors significantly influence the expression of key enzymes [21,22,23] and the accumulation of specific bioactive polyphenols [1,24,25,26]. This inherent variability presents both challenges and opportunities for research focused on enhancing plant abiotic stress tolerance through the identification and manipulation of potential molecular targets [3,23].
This review aims to integrate current knowledge regarding the modulation of the phenylpropanoid pathway and the role of MYB regulatory factors in regulating polyphenolic compounds to enhance plant stress tolerance under various abiotic stress conditions. We will examine the molecular mechanisms governing pathway regulation, the specific functions of different polyphenol classes in stress responses, specify the individual polyphenolic compounds that accumulate in response to specific abiotic stress, and highlight the role of phenolic compounds in neutralizing the reactive oxygen species and free radicals. This understanding has significant implications for agricultural sustainability. Deciphering these aspects is crucial for developing strategies to enhance crop resilience under increasing environmental challenges.
2. The MYB Family Emerges as Particularly Significant in Phenolic Metabolism Regulation and Abiotic Stress Tolerance
MYB transcription factors operate within complex regulatory networks, where individual factors can regulate multiple pathway genes, while single genes may be subject to regulation by MYB proteins [27]. These transcription factors frequently function within larger protein complexes, notably the MBW complex comprising MYB, bHLH, and WD40 transcription factors, and serve as master regulators of flavonoid biosynthesis, specifically in anthocyanin production [27,28,29]. Comprehensive functional characterization of these transcriptional regulators has been achieved within plant species, including Arabidopsis thaliana, Helianthus annuus L., Camellia sinensis, Medicago truncatula, Narcissus tazetta, and Vitis vinifera [30]. The elucidation of regulatory mechanisms governing phenolic metabolism offers substantial potential for enhancing the production of bioactive compounds, thereby improving both abiotic stress tolerance and the yield of medicinally valuable secondary metabolites in plants.
Transcription factors function as specialized proteins interacting with specific promoter regions to stimulate transcription initiation in response to endogenous and exogenous signals, including phytohormones and abiotic stresses [22]. The phenylpropanoid pathway is regulated at the transcriptional and post-transcriptional levels through various molecular mechanisms [22,23]. Multiple transcription factors, including the MYB, bHLH, and WRKY families, serve as key modulators to activate the expression of key enzymatic genes involved in this pathway, thereby accumulating increased levels of bioactive polyphenolic compounds in plant tissues [27]. The MYB family is particularly significant in phenolic metabolism regulation, functioning as an essential mechanism in plant stress responses. Studies have demonstrated that MYB genes exhibit multiple regulatory functions within the phenylpropanoid pathway and flavonoid biosynthesis pathway, serving as both activators and repressors of gene expression [31]. Recent research has revealed the distinct functional roles of MYB transcription factors across various plant species [32]. In the Musa cultivar Rasthal, the overexpression of the MusaMYB31 gene demonstrates suppressive effects on phenylpropanoid and flavonoid enzymatic pathway genes [33]. Conversely, studies in the Populus species identified the PtMYB115 gene as a positive regulator that promotes proanthocyanidin accumulation through direct interaction with the promoter regions of anthocyanidin reductase 1 (ANR1) and leucoanthocyanidin reductase 3 (LAR3) genes [34]. The Vitis vinifera VvMYB5a gene exhibits a unique regulatory mechanism by simultaneously promoting flavonoid synthesis while suppressing lignin production, maintaining metabolic homeostasis between these pathways [32]. The crucial role of MYB transcription factors in regulating secondary metabolite biosynthesis (especially phenolic compounds) and enhancing plant stress tolerance are documented in Table 1. The MYB transcription factors function through multiple regulatory mechanisms, primarily by modulating key enzymes in the phenylpropanoid pathway, including chalcone synthase (CHS), chalcone isomerase (CHI), flavanone-3-hydroxylase (F3H), and flavonol synthase (FLS). These factors orchestrate the accumulation of protective compounds such as flavonoids, anthocyanins, and other phenolic compounds that are powerful antioxidants under abiotic stress conditions (Table 1). Notable examples include AtMYB12, which enhances drought and salt tolerance through flavonoid biosynthesis (Table 1). Stress-protective mechanisms generally involve ROS scavenging, maintaining membrane integrity, and activating stress-responsive genes. This understanding opens new avenues to produce genetically engineered crops with enhanced stress tolerance through the targeted manipulation of MYB transcription factors.

Table 1.
Role of MYB transcription factors as master regulators of secondary metabolism and abiotic stress tolerance in plants.
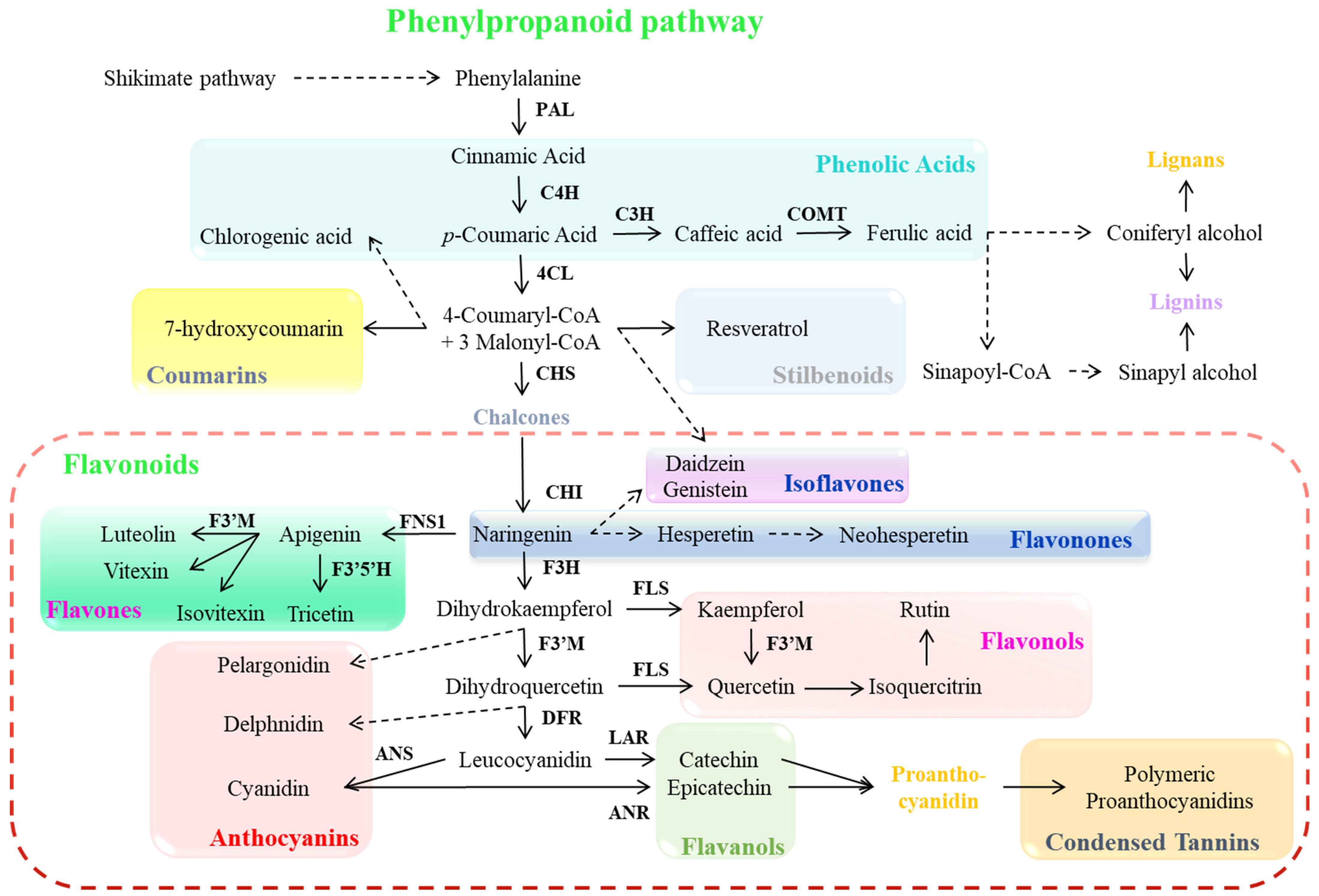
Plant secondary metabolites, such as phenolic compounds, are synthesized through the shikimate pathway, which derives its name from its key intermediate shikimic acid, facilitating the production of aromatic amino acids and phenolic compounds (Figure 2). This pathway initiates with primary metabolites, phosphoenolpyruvate and erythrose-4-phosphate, which combine to form 3-deoxy-D-arabinohexulose-7-phosphate and are then transformed into shikimic acid, ultimately yielding various hydroxybenzoic acids (including p-hydroxybenzoic, protocatechuic, and gallic acids) [3]. This metabolic sequence ends in the biosynthesis of phenylalanine, a crucial amino acid from which the phenylpropanoid pathway begins. The phenylalanine is converted into cinnamic acid by the action of the phenylalanine ammonia-lyase (PAL) enzyme (Figure 2) [61]. The phenylpropanoid pathway proceeds further via the cinnamate 4-hydroxylase (C4H) enzyme reaction that converts cinnamic acid into p-coumaric acid (also known as trans-p-hydroxycinnamate), generating hydroxycinnamic acids, a subgroup of phenolic acids [3,62]. These intermediates undergo various modifications within plant tissues, including β-oxidation to form hydroxybenzoic acids, reduction to produce lignin-associated cinnamic alcohols, formation of acyl derivatives and complex esters like chlorogenic acid, and coumarin synthesis [61,62]. The p-coumaroyl-CoA combines with three malonyl-CoA molecules to form chalcone, catalyzed by chalcone synthase (CHS). Chalcone isomerase (CHI) then converts chalcone naringenin to flavanone naringenin. Following redox modifications of the central heterocyclic ring, naringenin serves as a precursor for diverse flavonoid classes, including flavanols, flavanones, flavones, flavonols, and anthocyanins, though not chalcones or dihydrochalcones [61,62,63]. After that, a series of enzymatic reactions (flavanone-3-hydroxylase, flavone synthase 1, flavonol synthase, flavonoid 3′-monooxygenase, flavonoid 3′5′-hydroxylase, dihydroflavonol 4-reductase, anthocyanidin synthase, leucoanthocyanidin reductase, and anthocyanidin reductase) lead to the production of various phenolic compounds and pigmented anthocyanins and proanthocyanidins (Figure 2) [23,32,62,63].
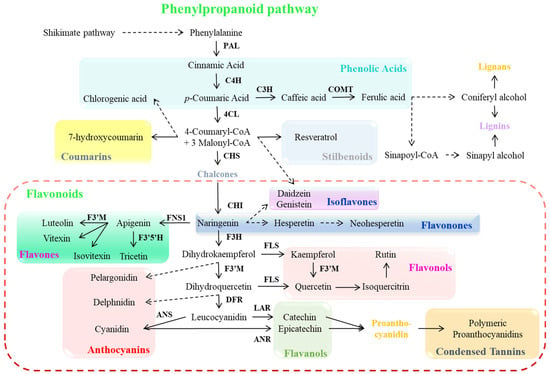
Figure 2.
Phenylpropanoid pathway and its biosynthesis genes. Dashed arrows indicate more than one step. Abbreviations: PAL (phenylalanine ammonia lyase), C4H (cinnamate 4-hydroxylase), C3H (cinnamate-3-hydroxylase), COMT (caffeic acid O-methyltransferase), 4CL (4-coumarate: CoA ligase), CHS (chalcone synthase), CHI (chalcone isomerase), F3H (flavanone-3-hydroxylase), FNS1 (flavone synthase 1), FLS (flavonol synthase), F3′M (flavonoid 3′-monooxygenase), F3′5′H (flavonoid 3′5′-hydroxylase), DFR (dihydroflavonol 4-reductase), ANS (anthocyanidin synthase), LAR (leucoanthocyanidin reductase), and ANR (anthocyanidin reductase).
The biosynthesis of phenolic compounds represents a complex metabolic network where monomeric forms serve as intermediates rather than terminal products. These compounds transform into more complicated oligomeric and polymeric structures [64]. A notable example is the formation of proanthocyanidins, which are complex derivatives synthesized from flavan-3-ol precursors and are widely distributed across plant species [65]. The final phase of the flavonoid pathway involves proanthocyanidins biosynthesis, though the precise mechanisms governing flavanol (such as catechin and epicatechin) condensation into proanthocyanidins remain to be fully elucidated [31]. Current evidence suggests two potential routes for this condensation process: an enzyme-mediated pathway utilizing peroxidase, polyphenol oxidase, and laccase, or a spontaneous non-enzymatic auto-condensation of flavanol units [66]. Recent advances in molecular biology and functional genomics have shifted the research focus toward understanding the genetic basis and regulation of phenolic metabolism, while acknowledging the continued importance of traditional biochemical approaches [31,62]. This molecular perspective has enabled significant progress in genetic engineering strategies aimed at optimizing phenolic profiles in plants, particularly for enhancing the production of bioactive compounds to enhance the abiotic stress tolerance of crops [23].
3. The Role of Polyphenolic Compounds in Abiotic Stress Tolerance
Abiotic stresses trigger the production of ROS within plant cells, which demonstrate significant reactivity with different cellular components, such as lipids, nucleic acids, proteins, and cell membranes, potentially leading to cellular dysfunction and death [67]. In response, plants stimulate the biosynthesis of antioxidant secondary metabolites, particularly polyphenolic compounds, as a defense mechanism and adaptive strategy in response to abiotic stresses [68]. These compounds significantly enhance plant tolerance against multiple abiotic stressors, including salinity, heavy metal toxicity, drought, heat stress, chilling injury, UV radiation, and other abiotic stresses [69,70]. The increased synthesis and rapid accumulation of these compounds having potent antioxidative properties during stress conditions can effectively quench the ROS, thereby reducing cellular membrane damage, and serving as a key indicator of plant tolerance and resistance capacity against oxidative stress [71].
Research demonstrates that plants under stress conditions trigger the biosynthesis of polyphenolic compounds compared to those growing in normal conditions [72,73,74,75,76]. The regulation of phenolic compound biosynthesis under stress involves complex enzymatic pathways, with key enzymes such as PAL and CHS playing crucial roles in modulating phenolic synthesis. Under different abiotic stresses, plants regulate multiple genes encoding essential enzymes, including PAL, C4H, C3H, 4CL, COMT, CHS, CHI, F3H, DFR, F3′M, FLS, ANR, and ANS [20]. The elevated expression of these genes results in the enhanced biosynthesis of diverse bioactive polyphenolic compounds, consequently enabling plant resilience through sophisticated stress tolerance mechanisms under adverse environmental conditions.
3.1. Temperature
Plants exhibit significant adaptability across a vast range of temperature conditions; nevertheless, temperature fluctuations can significantly impact plant morphology, growth, and development throughout the lifecycle [77]. Temperature stress, including heat and chilling conditions, induces plants to synthesize endogenous bioactive phenolic compounds. These compounds include various classes such as phenolic acids, anthocyanins, flavonoids, flavones, and flavonols, which serve as crucial protective molecules in plant cells during stress responses [78,79]. Phenolic compounds are crucial protective biomolecules against temperature-induced stress conditions [80]. Research has shown that chilling or cold stress exposure induces enhanced anthocyanin accumulation in plant tissues [81], concurrent with the increased expression of several flavonoid biosynthesis pathway genes including PAL, C4H, 4CL, CHI, DFR, and ANS (Figure 3). Brassica rapa has revealed strong associations between cold tolerance and the expression of anthocyanin biosynthesis pathway genes, specifically dihydroflavonol-4-reductase (DFR) and anthocyanidin synthase (ANS) [82,83]. In Malus sieversii, low-temperature exposure promotes anthocyanin accumulation through the regulatory action of the MdMYBPA1 transcription factor [65].
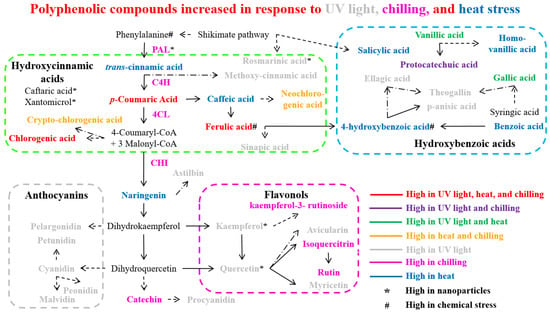
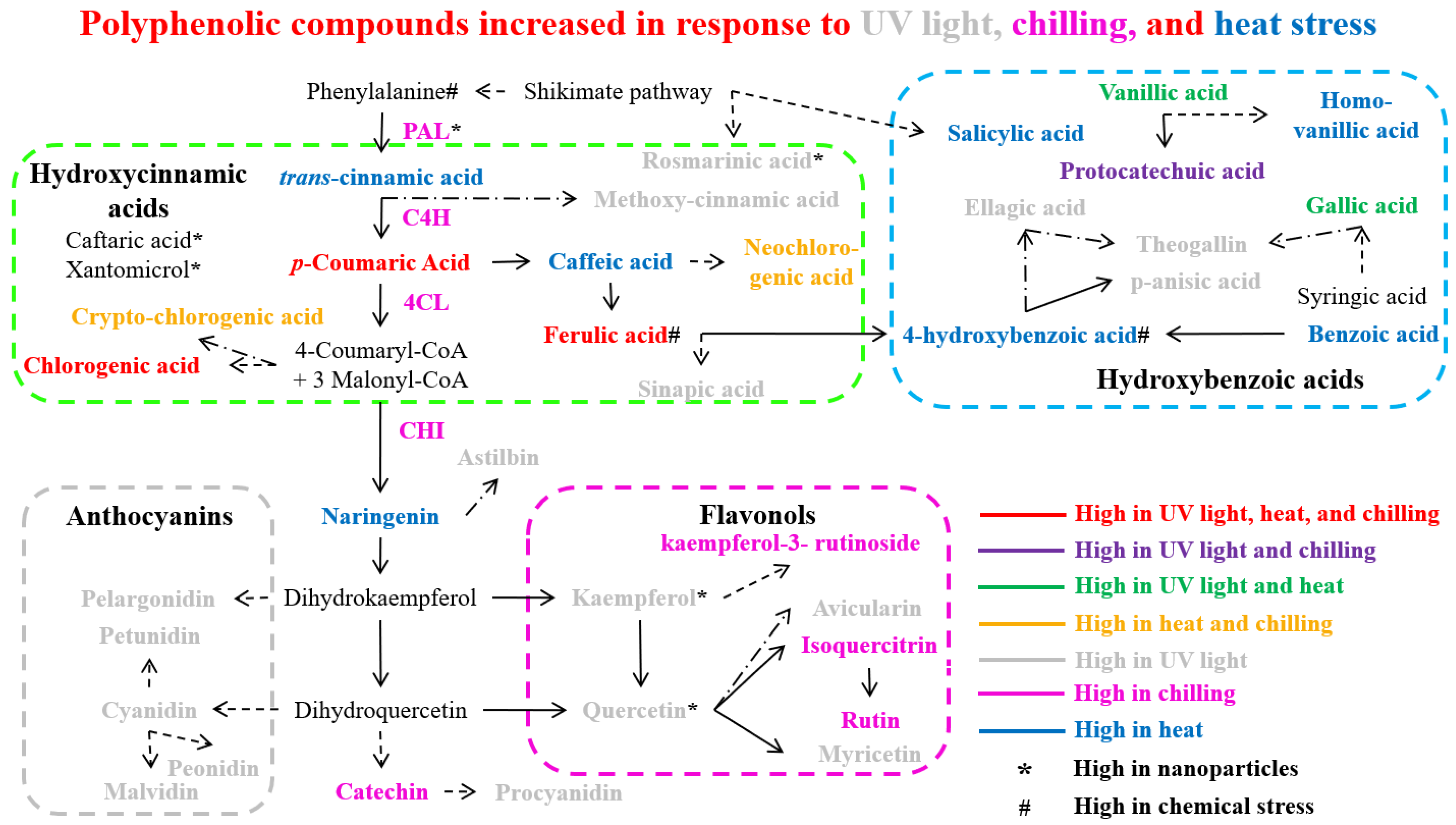
Figure 3.
Polyphenolic compounds accumulate in plants in response to abiotic stress such as light stress, temperature stress, nanoparticles, and chemical stress. Dashed arrows indicate more than one step, and dash-dot arrows denote an undefined pathway. Abbreviation: PAL (phenylalanine ammonia lyase), C4H (cinnamate 4-hydroxylase), 4CL (4-coumarate: CoA ligase), and CHI (chalcone isomerase).
Under chilling conditions, increased lignin deposition in epidermal cell layers enhances cell wall rigidity, providing a defense against chilling-induced cellular damage and dehydration [84]. Lignin biosynthesis during extreme temperature stress involves regulatory control by C2H2Zn and MYB transcription factors [85]. During chilling stress exposure, plants increase the production of cell wall-associated phenolics like suberin and lignin, which strengthen cellular barriers against chilling injury [86]. The enhanced cell wall thickness resulting from phenolic deposition helps prevent cellular collapse/damage under chilling conditions [19]. Cold-induced phenolic biosynthesis involves the upregulation of key enzymes including PAL, hydroxycinnamoyl transferase (HCT), and cinnamyl-alcohol dehydrogenase (CAD), and increased level of phenolic compounds, protecting the plant cells from chilling injury [87]. Research on peach trees has shown that 24-epibrassinolide-mediated phenolic accumulation aids in scavenging reactive oxygen species generated during cold stress [78]. In purple head Chinese cabbage (Brassica rapa L.) varieties, cold stress activates regulatory genes BrTT8 and BrMYB2, which stimulate the expression of different anthocyanin biosynthesis genes including BrDFR1, BrANS1, Br5MAT, BrUGT79B1, and DrUGT75C1 to accumulate large quantities of anthocyanin pigments in cabbage tissues [88]. In addition, lignin, a dominant complex phenolic polymer in plants, accumulates within cell walls and contributes to chilling tolerance [84].
High temperature or heat stress during plant growth influences antioxidant systems and polyphenol accumulation. In Solanum lycopersicon, flavonols accumulated under heat stress can efficiently scavenge the ROS and enhance the Solanum lycopersicon adaptability against high-temperature stress [89]. Similarly, elevated temperatures trigger increased flavonoid and phenylpropanoid production in Glycine max [90]. Likewise, Vitis vinifera revealed temperature-dependent responses varying between day and night cycles, with flavonol content remaining unchanged at 35 °C in darkness and declining at 45 °C regardless of photoperiod [91]. Festuca trachyphylla exposed to heat stress revealed significant increases in multiple polyphenols, including vanillic acid, 4-hydroxybenzoic acid, cinnamic acid, caffeic acid, coumaric acid, homovanillic acid, ferulic acid, gallic acid, salicylic acid, and benzoic acid [79]. This enhanced accumulation of phenolics correlates with improved heat stress tolerance [79].
Recent molecular studies of Chrysanthemum × morifolium cultivar ‘Fencui’ identified CmMYB012 (R2R3-MYB transcription factor), which suppresses flavonoid biosynthesis under prolonged heat exposure by downregulating the expression of flavonoid biosynthesis genes such as CmCHS, CmDFR, CmANS, and CmUFGT (UDP-glucose: flavonoid 3-O-glucosyltransferase) [92]. These results showed how different polyphenols respond to temperature stress: chilling conditions generally promote anthocyanin biosynthesis [88], while heat stress typically inhibits these pathways [92,93]. These temperature-mediated changes in polyphenol biosynthesis are governed by the coordinated expression of specific biosynthetic genes and regulatory elements. Research on carrots showed that specific phenolics—coumaric acid, caffeic acid, and anthocyanins—accumulate to reduce heat-induced oxidative damage [79]. Additionally, salicylic acid is a signaling molecule that promotes phenolic biosynthesis during high-temperature stress, thereby enhancing heat resistance by detoxifying ROS [94]. These results showed that plant species modify their phenolic metabolism in response to heat or chilling stress to tolerate unfavorable temperature conditions.
3.2. Light
Light represents a fundamental environmental factor that significantly influences plant growth and development [95,96,97]. This crucial factor coordinates with different metabolic pathways, particularly the synthesis of phenolic compounds [75,98,99]. Light regulates several enzymatic genes within the phenylpropanoid pathway, resulting in enhanced production and accumulation of antioxidative polyphenols [63]. The formation of chloroplasts, essential biosynthetic sites for secondary metabolites, is also light-dependent [100]. However, extreme light exposure can simultaneously function as abiotic stress, trigger cellular ROS levels, and potentially initiate oxidative stress responses [101,102].
Multiple studies have revealed a positive correlation between light exposure and antioxidant phenolic compound synthesis within plant tissues. Research has shown that light stimulates the production of various bioactive molecules, including flavonoids, anthocyanins, and proanthocyanidins [75,98,99], in both foliar tissues and Camellia sinensis in vitro cultures [103,104]. Notably, a strong correlation was revealed between proanthocyanidin accumulation in leaf tissues and the expression of key phenylpropanoid pathway genes, specifically PAL, F3H, F3′H, DFR, and ANR [64,66]. Moreover, a high accumulation of O-glycosylated flavonols is associated with the expression of CHS and F3′5′H, highlighting the complex regulatory network governing polyphenols in response to light [105,106]. The overexpression of the Citrus sinensis UGT gene enhanced the total content of flavonoids, anthocyanins, and proanthocyanidins in transgenic Arabidopsis thaliana leaves and induced high-light stress tolerance by elevating the antioxidant activity and capacities of transgenic plants [75].
Research has revealed significant interest in understanding how different light wavelengths influence polyphenol biosynthesis and accumulation in plant tissues [63]. Under blue light conditions, B. napus and B. oleracea have shown increased polyphenol biosynthesis [107]. In Camellia japonica (callus cultures), optimal phenols and flavonoid accumulation were achieved by both red-blue or blue-green light combinations [108]. The molecular mechanisms underlying these responses involve light-sensitive transcription factors. Notably, Fagopyrum tataricum FtMYB6 (R2R3-MYB transcription factor) exhibits light-induced promoter activity. This factor enhances flavonol biosynthesis by increasing the regulation of FtF3H and FtFLS1 promoters while downregulating the Ft4CL promoter [109].
Plant responses to light are mediated through specialized photoreceptors that detect specific spectral ranges: far-red (700–775 nm), red (620–700 nm), green (500–580 nm), and blue (445–500 nm) [110]. The efficacy of these photoreceptors depends on light intensity and duration [110]. This suggests potential applications in commercial plant cultivation through controlled artificial lighting systems to optimize bioactive polyphenols biomolecule production. Despite extensive research into light-mediated polyphenol accumulation and biosynthesis regulation, the precise mechanisms remain incompletely understood [96,104]. The current understanding primarily covers species–specific responses, which vary considerably based on environmental factors, physiological conditions, and light exposure parameters. This complexity highlights the continuing importance of researching the biochemical and molecular aspects of light-mediated polyphenol biosynthesis in plant biology and biochemistry research.
UV Radiation
To date, climate change is one of the most significant environmental challenges facing our planet. Of particular concern is the increasing exposure to harmful solar rays, specifically ultraviolet (UV) radiation, which is stimulated by various factors including greenhouse gas (CO2, CH4, and N2O) emissions, cloud dynamics, and surface albedo from ice and snow cover [111]. The UV light spectrum involves three distinct bands: UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (200–280 nm) [112,113]. UV-A readily penetrates the ozone layer, and UV-C radiation is completely blocked by our planet’s atmosphere. However, UV-B radiation, comprising 5% of total UV radiation, emerges as a significant stress factor for living organisms. UV-B radiation exposure is intense in mountainous regions and areas suffering from ozone depletion. Exposure to UV-B radiation induces changes in plant systems, affecting their physiological, morphological, biochemical, and genetic properties, with responses proportional to radiation strength and duration [112]. Common manifestations include impaired growth, reduced productivity, increased ROS production, decreased photosynthetic capacity, enhanced mutagenesis, and damaged DNA and protein structures (Figure 1). The harmful impact of UV-B radiation displays both immediate and delayed responses linked to altered biosynthetic pathways [114,115].
Interestingly, plants have evolved superior UV-B tolerance compared to other living organisms, largely due to the biosynthesis of protective, biologically active metabolites. Among these, polyphenol antioxidants are prominent due to their dual protective functions: neutralizing or quenching ROS and absorbing short-wavelength radiation, thereby providing metabolic and physical cellular protection [116,117]. For example, when Rosa damascena cell cultures were exposed to UV-B radiation they accumulated fifteen-fold flavonoids compared to the control conditions [118]. Similarly, olive leaves exposed to UV-B showed increased production of specific flavonoids like methoxy-luteolin derivatives and elevated levels of β-hydroxy-verbascoside [119]. Furthermore, in Mangifera indica, UV-B exposure triggered phenylpropanoid pathway-related genes, where specific chalcone synthase genes (MiCHS4, MiCHS1, MiCHS17) showed enhanced activity [120]. However, plant responses to UV-B radiation depend on inherent phenolic compound levels, their compositional diversity, and tissue distribution patterns.
Plants accumulate the endogenous antioxidant phenolic compounds that form protective shields beneath the epidermal layer. These compounds exhibit significant efficiency in inhibiting thymine dimerization and protecting crucial cellular enzymes, including NAD/NADP, from photooxidative damage. UV-B radiation induces cellular damage in plants through multiple mechanisms, primarily by disrupting protein structures, triggering DNA mutations, and stimulating the production of ROS [121]. In the past few decades, research has shown that plants respond to UV radiation by enhancing flavonoid biosynthesis, resulting in improved UV absorption capabilities and increased radiation tolerance [122]. Flavonoids serve dual protective functions by absorbing both visible light (through pigmented flavonoids such as anthocyanins) and UV radiation (via anthocyanins and non-pigmented flavonoids), effectively protecting the cellular organelles of plants from radiation stress [123]. This adaptation is particularly obvious in high-altitude plants, which synthesize significantly higher concentrations of bioactive polyphenolic molecules than temperate plants [124]. UV radiation stress regulates multiple flavonoid biosynthesis genes, including CHS, CHI, DFR, FLS, F3H, ANS, and PAL [125]. Furthermore, UV light influences polyphenol biosynthesis via both jasmonate-dependent and independent signaling cascades [126], with abscisic acid (ABA) playing a crucial role in regulating UV-induced polyphenols synthesis [127]. These complex molecular mechanisms collectively contribute to plants’ adaptive responses to UV stress.
Various plant species demonstrate significant alterations in phenolic compound profiles under different environmental conditions. In Arbutus unedo, elevated levels of specialized phenolics including theogallin, avicularin, and juglanin were observed [128]. Similarly, Broccoli sprouts exhibited an increased accumulation of gallic and sinapic acids [129]. Studies on Caryopteris mongolica revealed an enhanced biosynthesis of flavonoids and anthocyanidins, correlating with increased PAL and CHI enzymatic activities [130]. The total content of phenols was increased in tomatoes (Solanum lycopersicum) under UV stress [131]. In Cuminum cyminum, upregulation of DAHP and PAL gene expression led to higher total phenolic and anthocyanin contents [124]. Analysis of Fragaria x ananassa (strawberries) showed the accumulation of kaempferol, ellagic acid, and glucoside derivatives of cyanidin, pelargonidin, and quercetin, accompanied by the upregulation of key flavonoid pathway genes (CHS, CHI, DFR, FLS, UFGT) [125]. Correspondingly, Kalanchoe pinnata displayed elevated levels of total flavonoid and quercitrin content [132]. Studies in Lactuca sativa revealed increased levels of phenolic acids (methoxycinnamic, rosmarinic, p-anisic, vanillic, and chlorogenic acids) alongside enhanced PAL activity and expression [133,134]. In addition, the Ribes nigrum accumulated higher levels of flavonols, anthocyanins, and phenolic acids after UV-B stress [135]. Studies on Triticum aestivum showed temporal changes in phenolic profiles after UV exposure, with increases in ferulic, p-coumaric, and vanillic acids [136], and enhanced the activities of PAL, C4H, 4CL, and COMT [137]. In Vigna radiata, elevated flavonoid and phenol contents correlated with increased PAL and CHI activities [138]. Vitis vinifera exhibited an increased accumulation of various phenolic compounds including anthocyanins (cyanidin, petunidin, peonidin, and malvidin), flavonoids (quercetin, myricetin, and kaempferol) [139], and phenolic acids (gallic, protocatechuic, and vanillic acids) [127]. The accumulation patterns of bioactive polyphenol compounds (including phenolic acids, flavonoids, and anthocyanins) through the phenylpropanoid biosynthetic pathway in various plant species under UV light stress conditions are presented in Figure 3.
3.3. Nanoparticles and Agrochemical Applications
Environmental factors including nanoparticle exposure and agrochemical applications significantly impact phenolic compound metabolism in plants [87,140]. These stressors enhance the biosynthesis of phenolic compounds and play a crucial role in developing stress tolerance mechanisms. The application of various nanoparticles has also been shown to modulate phenylpropanoid metabolism across different plant species. In Dracocephalum kotschyi, silicon dioxide nanoparticles enhanced the accumulation of phenols, flavonoids, rosmarinic acid, and xantomicrol, accompanied by an increased expression of PAL and rosmarinic acid synthase (RAS) genes [141]. The titanium nanoparticle treatment of Vitis vinifera increased phenolic compounds, particularly caftaric acid and flavonol derivatives including quercetin and kaempferol [142]. Similarly, copper nanoparticles enhanced the accumulation of both phenolic and flavonoid compounds in Withania somnifera [140]. Silver nanoparticles induced higher phenolic content in Solanum Lycopersicon [143], while zinc nanoparticles elevated phenolic and anthocyanin levels in Solanum tuberosum [144]. Agrochemicals such as insecticide application also induce phenolic biosynthetic pathways to accumulate phenolic compounds that contribute to pesticide stress in different plant species [145]. In Brassica juncea, insecticide treatment led to the enhanced accumulation of phenolic and polyphenolic compounds [146]. Furthermore, increased levels of phenols and anthocyanins were observed in the leaves of Brassica juncea L., concurrent with the upregulated expression of key biosynthetic genes including PAL and chalcone synthase [145,147]. Similar effects were noted in Oryza sativa, where insecticide application elevated specific phenylpropanoid pathway metabolites, notably phenylalanine, p-hydroxybenzoic acid, and ferulic acid [148].
3.4. Drought Stress
Water deficit or drought conditions trigger ROS production, including both radical forms such as superoxide radical (O2•−), alkoxyl (RO•), and hydroxyl radicals (•OH), as well as non-radical species including singlet oxygen molecules (1O2) and hydrogen peroxide (H2O2) compounds [68]. Prolonged drought stress induces elevated levels of reactive oxygen species, specifically hydrogen peroxide and singlet oxygen [20]. These ROS accumulate as products of oxidative stress under drought conditions [149]. Transcriptomic and metabolomic analyses revealed that plants exhibit elevated accumulation of bioactive phenolic compounds, which serve a crucial function in mitigating the adverse effects of drought stress [150]. Water stress conditions induce the biosynthesis of bioactive flavonols, which is directly correlated with increased drought tolerance [151,152,153]. Furthermore, drought conditions modulate the biosynthetic pathways governing phenolic acids, flavonoids, and anthocyanins, resulting in increased levels of antioxidative biomolecules that serve as robust antioxidants to protect plants from drought stress damage [154,155]. Tomato (Solanum lycopersicum) plants have shown increased concentrations of specific polyphenols, particularly kaempferol and quercetin, corresponding with enhanced drought resistance [156].
The overexpression of the Citrus sinensis CsCYT75B1 (CYTOCHROME P450) gene increases the total contents of phenolics and flavonoids in transgenic Arabidopsis thaliana leaves and induces drought tolerance by elevating the antioxidant properties of transgenic plants [153]. Within plant cells, the increased concentration of flavonoids in the cytoplasm effectively neutralizes drought-induced H2O2 molecules [156]. These flavonoids undergo oxidation followed by ascorbic acid-dependent reconversion to primary metabolites. This drought-induced polyphenol accumulation primarily results from the activation of several genes that encode essential enzymes associated with the phenylpropanoid biosynthetic pathway, thereby promoting increased phenolic compound synthesis [151,153]. After 21 days of drought exposure, the Achillea species exhibited increased concentrations of various phenolic compounds, including luteolin-7-O-glycoside, apigenin, chlorogenic acid, rutin, kaempferol, caffeic acid, luteolin, and 1,3-dicaffeoylquinic acid. This was accompanied by enhanced transcription levels of key enzymes, PAL, CHS, CHI, F3H, F3′H, F3′5′H, and FLS [73], along with elevated total phenols and flavonoid content [157]. Thymus vulgaris responded with elevated total flavonoid and polyphenol concentrations [158]. The phenylpropanoid biosynthesis pathway and bioactive polyphenols accumulated in plants under drought stress are illustrated in Figure 4.
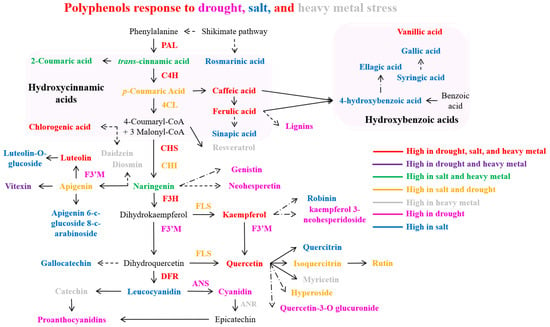
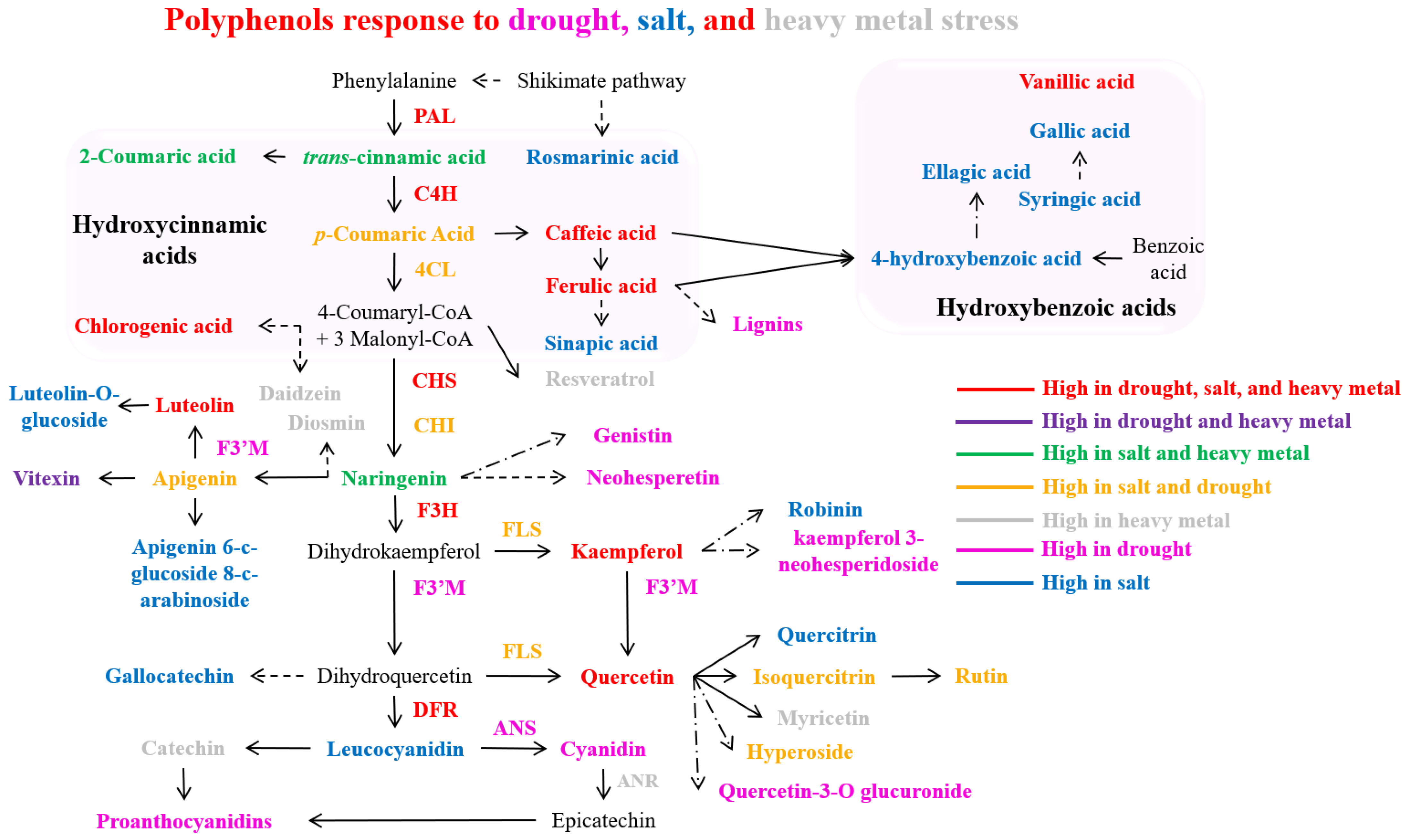
Figure 4.
Polyphenol accumulates in response to drought, salt, and heavy metal stress. Dashed arrows indicate more than one step, and dash-dot arrows denote an undefined pathway. Abbreviations: PAL (phenylalanine ammonia lyase), C4H (cinnamate 4-hydroxylase), 4CL (4-coumarate: CoA ligase), CHS (chalcone synthase), CHI (chalcone isomerase), F3H (flavanone3-hydroxylase), FLS (flavonol synthase), F3′M (flavonoid 3′-monooxygenase), DFR (dihydroflavonol 4-reductase), and ANS (anthocyanidin synthase).
During drought stress exposure, the expression of multiple phenylpropanoid pathway biosynthesis genes (PAL, 4CL, CHS, FLS1, F3H, DFR, and ANS) exhibited a progressive increase in hybrid Populus (P. tremula × P. alba) leaves, and, concurrently, a significant increase in the concentration of the salicylic acid (SA) was observed in the drought-stressed poplar leaves [20]. In Brassica napus, drought stress induced higher levels of total phenols, flavonoids, and flavonols, concurrent with increased PAL enzyme activity and gene expression [154]. Chrysanthemum morifolium demonstrated elevated concentrations of total phenolics, anthocyanins, chlorogenic acid, luteolin, rutin, ferulic acid, apigenin, and quercetin, with enhanced expression of PAL, CHI, and F3H, particularly evident in the Taraneh cultivar [159]. Drought stress in Cucumis sativus led to the upregulation of phenolic compounds, notably vanillic acid and 4-hydroxycinnamic acid [155]. Fragaria ananassa displayed enhanced transcript levels of PAL, C4H, 4CL, DFR, ANS, FLS, and UFGT [160]. Under drought conditions, Lactuca sativa exhibited elevated concentrations of phenolic compounds, particularly caftaric acid and rutin [161]. The Larrea species exhibited elevated levels of polyphenols, including flavonoids, proanthocyanidins, and flavonols [162]. Lotus japonicus showed increased kaempferol and quercetin content, accompanied by the upregulated expression of PAL, C4H, 4CL, CHS, CHI, DFR, and isoflavone synthase (IFS) genes [163]. Under drought stress, Nicotiana tabacum demonstrated enhanced PAL enzyme activity and lignin content [164], while the Ocimum species showed increased total phenol content [165]. In Triticum aestivum, increased total phenol content was observed, along with enhanced concentrations of flavonoids and anthocyanins, accompanied by elevated expression of CHS, CHI, F3H, FNS, FLS, DFR, and ANS genes [166]. Vitis vinifera (cv. Pinot noir) exhibited increased concentrations of various polyphenols, including 4-coumaric acid, caffeic acid, ferulic acid, cis- and trans-resveratrol-3-O-glucoside, catechin, epicatechin, caftaric acid, epicatechin gallate, kaempferol-3-O-glucoside, cyanidin-3-O-glucoside, quercetin-3-O-glucoside, and quercetin-3-O-glucuronide [17]. Additionally, enhanced anthocyanin content was observed, concurrent with the upregulation of biosynthetic genes including UFGT, CHS, and F3H [167]. To conclude, plant species demonstrated the elevated accumulation of bioactive polyphenols primarily through the activation of genes that encode enzymes associated with the phenylpropanoid pathway in response to water scarcity conditions (Figure 4). This activation resulted in enhanced concentrations of bioactive polyphenols under drought-stress conditions (Figure 4).
3.5. Salt Stress
Salt stress in plants occurs when elevated concentrations of sodium (Na+) and chloride (Cl−) ions are present in soil, which impairs the plant’s water absorption capabilities and causes detrimental effects on plants, including impeded growth, decreased photosynthetic efficiency, disrupted nutrient homeostasis, and, in severe cases, plant mortality (Figure 1) [168]. Salt stress induces the generation of ROS, including radical superoxide, hydrogen peroxide, and hydroxyl ions (OH¯) [169]. This oxidative stress forces plants to activate their antioxidant defense mechanisms to quench ROS [170,171]. This mechanism includes the increased production of bioactive polyphenol molecules that serve as potent antioxidants that effectively scavenge harmful ROS accumulated during salt stress conditions [172,173]. The phenylpropanoid biosynthetic pathway becomes activated under saline conditions, leading to the enhanced production of various phenolic biomolecules with potent antioxidative capabilities [174,175].
Specific genes regulate flavonoid biosynthesis under salt stress conditions [176]. A bHLH transcription factor from Vitis vinifera, the VvbHLH1 gene, enhances the total contents of flavonoids by regulating flavonoid biosynthetic pathway genes, thereby inducing salt tolerance [177]. In tobacco (Nicotiana tabacum L.), the NtCHS1 gene is crucial for flavonoid biosynthesis during salt stress, with increased flavonoid accumulation directly quenching the ROS generated under salt stress. Similarly, salinity stress upregulates the Glycine max flavone synthase genes (GmFNSII-1 and GmFNSII-2), promoting flavone biosynthesis and contributing to salinity tolerance [178]. Various phenolic acids, particularly hydroxycinnamic acids and hydroxybenzoic acids, such as vanillic acid, ferulic acid, caffeic acid, gallic acid, cinnamylmalic acid, and caftaric acid, accumulated in response to saline conditions [15,72,179]. Additionally, salt stress promotes the biosynthesis of pigmented flavonoids such as anthocyanin, further enhancing the plant’s antioxidative properties [180,181].
Several plant species improved polyphenol biosynthesis after salinity stress. Amaranthus tricolor exhibited increased concentrations of total phenolics, including hydroxybenzoic acids (ellagic, gallic, p-hydroxybenzoic, vanillic, and syringic acids), hydroxycinnamic acids (p-coumaric, caffeic, chlorogenic, m-coumaric, sinapic, ferulic, and trans-cinnamic acids), and flavonoids (hyperoside, iso-quercetin, and rutin) [179]. Chenopodium quinoa showed increased bioactive polyphenol and flavonoid content under saline conditions [182]. Asparagus aethiopicus demonstrated elevated levels of phenolic compounds, specifically caffeic acid, robinin, apigenin, chlorogenic acid, and rutin, when exposed to salinity stress [175]. Solanum lycopersicon showed a significant increment in total caffeoylquinic acid content under salinity stress [171]. The phenylpropanoid pathway and bioactive polyphenols accumulated in plants under salt stress are represented in Figure 4.
Cynara cardunculus presented higher concentrations of phenolic compounds, including apigenin 6-c-glucoside 8-c-arabinoside, quercitrin, luteolin-O-glucoside, leucocyanidin, and gallocatechin, while exhibiting decreased levels of ferulic acid, chrysin, daidzein, genistein, and apigenin [183]. Under saline conditions, Fragaria ananassa showed the upregulation of several phenylpropanoid pathway genes such as PAL, C4H, F3H, DFR, and FLS [160]. Salvia mirzayanii showed an increased total phenolic content [184], while both S. acrosiphon and S. mirzayanii species revealed enhanced total phenolic content and PAL activity [185]. Under salt stress, Mentha piperita and Hordeum vulgare exhibited increased total polyphenols contents [186,187]. Ocimum basilicum displayed elevated levels of various bioactive polyphenols, including quercetin-rutinoside, caffeic acid, cinnamyl malic acid, rosmarinic acid, feruloyl tartaric acid, and caftaric acid [72] Olea europaea showed enhanced concentrations of total phenolics, quercetin, and kaempferol derivatives, accompanied by regulated transcript levels of PAL, C4H, 4CL, CHI, and CHS [174]. Triticum aestivum showed increased total phenolic content when exposed to saline stress [188]. Thymus spp. exhibited superior concentrations of various phenolic compounds under salt stress, including rosmarinic acid, caffeic acid, quercitrin, trans-2-hydroxycinnamic acid, luteolin, cinnamic acid, gallic acid, rutin, apigenin, naringenin, syringic acid, and vanillic acid [173]. Solanum villosum demonstrated elevated total phenolic content, caffeic acid, and quercetin 3-β-D-glucoside levels, along with upregulated expression of PAL and FLS [15]. These findings provide valuable insights into plant adaptation strategies under saline conditions and highlight the potential for enhancing crop stress tolerance through targeted manipulation of phenylpropanoid metabolism.
3.6. Heavy Metals Toxicity
Heavy metal toxicity, primarily introduced through anthropogenic activities, represents significant abiotic stressors that adversely affect plant growth and metabolic functions [189]. The severity of heavy metal toxicity is determined by multiple factors, including their chemical properties, bioavailability in soil solutions or growth media, concentration levels, and the duration of metal stress [189,190]. While certain heavy metals such as zinc, copper, and molybdenum are essential micronutrients at low concentrations but become toxic at elevated levels, others like cadmium, lead, and mercury exhibit toxicity even at minimal concentrations due to their non-participation in metabolic processes [190,191,192,193]. Some key genes showed a high expression that encodes phenylpropanoid biosynthesis enzymes including PAL, CHS, F3H, DFR, and ANR. Critical enzymes such as shikimate dehydrogenase (SKDH) and glucose-6-phosphate dehydrogenase (G6PDH) catalyze essential precursor molecules of the phenylpropanoid pathway [194]. The cinnamyl alcohol dehydrogenase (CADH) enzyme enables lignin precursor synthesis, while metal exposure stimulates phenylpropanoid metabolism through enhanced activities of PAL, SKDH, G6PDH, and CADH [195]. Polyphenol oxidase (PPO) contributes to quenching ROS and induces heavy metal stress tolerance [196]. This enzymatic upregulation occurs through transcriptional modulation of corresponding biosynthetic genes under metal stress conditions. Flavonoids contribute significantly to stress tolerance through scavenging H2O2 and play a significant role in the phenolic/ascorbate-peroxidase cycle [197]. These biochemical adaptations collectively enhance plant resilience under metal stress conditions.
Transcriptomic analysis revealed that AoMYB12, AoWRKY5, and AoWRKY6 were involved in Cd heavy metal stress tolerance in Alisma orientale [198]. The expression profiling identified several stress-responsive MYB transcription factors in response to cold and cadmium stress in Apocynum venetum [199]. Specifically, the AvMYB48, AvMYB97, AvMYB8, and AvMYB4 genes showed significant transcriptional changes, suggesting their involvement in abiotic stress responses. Additionally, AvMYB10 and AvMYB11 were found to regulate proanthocyanidin biosynthesis pathways, displaying functional conservation with their orthologous counterparts in Arabidopsis thaliana [199]. Transcriptomic, qPCR, and phylogenetic analyses identified six BnGMYB genes (BnGMYB9, BnGMYB10, BnGMYB12, BnGMYB28, BnGMYB41, and BnGMYB78) that exhibited significant responsiveness to cadmium stress conditions [200]. Notably, the BnGMYB10, BnGMYB12, and BnGMYB41 genes were significantly upregulated across all plant tissues, with increasing expression levels according to the degree of cadmium stress. Protein interaction network analysis revealed potential molecular interactions between these transcription factors and key regulatory elements involved in flavonoid biosynthesis, suggesting potential mechanistic linkages between cadmium-induced stress responses and the regulation of flavonoid metabolism [200].
Heavy metal stress induces oxidative damage in plants by activating ROS production, causing growth inhibition and cellular toxicity [201]. In response to heavy metal stress, plants enhanced the synthesis of phenolic compounds (served as crucial antioxidants) to protect themselves from metal stress-induced oxidative damage [202,203]. Notably, flavonoids not only neutralize ROS, but, due to metal chelating properties, they form chelate complexes with heavy metals, playing a key role in metal stress tolerance, and effectively reducing metal-catalyzed oxidation reactions within plant cells [204,205]. This chelating ability stems from the nucleophilic nature of their benzene rings, with efficiency varying based on hydroxyl group configuration [193,206]. Notably, polyphenols including flavonoids reveal significant metal-chelating capabilities, as evidenced in Gynura pseudochina, where they effectively chelate zinc and cadmium, while catechins specifically chelate iron [207]. In response to cadmium (Cd) stress, plants exhibited increased flavonoid and anthocyanin biosynthesis, accompanied by elevated PAL and CHS expression [208,209]. Prolonged Cd exposure enhanced the accumulation of phenols, polyphenols, and related compounds [209]. The phenylpropanoid pathway and individual bioactive compounds elevated in plants under heavy metal stress are represented in Figure 4.
Studies on Brassica juncea have revealed significant modifications in secondary metabolite profiles in response to various metal stresses. Under copper (Cu) exposure, substantial increases were observed in total phenolic compounds, specifically catechin, caffeic acid, coumaric acid, kaempferol, and anthocyanins [197]. Lead (Pb) stress similarly elicited comprehensive increases in phenolic compounds, flavonoids, and anthocyanins, accompanied by corresponding upregulation of PAL and CHS genes [202]. These findings demonstrate consistent patterns of secondary metabolite modulation as a stress response mechanism in B. juncea exposed to heavy metals [208,210]. Red cabbage (Brassica oleracea) sprouts exposed to copper metal stress showed concurrent increases in total phenolic content and PAL activity [211]. In wheat (Triticum aestivum), both PAL and tyrosine ammonia-lyase activities were enhanced under lead and copper metal stress, with more obvious effects observed with copper stress [212]. Chromium (Cr) stress induced elevated concentrations of phenols, flavonoids, and anthocyanins, coinciding with the enhanced expression of key biosynthetic genes, PAL and CHS. Further investigations of Cr stress confirmed anthocyanin accumulation, which specifically correlated with CHS gene upregulation [213]. The regulatory transcription factors, particularly the MYB family, with the R2R3MYB subfamily, play a crucial role in stress responses through the MBW complex-mediated regulation of flavonoid and anthocyanin biosynthesis [189,190,214]. In Artemisia annua, the overexpression of metabolite enzymatic genes enhances artemisinin production under copper and silver stress, while elevated PAL and CHS gene activity increases flavonoid and anthocyanin levels [207].
Multiple plant species exhibit enhanced phenolic metabolism under metal stress conditions. In Fagopyrum esculentum, aluminum exposure led to elevated phenolic compounds, flavonoids, anthocyanins, and PAL activity [215]. Similarly, Kandelia obovata showed increased phenolic accumulation when exposed to cadmium and zinc, accompanied by the upregulation of key enzymes including shikimate dehydrogenase, cinnamyl alcohol dehydrogenase, and polyphenol oxidase [216]. Copper stress in Vitis vinifera induced transcriptional changes in phenylpropanoid pathway genes, upregulating PAL, C4H, CHS, F3H, and DFR while suppressing UFGT and ANR expression [217]. Cadmium exposure in Withania somnifera enhanced flavonoid and phenolic accumulation [195]. In Zea mays, multiple heavy metals (Cu, Pb, and Cd) triggered increases in total phenols and specific compounds like chlorogenic and vanillic acids [214]. Heavy metal stress increased catechins and quercetin levels in Pinus and Zea mays root systems [218]. Similarly, copper stress triggered flavonoid production in Amaranthus caudatus and Ginkgo biloba callus cultures [219]. Under lead stress, Prosopis farcta displayed higher phenolic content and PAL activity, with increases in specific compounds like ferulic acid, cinnamic acid, caffeic acid, and various flavonoids including daidzein, vitexin, resveratrol, and quercetin [220]. However, some phenolic compounds such as anthocyanin significantly decreased after excessive nickel stress in Lactuca sativa sprouts, while certain Asteraceae family members showed reduced secondary metabolite production under metal stress [19]. This reduction likely results from severe heavy metal toxicity including both antioxidant systems and metabolite biosynthesis pathways. These studies revealed that the activation of phenylpropanoid pathways correlates with increased heavy metal stress tolerance in plants.
3.7. Polyphenols Pathway Responds to Multiple Environmental Stressors
The complex interrelationships between individual abiotic stresses and their regulatory effects on polyphenol metabolism across diverse plant species have been extensively documented (Figure 3 and Figure 4). Notably, under natural or field conditions, plants rarely experience isolated stress factors. Rather, they are typically subjected to multiple concurrent environmental stresses, resulting in distinct metabolic responses that differ significantly from those observed under single-stress conditions. In Eucalyptus globulus, combined drought and heat stress led to significantly elevated levels of cinnamate (trans-cinnamic acid), whereas individual drought or heat stress did not produce comparable increases [221]. Given that cinnamate serves as a precursor for various polyphenols, elevated concentrations of this compound may generate diverse phenylpropanoids that potentially contribute to stress tolerance against combined drought and heat conditions in E. globulus [221]. Interestingly, under combined drought and heat stress, total anthocyanin content remained relatively stable in both Pinus elliottii var. elliottii and the hybrid Pinus elliottii var. elliottii × Pinus caribaea var. hondurensis [222]. These findings underscore the importance of investigating stress combinations rather than individual stress; such studies would significantly enhance our understanding of plant adaptation mechanisms in natural environments.
Under combined drought and heat stress conditions, the ‘Cobrançosa’ olive (Olea europaea) cultivar exhibited reduced levels of luteolin-4-O-glucoside, apigenin-7-O-glucoside, and quercetin-3-rutinoside compared to control conditions [223]. However, p-coumaric acid levels increased by more than 70% under combined stress conditions. While drought and heat stress individually enhance the accumulation of phenolic compounds in plants (Figure 3 and Figure 4), the polyphenols decreased under combined stress conditions in olives, except p-coumaric acid [223]. These findings suggest that the combination of drought and heat stress may operate through distinct mechanisms, potentially by suppressing endogenous enzymes such as oxidoreductases, polyphenol oxidase, and peroxidase, which are responsible for phenolic compound oxidation [224], or by downregulating polyphenol-related genes including PAL, CHS, and dihydroflavonol reductase [225]. Notably, under combined stress conditions, the ‘C.C.Branco’ olive cultivar demonstrated suppressed flavonoid production while accumulating elevated levels of oleuropein, another bioactive compound [223]. These metabolic modifications underscore the complex nature of stress responses across different olive cultivars and suggest the existence of diverse adaptive mechanisms within the species. This phenomenon highlights the importance of investigating stress combinations rather than isolated stresses to gain a more comprehensive understanding of plant adaptation mechanisms in natural environments.
4. Polyphenols Antioxidant Properties to Scavenge Free Radicals and Reactive Oxygen Species
Under abiotic stress conditions, various phenolic compounds play crucial roles in enhancing plant resilience by exhibiting antioxidant activity during abiotic stress or unfavorable environmental conditions [19]. The effectiveness of polyphenols to scavenge free radicals or reactive oxygen species can vary based on the type of abiotic stress (e.g., drought, salinity, or heat), duration, and specific plant species [5,67]. In our review, we have found that several phenolic compounds such as quercetin, kaempferol, luteolin, apigenin, naringenin, ferulic acid, chlorogenic acid, and caffeic acid commonly respond to different abiotic stresses in various plant species (Figure 3 and Figure 4). Among these, quercetin is one of the most potent antioxidants in plant species and can effectively quench the ROS and mitigate oxidative stress caused by abiotic factors. Quercetin donates hydrogen atoms to free radicals, especially superoxide anions, hydroxyl radicals, and hydrogen peroxide, and inhibits lipid peroxidation [226]. Thus, stabilizing the free radicals enhances plant tolerance to various stresses and prevents plants’ cellular organelles from oxidative damage [226]. Like quercetin, kaempferol also exhibits strong antioxidant properties, enhances the activity of endogenous antioxidant enzymes, reduces ROS levels, and protects plants from oxidative damage, thereby improving abiotic stress tolerance [227,228,229,230]. Kaempferol acts as a hydrogen donor, and high biosynthesis of this compound under abiotic stress can efficiently neutralize the superoxide and hydroxyl radicals during abiotic stress [228,231,232].
Luteolin and apigenin are recognized for their antioxidant properties that scavenge free radicals (superoxide radicals and hydroxyl radicals by donating hydrogen), which have been shown to improve plant resilience under abiotic stress conditions [233,234]. Additionally, luteolin also chelates metal ions, which may contribute to its antioxidant activity by preventing the Fenton reaction that generates hydroxyl radicals; besides this, it has a wide range of bioactivities, including anti-inflammatory, antioxidant, antibacterial, neuroprotective, antiviral, and cardioprotective effects [204,233]. Naringenin exhibits potent antioxidant properties through its ability to donate hydrogen atoms, neutralize superoxide anions, and scavenge hydroxyl radicals and hydrogen peroxide molecules [232,235]. Ferulic acid levels increased in several plant species in response to different abiotic stresses such as drought, heat, chilling, salt, UV light, and heavy metal stress (Figure 3 and Figure 4). Ferulic acid exhibits antioxidant activity by hydrogen donation to scavenge the hydroxyl radicals and peroxyl radicals (ROO•) and triggers the activity of other antioxidants [236,237]. Chlorogenic acid is a robust antioxidant that can scavenge free radicals by donating electrons and hydrogen atoms to neutralize free radicals (hydroxyl radicals, hydrogen peroxide, and superoxide radicals) produced during abiotic stress [238]. Caffeic acid has moderate antioxidant activity compared to the phenols mentioned above. It can stabilize the superoxide radicals and make them least reactive to protect them from oxidative damage during plant abiotic stress [239]. Among these compounds, quercetin, kaempferol, and luteolin are often recognized for their high antioxidant activity, followed by naringenin, chlorogenic acid, and ferulic acid, which also showed significant antioxidant properties, protecting plants from oxidative damage, and contribute to stress tolerance; however, apigenin and caffeic acid have moderate antioxidant activities [204,232,237,239]. However, the efficacy of these phenols can depend on the plant species and type of abiotic stress.
5. Conclusions
This review highlights the crucial role of polyphenolic compounds in plant stress tolerance mechanisms. The phenylpropanoid pathway emerges as a central metabolic route that produces diverse bioactive polyphenols essential for plant adaptation to various environmental stresses. These compounds exhibit significant antioxidant properties, effectively neutralizing reactive oxygen species and free radicals generated during abiotic stress. MYB transcription factors have been identified as master regulators of phenylpropanoid metabolism, controlling the expression of key biosynthetic genes and subsequently influencing polyphenol accumulation patterns under stress conditions. The regulatory networks involving these transcription factors represent promising targets for enhancing crop stress tolerance through genetic engineering approaches. This review reveals that plants accumulate specific phenolic compounds in response to different abiotic stresses, with some compounds like quercetin, kaempferol, and luteolin showing consistently high antioxidant activity across various stress conditions. However, plant responses to combined stresses often differ from single-stress scenarios, highlighting the complexity of stress adaptation mechanisms in natural environments. Understanding these intricate regulatory mechanisms and stress response patterns provides valuable insights for developing stress-resistant crops. Future research should focus on elucidating the precise molecular mechanisms governing polyphenol-mediated stress tolerance and exploring the potential of targeted genetic modifications to enhance crop resilience in the face of increasing environmental challenges.
Author Contributions
Conceptualization, M.J.R. and B.Z.; methodology, M.J.R.; software, M.J.R., writing—original draft preparation, M.J.R.; writing—review and editing, M.J.R. and B.Z., supervision, B.Z.; project administration, M.J.R.; funding acquisition, B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Provincial Key Laboratory for Non-wood Forest and Quality Control and Utilization of Its Products (Zhejiang A&F University, Hangzhou 311300, China), Key Funded Project of Zhejiang 151 Talent Engineering (for Bingsong Zheng), and the Overseas Expertise Introduction Project for Discipline Innovation (111 Project D18008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available in the main text.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, Y.; Tilman, D.; Jin, Z.; Smith, P.; Barrett, C.B.; Zhu, Y.-G.; Burney, J.; D’Odorico, P.; Fantke, P.; Fargione, J. Climate Change Exacerbates the Environmental Impacts of Agriculture. Science 2024, 385, eadn3747. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, S.; Chen, J.; Yu, H.; Yang, T.; Wang, C.; Huang, S.; Chen, H.; Ao, X. Impacts of Global Climate Change on Agricultural Production: A Comprehensive Review. Agronomy 2024, 14, 1360. [Google Scholar] [CrossRef]
- Paul, A.; Acharya, K.; Chakraborty, N. Involvement of Phenylpropanoid Pathway and Shikimic Acid Pathway in Environmental Stress Response. In Biology and Biotechnology of Environmental Stress Tolerance in Plants; Apple Academic Press: Williston, VT, USA, 2023; pp. 27–66. [Google Scholar]
- Saini, N.; Anmol, A.; Kumar, S.; Bakshi, M.; Dhiman, Z. Exploring Phenolic Compounds as Natural Stress Alleviators in Plants-a Comprehensive Review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications. Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Yang, T.; Saad, A.M.; Alkafaas, S.S.; Elkafas, S.S.; Eldeeb, G.S.; Mohammed, D.M.; Salem, H.M.; Korma, S.A.; Loutfy, S.A. Chemistry, Bioavailability, Bioactivity, Nutritional Aspects and Human Health Benefits of Polyphenols: A Comprehensive Review. Int. J. Biol. Macromol. 2024, 277, 134223. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Aires, A.; Falco, V.; Valentão, P.; Poeta, P. Phenolic Compounds Classification and Their Distribution in Winemaking By-Products. Eur. Food Res. Technol. 2023, 249, 207–239. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Nehra, A.; Kalwan, G.; Gill, R.; Nehra, K.; Agarwala, N.; Jain, P.K.; Naeem, M.; Tuteja, N.; Pudake, R.N.; Gill, S.S. Status of Impact of Abiotic Stresses on Global Agriculture. In Nanotechnology for Abiotic Stress Tolerance and Management in Crop Plants; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–21. [Google Scholar]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Dangles, O. A Comprehensive Review on Flavanones, the Major Citrus Polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Lattanzio, V. Bioactive Polyphenols: Their Role in Quality and Storability of Fruit and Vegetables. J. Appl. Bot. 2003, 77, 128–146. [Google Scholar]
- Yang, S.; Wang, C.; Li, X.; Wu, C.; Liu, C.; Xue, Z.; Kou, X. Investigation on the Biological Activity of Anthocyanins and Polyphenols in Blueberry. J. Food Sci. 2021, 86, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Ben-Abdallah, S.; Zorrig, W.; Amyot, L.; Renaud, J.; Hannoufa, A.; Lachâal, M.; Karray-Bouraoui, N. Potential Production of Polyphenols, Carotenoids and Glycoalkaloids in Solanum villosum Mill. under Salt Stress. Biologia 2019, 74, 309–324. [Google Scholar] [CrossRef]
- Lacikova, L.; Jancova, M.; Muselik, J.; Masterova, I.; Grancai, D.; Fickova, M. Antiproliferative, Cytotoxic, Antioxidant Activity and Polyphenols Contents in Leaves of Four Staphylea L. Species. Molecules 2009, 14, 3259–3267. [Google Scholar] [CrossRef]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe Drought Stress Is Affecting Selected Primary Metabolites, Polyphenols, and Volatile Metabolites in Grapevine Leaves (Vitis vinifera cv. Pinot Noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, T.; Duan, W.; Zhang, Z.; Li, Y.; Fang, B.; Xia, M.; Wang, M. Effects of Organic Acids, Amino Acids and Phenolic Compounds on Antioxidant Characteristic of Zhenjiang Aromatic Vinegar. Molecules 2019, 24, 3799. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules; Woodhead Publishing: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar]
- Ahmed, U.; Rao, M.J.; Qi, C.; Xie, Q.; Noushahi, H.A.; Yaseen, M.; Shi, X.; Zheng, B. Expression Profiling of Flavonoid Biosynthesis Genes and Secondary Metabolites Accumulation in Populus under Drought Stress. Molecules 2021, 26, 5546. [Google Scholar] [CrossRef] [PubMed]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide Analysis of Phenylpropanoid Defence Pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Chen, L.; Xiao, Y.; Wu, J.; Zeng, L.; Li, H.; Wu, Q.; Hu, Z. Recent Advanced Metabolic and Genetic Engineering of Phenylpropanoid Biosynthetic Pathways. Int. J. Mol. Sci. 2021, 22, 9544. [Google Scholar] [CrossRef]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach towards Plant Defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef]
- Alami, M.M.; Guo, S.; Mei, Z.; Yang, G.; Wang, X. Environmental Factors on Secondary Metabolism in Medicinal Plants: Exploring Accelerating Factors. Med. Plant Biol. 2024, 3, e016. [Google Scholar] [CrossRef]
- Tang, H.; Wang, Q.; Xie, H.; Li, W. The Function of Secondary Metabolites in Resisting Stresses in Horticultural Plants. Fruit Res. 2024, 4, e021. [Google Scholar] [CrossRef]
- Oyebamiji, Y.O.; Adigun, B.A.; Shamsudin, N.A.A.; Ikmal, A.M.; Salisu, M.A.; Malike, F.A.; Lateef, A.A. Recent Advancements in Mitigating Abiotic Stresses in Crops. Horticulturae 2024, 10, 156. [Google Scholar] [CrossRef]
- Kashyap, A.K.; Dubey, S.K.; Shah, S.; Kumar, A. A Short Review on Genes Regulating Biosynthesis of Major Secondary Metabolites. In Phytochemical Genomics Plant Metabolomics and Medicinal Plant Genomics; Springer: Singapore, 2023; pp. 501–519. [Google Scholar]
- Rao, M.J.; Duan, M.; Yang, M.; Fan, H.; Shen, S.; Hu, L.; Wang, L. Novel Insights into Anthocyanin Metabolism and Molecular Characterization of Associated Genes in Sugarcane Rinds Using the Metabolome and Transcriptome. Int. J. Mol. Sci. 2022, 23, 338. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Wang, J.; Han, S.; Ma, L.; Mo, X.; Li, M.; Hu, L.; Wang, L. Transcriptomic and Widely Targeted Metabolomic Approach Identified Diverse Group of Bioactive Compounds, Antiradical Activities, and Their Associated Genes in Six Sugarcane Varieties. Antioxidants 2022, 11, 1319. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Ng, M.-S.; Cheng, S.-S.; Lo, A.W.-Y.; Xiao, Z.; Shin, T.-S.; Chung, G.; Lam, H.-M. Understanding the Composition, Biosynthesis, Accumulation and Transport of Flavonoids in Crops for the Promotion of Crops as Healthy Sources of Flavonoids for Human Consumption. Nutrients 2020, 12, 1717. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Li, H.; Chiang, V.L.; Fu, Y. Cooperative Regulation of Flavonoid and Lignin Biosynthesis in Plants. Crit. Rev. Plant Sci. 2021, 40, 109–126. [Google Scholar] [CrossRef]
- Tak, H.; Negi, S.; Ganapathi, T.R. Overexpression of MusaMYB31, a R2R3 Type MYB Transcription Factor Gene Indicate Its Role as a Negative Regulator of Lignin Biosynthesis in Banana. PLoS ONE 2017, 12, e0172695. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ran, L.; Hou, Y.; Tian, Q.; Li, C.; Liu, R.; Fan, D.; Luo, K. The Transcription Factor MYB115 Contributes to the Regulation of Proanthocyanidin Biosynthesis and Enhances Fungal Resistance in Poplar. New Phytol. 2017, 215, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kong, W.; Wong, G.; Fu, L.; Peng, R.; Li, Z.; Yao, Q. AtMYB12 Regulates Flavonoids Accumulation and Abiotic Stress Tolerance in Transgenic Arabidopsis thaliana. Mol. Genet. Genom. 2016, 291, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mitra, M.; Roy, S. Study of Changes in Folding/Unfolding Properties and Stability of Arabidopsis thaliana MYB12 Transcription Factor Following UV-B Exposure in Vitro. Heliyon 2024, 10, 13. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Meng, Z.; Jia, Z.; Fu, F.; Jin, B.; Cao, F.; Wang, L. LncNAT11-GbMYB11-GbF3′H/GbFLS Module Mediates Flavonol Biosynthesis to Regulate Salt Stress Tolerance in Ginkgo biloba. J. Exp. Bot. 2024, erae438. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, L.J.; Gao, H.N.; Wang, X.F.; Li, Z.W.; Li, Y.Y. The Transcription Factor MdMYB2 Influences Cold Tolerance and Anthocyanin Accumulation by Activating SUMO E3 Ligase MdSIZ1 in Apple. Plant Physiol. 2022, 189, 2044–2060. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.; Zhang, L.; Shu, J.; Court, M.H.; Sun, Z.; Jiang, L.; Zheng, C.; Shu, H.; Ji, L.; et al. Genome-Wide Analysis of the Apple Family 1 Glycosyltransferases Identified a Flavonoid-Modifying UGT, MdUGT83L3, Which Is Targeted by MdMYB88 and Contributes to Stress Adaptation. Plant Sci. 2022, 321, 111314. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Zhang, X.W.; Xu, H.F.; Bi, S.Q.; You, C.X.; Hao, Y.J. An Apple MYB Transcription Factor Regulates Cold Tolerance and Anthocyanin Accumulation and Undergoes MIEL1-Mediated Degradation. Plant Biotechnol. J. 2020, 18, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Quan, R.; Chen, G.; Yu, G.; Li, X.; Han, Z.; Xu, W.; Li, G.; Shi, J.; Li, B. An R2R3-MYB Transcription Factor VyMYB24, Isolated from Wild Grape Vitis yanshanesis JX Chen., Regulates the Plant Development and Confers the Tolerance to Drought. Front. Plant Sci. 2022, 13, 966641. [Google Scholar]
- Wang, J.; Wang, F.; Jin, C.; Tong, Y.; Wang, T. A R2R3-MYB Transcription Factor VvMYBF1 from Grapevine (Vitis vinifera L.) Regulates Flavonoids Accumulation and Abiotic Stress Tolerance in Transgenic Arabidopsis. J. Hortic. Sci. Biotechnol. 2020, 95, 147–161. [Google Scholar] [CrossRef]
- Han, J.; Dai, J.; Chen, Z.; Li, W.; Li, X.; Zhang, L.; Yao, A.; Zhang, B.; Han, D. Overexpression of a ‘Beta’MYB Factor Gene, VhMYB15, Increases Salinity and Drought Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 1534. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Agarwal, P.; Choudhury, S.R.; Roy, S. MYB4, a Member of R2R3-Subfamily of MYB Transcription Factor Functions as a Repressor of Key Genes Involved in Flavonoid Biosynthesis and Repair of UV-B Induced DNA Double Strand Breaks in Arabidopsis. Plant Physiol. Biochem. 2024, 211, 108698. [Google Scholar] [CrossRef]
- Qin, J.; Zhao, C.; Wang, S.; Gao, N.; Wang, X.; Na, X.; Wang, X.; Bi, Y. PIF4-PAP1 Interaction Affects MYB-BHLH-WD40 Complex Formation and Anthocyanin Accumulation in Arabidopsis. J. Plant Physiol. 2021, 268, 153558. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gu, X.; Jiang, Y.; Wang, L.; Xiao, N.; Chen, Y.; Jin, B.; Wang, L.; Li, W. UV-B Promotes Flavonoid Biosynthesis in Ginkgo Biloba by Inducing the GbHY5-GbMYB1-GbFLS Module. Hortic. Res. 2023, 10, uhad118. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Wang, Z.; Su, L.; Gong, L.; Xin, H. Vitis Myb14 Confer Cold and Drought Tolerance by Activating Lipid Transfer Protein Genes Expression and Reactive Oxygen Species Scavenge. Gene 2024, 890, 147792. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhao, W.; Zhou, R.; Wang, B.; Liu, Y.; Ge, W.; Liang, J.; Zhao, Q.; Wen, P. VviMYB24 Positively Regulates Flavonol Biosynthesis in Response to Moderate Drought Stress in ‘Cabernet Sauvignon’ Grape Seedlings. Sci. Hortic. 2024, 338, 113769. [Google Scholar] [CrossRef]
- Raziq, A.; Zhang, K.; Sun, W.; Ahmad, N.; Zhao, H.; Raza, M.A.; Ahmed, S.; Din, A.M.U.; Zhao, S.; Pan, J.; et al. Transcriptome Profiling of MYB-Overexpressed Transgenic Lines Provides Crucial Molecular Insights into Anthocyanin and Remodel the Biosynthesis Regulatory Network in Nicotiana tabacum. Ind. Crops Prod. 2024, 213, 118374. [Google Scholar] [CrossRef]
- Yong, Y.; Zhang, Y.; Lyu, Y. A MYB-Related Transcription Factor from Lilium lancifolium L. (LlMYB3) Is Involved in Anthocyanin Biosynthesis Pathway and Enhances Multiple Abiotic Stress Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 3195. [Google Scholar] [CrossRef]
- Yang, J.; Guo, C.; Chen, F.; Lv, B.; Song, J.; Ning, G.; He, Y.; Lin, J.; He, H.; Yang, Y.; et al. Heat-Induced Modulation of Flavonoid Biosynthesis via a LhMYBC2-Mediated Regulatory Network in Oriental Hybrid Lily. Plant Physiol. Biochem. 2024, 214, 108966. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Zuo, H.; Peng, A.; Lin, J.; Li, P.; Wang, K.; Tang, Q.; Tadege, M.; Liu, Z. CsMYBL2 Homologs Modulate the Light and Temperature Stress-regulated Anthocyanin and Catechins Biosynthesis in Tea Plants (Camellia sinensis). Plant J. 2023, 115, 1051–1070. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.N.; Naing, A.H.; Yun, B.-W.; Lim, S.H.; Kim, C.K. Overexpression of RsMYB1 Enhances Anthocyanin Accumulation and Heavy Metal Stress Tolerance in Transgenic Petunia. Front. Plant Sci. 2018, 9, 01388. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Peng, R.; Yao, Q. SlMYB14 Promotes Flavonoids Accumulation and Confers Higher Tolerance to 2,4,6-Trichlorophenol in Tomato. Plant Sci. 2021, 303, 110796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yu, Z.; Yao, B.; Teixeira da Silva, J.A.; Wen, D. SsMYB113, a Schima superba MYB Transcription Factor, Regulates the Accumulation of Flavonoids and Functions in Drought Stress Tolerance by Modulating ROS Generation. Plant Soil. 2022, 478, 427–444. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, L.; Wang, A.; Yu, L.; Liu, Y.; Zhang, X.; Wang, R.; Li, X.; Yang, Y.; Li, X.; et al. An R2R3-MYB FtMYB11 from Tartary Buckwheat Has Contrasting Effects on Abiotic Tolerance in Arabidopsis. J. Plant Physiol. 2023, 280, 153842. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Killiny, N.; Dutt, M. Physiological and Molecular Responses of ‘Hamlin’ Sweet Orange Trees Expressing the VvmybA1 Gene under Cold Stress Conditions. Planta 2024, 260, 67. [Google Scholar] [CrossRef]
- D’Amelia, V.; Aversano, R.; Ruggiero, A.; Batelli, G.; Appelhagen, I.; Dinacci, C.; Hill, L.; Martin, C.; Carputo, D. Subfunctionalization of Duplicate MYB Genes in Solanum commersonii Generated the Cold-induced ScAN2 and the Anthocyanin Regulator ScAN1. Plant Cell Environ. 2018, 41, 1038–1051. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, S.; Li, Y.; Zhang, L.; Song, W.; Chen, C. Overexpression of AtMYB2 Promotes Tolerance to Salt Stress and Accumulations of Tanshinones and Phenolic Acid in Salvia miltiorrhiza. Int. J. Mol. Sci. 2024, 25, 4111. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, S.J.; Yanders, S.; Park, S.; Kim, H.S.; Kim, S. MYB3 Plays an Important Role in Lignin and Anthocyanin Biosynthesis under Salt Stress Condition in Arabidopsis. Plant Cell Rep. 2022, 41, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Dixon, R.A. Plant Phenylalanine/Tyrosine Ammonia-Lyases. Trends Plant Sci. 2020, 25, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.D.P.; Abrahão, J.; et al. Biosynthesis and Metabolic Actions of Simple Phenolic Acids in Plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of Anthocyanin and Proanthocyanin Biosynthesis in Land Plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Qu, C.; Jiang, S.; Chen, Z.; Xu, H.; Fang, H.; Su, M.; Zhang, J.; Wang, Y.; Liu, W. The Proanthocyanidin-specific Transcription Factor Md MYBPA 1 Initiates Anthocyanin Synthesis under Low-temperature Conditions in Red-fleshed Apples. Plant J. 2018, 96, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic Stress and Reactive Oxygen Species: Generation, Signaling, and Defense Mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Syvertsen, J.P.; Garcia-Sanchez, F. Multiple Abiotic Stresses Occurring with Salinity Stress in Citrus. Environ. Exp. Bot. 2014, 103, 128–137. [Google Scholar] [CrossRef]
- Nawaz, M.; Sun, J.; Shabbir, S.; Khattak, W.A.; Ren, G.; Nie, X.; Bo, Y.; Javed, Q.; Du, D.; Sonne, C. A Review of Plants Strategies to Resist Biotic and Abiotic Environmental Stressors. Sci. Total Environ. 2023, 900, 165832. [Google Scholar] [CrossRef]
- Mayor, R. Oxidative Stress and Antioxidant Defense System. J. Inst. Med. Trop. 2010, 5, 23–39. [Google Scholar]
- Scagel, C.F.; Lee, J.; Mitchell, J.N. Salinity from NaCl Changes the Nutrient and Polyphenolic Composition of Basil Leaves. Ind. Crops Prod. 2019, 127, 119–128. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Talebi, M.; Matkowski, A. The Effect of Drought Stress on Polyphenolic Compounds and Expression of Flavonoid Biosynthesis Related Genes in Achillea pachycephala Rech. f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Rao, M.J.; Ding, F.; Wang, N.; Deng, X.; Xu, Q. Metabolic Mechanisms of Host Species Against Citrus Huanglongbing (Greening Disease). Crit. Rev. Plant Sci. 2018, 37, 1544843. [Google Scholar] [CrossRef]
- Rao, M.J.; Xu, Y.; Huang, Y.; Tang, X.; Deng, X.; Xu, Q. Ectopic Expression of Citrus UDP-GLUCOSYL TRANSFERASE Gene Enhances Anthocyanin and Proanthocyanidins Contents and Confers High Light Tolerance in Arabidopsis. BMC Plant Biol. 2019, 19, 603. [Google Scholar] [CrossRef]
- Rao, M.J.; Ahmed, U.; Ahmed, M.H.; Duan, M.; Wang, J.; Wang, Y.; Wang, L. Comparison and Quantification of Metabolites and Their Antioxidant Activities in Young and Mature Leaves of Sugarcane. ACS Food Sci. Technol. 2021, 1, 362–373. [Google Scholar] [CrossRef]
- Kerbler, S.M.; Wigge, P.A. Temperature Sensing in Plants. Annu. Rev. Plant Biol. 2023, 74, 341–366. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Z.; Lv, X.; Cheng, N.; Peng, B.; Cao, W. Effect of 24-Epibrassinolide on Chilling Injury of Peach Fruit in Relation to Phenolic and Proline Metabolisms. Postharvest Biol. Technol. 2016, 111, 390–397. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, B.; Huang, B. Differential Heat-induced Changes in Phenolic Acids Associated with Genotypic Variations in Heat Tolerance for Hard Fescue. Crop Sci. 2019, 59, 667–674. [Google Scholar] [CrossRef]
- Semmar, N. Temperature-Linked Constraints and Plant Protection Responses. In Secondary Metabolites in Plant Stress Adaptation: Analytic Space of Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2024; pp. 155–219. [Google Scholar]
- He, J.; Yao, L.; Pecoraro, L.; Liu, C.; Wang, J.; Huang, L.; Gao, W. Cold Stress Regulates Accumulation of Flavonoids and Terpenoids in Plants by Phytohormone, Transcription Process, Functional Enzyme, and Epigenetics. Crit. Rev. Biotechnol. 2023, 43, 680–697. [Google Scholar] [CrossRef]
- Ahmed, N.U.; Park, J.-I.; Jung, H.-J.; Hur, Y.; Nou, I.-S. Anthocyanin Biosynthesis for Cold and Freezing Stress Tolerance and Desirable Color in Brassica Rapa. Funct. Integr. Genom. 2015, 15, 383–394. [Google Scholar] [CrossRef]
- Ahmed, A.; Tariq, A.; Habib, S. Interactive Biology of Auxins and Phenolics in Plant Environment. In Plant Phenolics in Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1, pp. 117–133. [Google Scholar]
- Kavi, K.P.B.; Srinivas, B.; Prashant, S.; Sahitya, G.; Tulya, R.S.V.; Rajasheker, G.; Prashanth, S. Modulation of Lignin and Its Implications in Salt, Drought and Temperature Stress Tolerance. Curr. Chem. Biol. 2023, 17, 2–12. [Google Scholar] [CrossRef]
- Singh, A.K.; Dhanapal, S.; Yadav, B.S. The Dynamic Responses of Plant Physiology and Metabolism during Environmental Stress Progression. Mol. Biol. Rep. 2020, 47, 1459–1470. [Google Scholar] [CrossRef]
- Gupta, R.; Deswal, R. Antifreeze Proteins Enable Plants to Survive in Freezing Conditions. J. Biosci. 2014, 39, 931–944. [Google Scholar] [CrossRef]
- Zhou, P.; Li, Q.; Liu, G.; Xu, N.; Yang, Y.; Zeng, W.; Chen, A.; Wang, S. Integrated Analysis of Transcriptomic and Metabolomic Data Reveals Critical Metabolic Pathways Involved in Polyphenol Biosynthesis in Nicotiana tabacum under Chilling Stress. Funct. Plant Biol. 2018, 46, 30–43. [Google Scholar] [CrossRef]
- He, Q.; Ren, Y.; Zhao, W.; Li, R.; Zhang, L. Low Temperature Promotes Anthocyanin Biosynthesis and Related Gene Expression in the Seedlings of Purple Head Chinese Cabbage (Brassica rapa L.). Genes 2020, 11, 81. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Younts, T.L.B.; Muday, G.K. Flavonols Control Pollen Tube Growth and Integrity by Regulating ROS Homeostasis during High-Temperature Stress. Proc. Natl. Acad. Sci. USA 2018, 115, 11188–11197. [Google Scholar] [CrossRef]
- Chebrolu, K.K.; Fritschi, F.B.; Ye, S.; Krishnan, H.B.; Smith, J.R.; Gillman, J.D. Impact of Heat Stress during Seed Development on Soybean Seed Metabolome. Metabolomics 2016, 12, 28. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape Berry Flavonoids: A Review of Their Biochemical Responses to High and Extreme High Temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- Zhou, L.-J.; Geng, Z.; Wang, Y.; Wang, Y.; Liu, S.; Chen, C.; Song, A.; Jiang, J.; Chen, S.; Chen, F. A Novel Transcription Factor CmMYB012 Inhibits Flavone and Anthocyanin Biosynthesis in Response to High Temperatures in Chrysanthemum. Hortic. Res. 2021, 8, 248. [Google Scholar] [CrossRef]
- Yamagishi, M. Mechanisms by Which High Temperatures Suppress Anthocyanin Coloration in Flowers and Fruits, and Discovery of Floricultural Crops That Exhibit High-Temperature-Tolerant Flower Pigmentation. Hortic. J. 2024, 93, 203–215. [Google Scholar] [CrossRef]
- Cingoz, G.S.; Gurel, E. Effects of Salicylic Acid on Thermotolerance and Cardenolide Accumulation under High Temperature Stress in Digitalis trojana Ivanina. Plant Physiol. Biochem. 2016, 105, 145–149. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant Growth Regulation in Cell and Tissue Culture in Vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, Z.; Ren, X.; Li, Y.; Zhang, N.; Liu, Q. Effects of Red and Blue Light on Red Clover (Trifolium pratense L.) Growth and Secondary Metabolism. Plant Growth Regul. 2024, 104, 1087–1106. [Google Scholar] [CrossRef]
- Hong, J.; Meng, K.; Thomas, H.R.; Yang, Y.; Williams, B.; Kang, H.; Zhou, Y. Reframing Agriculture by Light: The Role of Light-Mediated Jasmonates/Salicylic Acid Regulation in Plant Defense, Development and Beyond. Veg. Res. 2024, 4, e027. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light Induced Regulation Pathway of Anthocyanin Biosynthesis in Plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef]
- Guo, X.L.; Hu, J.B.; Wang, D.L. Effect of Light Intensity on Blueberry Fruit Coloration, Anthocyanin Synthesis Pathway Enzyme Activity, and Gene Expression. Russ. J. Plant Physiol. 2023, 70, 136. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, H.; Yang, C.; Shi, B.; Zheng, B.; Ma, X.; Zhou, K.; Qian, M. Postharvest Light-Induced Flavonoids Accumulation in Mango (Mangifera indica L.) Peel Is Associated with the up-Regulation of Flavonoids-Related and Light Signal Pathway Genes. Front. Plant Sci. 2023, 14, 1136281. [Google Scholar] [CrossRef]
- Balarynová, J.; Danihlík, J.; Fellner, M. Changes in Plasma Membrane Aquaporin Gene Expression under Osmotic Stress and Blue Light in Tomato. Acta Physiol. Plant 2018, 40, 27. [Google Scholar] [CrossRef]
- Hussain, S.; Shuxian, L.; Mumtaz, M.; Shafiq, I.; Iqbal, N.; Brestic, M.; Shoaib, M.; Sisi, Q.; Li, W.; Mei, X. Foliar Application of Silicon Improves Stem Strength under Low Light Stress by Regulating Lignin Biosynthesis Genes in Soybean (Glycine max (L.) Merr.). J. Hazard. Mater. 2021, 401, 123256. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Shan, Y.; Liu, Y.; Tian, Y.; Xia, T. Influence of Shade on Flavonoid Biosynthesis in Tea (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2012, 141, 7–16. [Google Scholar] [CrossRef]
- Zubova, M.Y.; Nechaeva, T.L.; Kartashov, A.V.; Zagoskina, N.V. Regulation of the Phenolic Compounds Accumulation in the Tea-Plant Callus Culture with a Separate and Combined Effect of Light and Cadmium Ions. Biol. Bull. 2020, 47, 593–604. [Google Scholar] [CrossRef]
- Jaakola, L.; Määttä, K.; Pirttilä, A.M.; Törrönen, R.; Kärenlampi, S.; Hohtola, A. Expression of Genes Involved in Anthocyanin Biosynthesis in Relation to Anthocyanin, Proanthocyanidin, and Flavonol Levels during Bilberry Fruit Development. Plant Physiol. 2002, 130, 729–739. [Google Scholar] [CrossRef]
- Wang, Y.; Fong, S.K.; Singh, A.P.; Vorsa, N.; Johnson-Cicalese, J. Variation of Anthocyanins, Proanthocyanidins, Flavonols, and Organic Acids in Cultivated and Wild Diploid Blueberry Species. HortScience 2019, 54, 576–585. [Google Scholar] [CrossRef]
- Park, W.T.; Yeo, S.K.; Sathasivam, R.; Park, J.S.; Kim, J.K.; Park, S.U. Influence of Light-Emitting Diodes on Phenylpropanoid Biosynthetic Gene Expression and Phenylpropanoid Accumulation in Agastache Rugosa. Appl. Biol. Chem. 2020, 63, 25. [Google Scholar] [CrossRef]
- Jang, E.B.; Ho, T.-T.; Park, S.-Y. Effect of Light Quality and Tissue Origin on Phenolic Compound Accumulation and Antioxidant Activity in Camellia japonica Calli. In Vitro Cell. Dev. Biol.-Plant 2020, 56, 567–577. [Google Scholar] [CrossRef]
- Yao, P.; Huang, Y.; Dong, Q.; Wan, M.; Wang, A.; Chen, Y.; Li, C.; Wu, Q.; Chen, H.; Zhao, H. FtMYB6, a Light-Induced SG7 R2R3-MYB Transcription Factor, Promotes Flavonol Biosynthesis in Tartary Buckwheat (Fagopyrum tataricum). J. Agric. Food Chem. 2020, 68, 13685–13696. [Google Scholar] [CrossRef]
- Voitsekhovskaja, O.V. Phytochromes and Other (Photo) Receptors of Information in Plants. Russ. J. Plant Physiol. 2019, 66, 351–364. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Castillejo, N.; Martínez-Zamora, L. UV and Visible Spectrum Led Lighting as Abiotic Elicitors of Bioactive Compounds in Sprouts, Microgreens, and Baby Leaves—A Comprehensive Review Including Their Mode of Action. Foods 2022, 11, 265. [Google Scholar] [CrossRef]
- Skowron, E.; Trojak, M.; Pacak, I. Effects of UV-B and UV-C Spectrum Supplementation on the Antioxidant Properties and Photosynthetic Activity of Lettuce Cultivars. Int. J. Mol. Sci. 2024, 25, 9298. [Google Scholar] [CrossRef] [PubMed]
- Gąstoł, M.; Błaszczyk, U. Effect of Magnetic Field and UV-C Radiation on Postharvest Fruit Properties. Agriculture 2024, 14, 1167. [Google Scholar] [CrossRef]
- Liaqat, W.; Altaf, M.T.; Barutçular, C.; Nawaz, H.; Ullah, I.; Basit, A.; Mohamed, H.I. Ultraviolet-B Radiation in Relation to Agriculture in the Context of Climate Change: A Review. Cereal Res. Commun. 2024, 52, 1–24. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Zu, Y.; He, Y.; Li, Z.; Li, Y. Effects of UV-B Radiation Exposure on Transgenerational Plasticity in Grain Morphology and Proanthocyanidin Content in Yuanyang Red Rice. Int. J. Mol. Sci. 2024, 25, 4766. [Google Scholar] [CrossRef]
- Leonardelli, M.; Tissot, N.; Podolec, R.; Ares-Orpel, F.; Glauser, G.; Ulm, R.; Demarsy, E. Photoreceptor-Induced Sinapate Synthesis Contributes to Photoprotection in Arabidopsis. Plant Physiol. 2024, 196, 1518–1533. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Kaur, P.; Soni, R.; Madan, A.; Agarwal, P.; Singh, G. Decoding the Photoprotection Strategies and Manipulating Cyanobacterial Photoprotective Metabolites, Mycosporine-like Amino Acids, for next-Generation Sunscreens. Plant Physiol. Biochem. 2024, 212, 108744. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.M.; Hamilton, C.M.; Street, H.E. A Strain of Rosa Damascena Cultured Cells Resistant to Ultraviolet Light. Plant Physiol. 1979, 64, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Freitas, H.; Santos, C.; Silva, A.M.S. The Antioxidant System in Olea Europaea to Enhanced UV-B Radiation Also Depends on Flavonoids and Secoiridoids. Phytochemistry 2020, 170, 112199. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Shi, B.; Zhu, W.; Zheng, B.; Zhou, K.; Qian, M.; Wu, H. Genome-Wide Identification, Characterization and Expression Analysis of Mango (Mangifera indica L.) Chalcone Synthase (CHS) Genes in Response to Light. Horticulturae 2022, 8, 968. [Google Scholar] [CrossRef]
- Dini, I.; Grumetto, L. Recent Advances in Natural Polyphenol Research. Molecules 2022, 27, 8777. [Google Scholar] [CrossRef]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple Functional Roles of Anthocyanins in Plant-Environment Interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Ghasemi, S.; Kumleh, H.H.; Kordrostami, M. Changes in the Expression of Some Genes Involved in the Biosynthesis of Secondary Metabolites in Cuminum cyminum L. under UV Stress. Protoplasma 2019, 256, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Veronneau, P.-Y.; Rolland, D.; Roussel, D. Preharvest Ultraviolet C Irradiation Increased the Level of Polyphenol Accumulation and Flavonoid Pathway Gene Expression in Strawberry Fruit. J. Agric. Food Chem. 2017, 65, 9970–9979. [Google Scholar] [CrossRef]
- Demkura, P.V.; Abdala, G.; Baldwin, I.T.; Ballareݩ, C.L. Jasmonate-Dependent and-Independent Pathways Mediate Specific Effects of Solar Ultraviolet B Radiation on Leaf Phenolics and Antiherbivore Defense. Plant Physiol. 2010, 152, 1084–1095. [Google Scholar] [CrossRef]
- Berli, F.J.; Fanzone, M.; Piccoli, P.; Bottini, R. Solar UV-B and ABA Are Involved in Phenol Metabolism of Vitis vinifera L. Increasing Biosynthesis of Berry Skin Polyphenols. J. Agric. Food Chem. 2011, 59, 4874–4884. [Google Scholar] [CrossRef]
- Nenadis, N.; Llorens, L.; Koufogianni, A.; Diaz, L.; Font, J.; Gonzalez, J.A.; Verdaguer, D. Interactive Effects of UV Radiation and Reduced Precipitation on the Seasonal Leaf Phenolic Content/Composition and the Antioxidant Activity of Naturally Growing Arbutus Unedo Plants. J. Photochem. Photobiol. B 2015, 153, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Cao, B.; Zhou, S.; Liu, Y. Responses of the Flavonoid Pathway to UV-B Radiation Stress and the Correlation with the Lipid Antioxidant Characteristics in the Desert Plant Caryopteris mongolica. Acta Ecol. Sin. 2012, 32, 150–155. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; De Oliveira, J.M.P.F.; Melo, P.; Santos, C. Tomato Plants Use Non-Enzymatic Antioxidant Pathways to Cope with Moderate UV-A/B Irradiation: A Contribution to the Use of UV-A/B in Horticulture. J. Plant Physiol. 2018, 221, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Nascimento, L.B.; Leal-Costa, M.V.; Menezes, E.A.; Lopes, V.R.; Muzitano, M.F.; Costa, S.S.; Tavares, E.S. Ultraviolet-B Radiation Effects on Phenolic Profile and Flavonoid Content of Kalanchoe Pinnata. J. Photochem. Photobiol. B 2015, 148, 73–81. [Google Scholar] [CrossRef]
- Sytar, O.; Zivcak, M.; Bruckova, K.; Brestic, M.; Hemmerich, I.; Rauh, C.; Simko, I. Shift in Accumulation of Flavonoids and Phenolic Acids in Lettuce Attributable to Changes in Ultraviolet Radiation and Temperature. Sci. Hortic. 2018, 239, 193–204. [Google Scholar] [CrossRef]
- Lee, M.; Son, J.E.; Oh, M. Growth and Phenolic Compounds of Lactuca sativa L. Grown in a Closed-type Plant Production System with UV-A,-B, Or-C Lamp. J. Sci. Food Agric. 2014, 94, 197–204. [Google Scholar] [CrossRef]
- Huyskens-Keil, S.; Eichholz, I.; Kroh, L.W.; Rohn, S. UV-B Induced Changes of Phenol Composition and Antioxidant Activity in Black Currant Fruit (Ribes nigrum L.). J. Appl. Bot. Food Qual. 2012, 81, 140–144. [Google Scholar]
- Chen, Z.; Ma, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of Exogenous Ca2+ on Phenolic Accumulation and Physiological Changes in Germinated Wheat (Triticum aestivum L.) under UV-B Radiation. Food Chem. 2019, 288, 368–376. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Weng, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of UV-B Radiation on Phenolic Accumulation, Antioxidant Activity and Physiological Changes in Wheat (Triticum aestivum L.) Seedlings. Food Biosci. 2019, 30, 100409. [Google Scholar] [CrossRef]
- Goyal, A.; Siddiqui, S.; Upadhyay, N.; Soni, J. Effects of Ultraviolet Irradiation, Pulsed Electric Field, Hot Water and Ethanol Vapours Treatment on Functional Properties of Mung Bean Sprouts. J. Food Sci. Technol. 2014, 51, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Berli, F.J.; Fontana, A.; Piccoli, P.; Bottini, R. Malbec Grape (Vitis vinifera L.) Responses to the Environment: Berry Phenolics as Influenced by Solar UV-B, Water Deficit and Sprayed Abscisic Acid. Plant Physiol. Biochem. 2016, 109, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.S.; Pant, N.C.; Laishram, L.; Tewari, M.; Dhoundiyal, R.; Joshi, K.; Pandey, C.S. Effect of CuO Nanoparticles on Polyphenols Content and Antioxidant Activity in Ashwagandha (Withania somnifera L. Dunal). J. Pharmacogn. Phytochem. 2018, 7, 3433–3439. [Google Scholar]
- Nourozi, E.; Hosseini, B.; Maleki, R.; Mandoulakani, B.A. Pharmaceutical Important Phenolic Compounds Overproduction and Gene Expression Analysis in Dracocephalum kotschyi Hairy Roots Elicited by SiO2 Nanoparticles. Ind. Crops Prod. 2019, 133, 435–446. [Google Scholar] [CrossRef]
- Kőrösi, L.; Bouderias, S.; Csepregi, K.; Bognár, B.; Teszlák, P.; Scarpellini, A.; Castelli, A.; Hideg, É.; Jakab, G. Nanostructured TiO2-Induced Photocatalytic Stress Enhances the Antioxidant Capacity and Phenolic Content in the Leaves of Vitis Vinifera on a Genotype-Dependent Manner. J. Photochem. Photobiol. B 2019, 190, 137–145. [Google Scholar] [CrossRef]
- Girilal, M.; Fayaz, A.M.; Elumalai, L.K.; Sathiyaseelan, A.; Gandhiappan, J.; Kalaichelvan, P.T. Comparative Stress Physiology Analysis of Biologically and Chemically Synthesized Silver Nanoparticles on Solanum lycopersicum L. Colloid. Interface Sci. Commun. 2018, 24, 1–6. [Google Scholar] [CrossRef]
- Raigond, P.; Raigond, B.; Kaundal, B.; Singh, B.; Joshi, A.; Dutt, S. Effect of Zinc Nanoparticles on Antioxidative System of Potato Plants. J. Environ. Biol. 2017, 38, 435. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Thukral, A.K.; Bhardwaj, R. Epibrassinolide-Imidacloprid Interaction Enhances Non-Enzymatic Antioxidants in Brassica juncea L. Indian J. Plant Physiol. 2016, 21, 70–75. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, S.; Kumar, V.; Kanwar, M.K.; Kesavan, A.K.; Thukral, A.K.; Bhardwaj, R.; Alam, P.; Ahmad, P. Pre-Sowing Seed Treatment with 24-Epibrassinolide Ameliorates Pesticide Stress in Brassica juncea L. through the Modulation of Stress Markers. Front. Plant Sci. 2016, 7, 1569. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yuan, H.; Kumar, V.; Ramakrishnan, M.; Kohli, S.K.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Zheng, B. Castasterone Attenuates Insecticide Induced Phytotoxicity in Mustard. Ecotoxicol. Environ. Saf. 2019, 179, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, V.; Farimani, M.M.; Fathi, F.; Ghassempour, A. A Targeted Metabolomics Approach toward Understanding Metabolic Variations in Rice under Pesticide Stress. Anal. Biochem. 2015, 478, 65–72. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K. Enhancement of Oxidative and Drought Tolerance in Arabidopsis by Overaccumulation of Antioxidant Flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Duan, M.; Eman, M.; Yuan, H.; Sharma, A.; Zheng, B. Comparative Analysis of Citrus Species’ Flavonoid Metabolism, Gene Expression Profiling, and Their Antioxidant Capacity under Drought Stress. Antioxidants 2024, 13, 1149. [Google Scholar] [CrossRef]
- Rao, M.J.; Feng, B.; Ahmad, M.H.; Tahir Ul Qamar, M.; Aslam, M.Z.; Khalid, M.F.; Hussain, S.B.; Zhong, R.; Ali, Q.; Xu, Q.; et al. LC-MS/MS-Based Metabolomics Approach Identified Novel Antioxidant Flavonoids Associated with Drought Tolerance in Citrus Species. Front. Plant Sci. 2023, 14, 1150854. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Xu, Y.; Tang, X.; Huang, Y.; Liu, J.; Deng, X. CsCYT75B1, a Citrus CYTOCHROME P450 Gene, Is Involved in Accumulation of Antioxidant Flavonoids and Induces Drought Tolerance in Transgenic Arabidopsis. Antioxidants 2020, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Differential Responses of Phenolic Compounds of Brassica napus under Drought Stress. Iran. J. Plant Physiol. 2018, 8, 2417–2425. [Google Scholar]
- Li, M.; Li, Y.; Zhang, W.; Li, S.; Gao, Y.; Ai, X.; Zhang, D.; Liu, B.; Li, Q. Metabolomics Analysis Reveals That Elevated Atmospheric CO2 Alleviates Drought Stress in Cucumber Seedling Leaves. Anal. Biochem. 2018, 559, 71–85. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; del Mar Rubio-Wilhelmi, M.; Ruiz, J.M. Differential Responses of Five Cherry Tomato Varieties to Water Stress: Changes on Phenolic Metabolites and Related Enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.; Fekry, M.; Bishr, M.; El-Zalabani, S.; Salama, O. Foliar Spraying of Salicylic Acid Induced Accumulation of Phenolics, Increased Radical Scavenging Activity and Modified the Composition of the Essential Oil of Water Stressed Thymus vulgaris L. Plant Physiol. Biochem. 2018, 123, 65–74. [Google Scholar] [CrossRef]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The Effect of Water Stress on Phytochemical Accumulation, Bioactive Compounds and Expression of Key Genes Involved in Flavonoid Biosynthesis in Chrysanthemum morifolium L. Ind. Crops Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Perin, E.C.; da Silva Messias, R.; Borowski, J.M.; Crizel, R.L.; Schott, I.B.; Carvalho, I.R.; Rombaldi, C.V.; Galli, V. ABA-Dependent Salt and Drought Stress Improve Strawberry Fruit Quality. Food Chem. 2019, 271, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Galieni, A.; Di Mattia, C.; De Gregorio, M.; Speca, S.; Mastrocola, D.; Pisante, M.; Stagnari, F. Effects of Nutrient Deficiency and Abiotic Environmental Stresses on Yield, Phenolic Compounds and Antiradical Activity in Lettuce (Lactuca sativa L.). Sci. Hortic. 2015, 187, 93–101. [Google Scholar] [CrossRef]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic Compounds as Indicators of Drought Resistance in Shrubs from Patagonian Shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef] [PubMed]
- García-Calderón, M.; Pons-Ferrer, T.; Mrazova, A.; Pal’ove-Balang, P.; Vilkova, M.; Pérez-Delgado, C.M.; Vega, J.M.; Eliášová, A.; Repčák, M.; Márquez, A.J. Modulation of Phenolic Metabolism under Stress Conditions in a Lotus japonicus Mutant Lacking Plastidic Glutamine Synthetase. Front. Plant Sci. 2015, 6, 760. [Google Scholar] [CrossRef]
- Silva, F.L.B.; Vieira, L.G.E.; Ribas, A.F.; Moro, A.L.; Neris, D.M.; Pacheco, A.C. Proline Accumulation Induces the Production of Total Phenolics in Transgenic Tobacco Plants under Water Deficit without Increasing the G6PDH Activity. Theor. Exp. Plant Physiol. 2018, 30, 251–260. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous Application of Chitosan on Biochemical and Physiological Characteristics, Phenolic Content and Antioxidant Activity of Two Species of Basil (Ocimum ciliatum and Ocimum basilicum) under Reduced Irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of Flavonoid Biosynthesis Genes and Accumulation of Flavonoid in Wheat Leaves in Response to Drought Stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional Regulation of Anthocyanin Biosynthesis in Ripening Fruits of Grapevine under Seasonal Water Deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Taïbi, K.; Taïbi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of Salt Stress on Growth, Chlorophyll Content, Lipid Peroxidation and Antioxidant Defence Systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; de Abreu, C.E.B.; Gomes-Filho, E. Effect of Salt Stress on Antioxidative Enzymes and Lipid Peroxidation in Leaves and Roots of Salt-Tolerant and Salt-Sensitive Maize Genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of Flavonols over Hydroxycinnamic Acids Favors Oxidative Damage Protection under Abiotic Stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H. NtMYB4 and NtCHS1 Are Critical Factors in the Regulation of Flavonoid Biosynthesis and Are Involved in Salinity Responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of Salinity Stress on the Physiological Characteristics, Phenolic Compounds and Antioxidant Activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Rossi, L.; Borghi, M.; Francini, A.; Lin, X.; Xie, D.-Y.; Sebastiani, L. Salt Stress Induces Differential Regulation of the Phenylpropanoid Pathway in Olea europaea Cultivars Frantoio (Salt-Tolerant) and Leccino (Salt-Sensitive). J. Plant Physiol. 2016, 204, 8–15. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Elansary, H.O. Synergetic Effects of 5-Aminolevulinic Acid and Ascophyllum Nodosum Seaweed Extracts on Asparagus Phenolics and Stress Related Genes under Saline Irrigation. Plant Physiol. Biochem. 2018, 129, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Golkar, P.; Taghizadeh, M. In Vitro Evaluation of Phenolic and Osmolite Compounds, Ionic Content, and Antioxidant Activity in Safflower (Carthamus tinctorius L.) under Salinity Stress. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 134, 357–368. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, H.; Chen, D.; Li, Z.; Peng, R.; Yao, Q. A Grape BHLH Transcription Factor Gene, VvbHLH1, Increases the Accumulation of Flavonoids and Enhances Salt and Drought Tolerance in Transgenic Arabidopsis thaliana. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 125, 387–398. [Google Scholar] [CrossRef]
- Yan, J.; Wang, B.; Jiang, Y.; Cheng, L.; Wu, T. GmFNSII-Controlled Soybean Flavone Metabolism Responds to Abiotic Stresses and Regulates Plant Salt Tolerance. Plant Cell Physiol. 2014, 55, 74–86. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Augmentation of Leaf Color Parameters, Pigments, Vitamins, Phenolic Acids, Flavonoids and Antioxidant Activity in Selected Amaranthus Tricolor under Salinity Stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef] [PubMed]
- Ben Dkhil, B.; Denden, M. Effect of Salt Stress on Growth, Anthocyanins, Membrane Permeability and Chlorophyll Fluorescence of Okra (Abelmoschus esculentus L.) Seedlings. Am. J. Plant Physiol. 2012, 7, 174–183. [Google Scholar] [CrossRef]
- Eryılmaz, F. The Relationships between Salt Stress and Anthocyanin Content in Higher Plants. Biotechnol. Biotechnol. Equip. 2006, 20, 47–52. [Google Scholar] [CrossRef]
- Aloisi, I.; Parrotta, L.; Ruiz, K.B.; Landi, C.; Bini, L.; Cai, G.; Biondi, S.; Del Duca, S. New Insight into Quinoa Seed Quality under Salinity: Changes in Proteomic and Amino Acid Profiles, Phenolic Content, and Antioxidant Activity of Protein Extracts. Front. Plant Sci. 2016, 7, 656. [Google Scholar] [CrossRef]
- Lucini, L.; Borgognone, D.; Rouphael, Y.; Cardarelli, M.; Bernardi, J.; Colla, G. Mild Potassium Chloride Stress Alters the Mineral Composition, Hormone Network, and Phenolic Profile in Artichoke Leaves. Front. Plant Sci. 2016, 7, 948. [Google Scholar] [CrossRef] [PubMed]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of Salt Stress on Volatile Compounds, Total Phenolic Content and Antioxidant Activities of Salvia mirzayanii. S. Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Niazi, A.; Moghadam, A. Phenylalanine Ammonia Lyase Isolation and Functional Analysis of Phenylpropanoid Pathway under Salinity Stress in ’Salvia’ species. Aust. J. Crop Sci. 2015, 9, 656–665. [Google Scholar]
- Ma, Y.; Wang, P.; Gu, Z.; Tao, Y.; Shen, C.; Zhou, Y.; Han, Y.; Yang, R. Ca2+ Involved in GABA Signal Transduction for Phenolics Accumulation in Germinated Hulless Barley under NaCl Stress. Food Chem. X 2019, 2, 100023. [Google Scholar] [CrossRef] [PubMed]
- Çoban, Ö.; Baydar, N.G. Brassinosteroid Effects on Some Physical and Biochemical Properties and Secondary Metabolite Accumulation in Peppermint (Mentha piperita L.) under Salt Stress. Ind. Crops Prod. 2016, 86, 251–258. [Google Scholar] [CrossRef]
- Kaur, L.; Zhawar, V.K. Phenolic Parameters under Exogenous ABA, Water Stress, Salt Stress in Two Wheat Cultivars Varying in Drought Tolerance. Indian J. Plant Physiol. 2015, 20, 151–156. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy Metal Stress and Responses in Plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Zhang, H.; Reynolds, M. Cadmium Exposure in Living Organisms: A Short Review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, E.; Labudda, M. Dual Role of Metallic Trace Elements in Stress Biology—From Negative to Beneficial Impact on Plants. Int. J. Mol. Sci. 2019, 20, 3117. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Mukherjee, A.G.; Gopalakrishnan, A.V.; Wanjari, U.R.; Kannampuzha, S.; Murali, R.; Veeraraghavan, V.P.; Vinayagam, S.; Paz-Montelongo, S.; George, A. Protective Effects of Macromolecular Polyphenols, Metal (Zinc, Selenium, and Copper)-Polyphenol Complexes, and Pectin in Different Organs with an Emphasis on Arsenic Poisoning: A Review. Int. J. Biol. Macromol. 2023, 23, 126715. [Google Scholar]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Štork, F.; Bačkor, M. Comparison of Cadmium and Copper Effect on Phenolic Metabolism, Mineral Nutrients and Stress-Related Parameters in Matricaria chamomilla Plants. Plant Soil 2009, 320, 231–242. [Google Scholar] [CrossRef]
- Mishra, B.; Singh Sangwan, N. Amelioration of Cadmium Stress in Withania somnifera by ROS Management: Active Participation of Primary and Secondary Metabolism. Plant Growth Regul. 2019, 87, 403–412. [Google Scholar] [CrossRef]
- Mishra, B.; Sangwan, R.S.; Mishra, S.; Jadaun, J.S.; Sabir, F.; Sangwan, N.S. Effect of Cadmium Stress on Inductive Enzymatic and Nonenzymatic Responses of ROS and Sugar Metabolism in Multiple Shoot Cultures of Ashwagandha (Withania somnifera Dunal). Protoplasma 2014, 251, 1031–1045. [Google Scholar] [CrossRef]
- Kaur, R.; Bhardwaj, R.; Sirhindi, G. Castasterone Regulated Polyphenolic Metabolism and Photosynthetic System in Brassica juncea Plants under Copper Stress. J. Pharmacogn. Phytochem. 2015, 4, 282–289. [Google Scholar]
- Chang, X.; Li, J.; Wei, S.; Ying, J.; Nevill, P.; Qi, Z.; Lu, Q.; You, Z. Integrated Comparative Transcriptome and Weighted Gene Co-Expression Network Analysis Provide Valuable Insights into the Response Mechanisms of Alisma Orientale to Cadmium Stress. Sci. Total Environ. 2024, 957, 177401. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, A.S.; Feng, X.; Gao, G.; Yu, C.; Chen, J.; Chen, K.; Wang, X.; Mou, P.; Shao, D.; Chen, P. Genome Wide Characterization of R2R3 MYB Transcription Factor from Apocynum venetum Revealed Potential Stress Tolerance and Flavonoid Biosynthesis Genes. Genomics 2022, 114, 110275. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Abubakar, A.S.; Chen, K.; Yu, C.; Zhu, A.; Chen, J.; Gao, G.; Wang, X.; Mou, P.; Chen, P. Genome-Wide Analysis of R2R3-MYB Transcription Factors in Boehmeria nivea (L.) Gaudich Revealed Potential Cadmium Tolerance and Anthocyanin Biosynthesis Genes. Front. Genet. 2023, 14, 1080909. [Google Scholar] [CrossRef]
- Villiers, F.; Ducruix, C.; Hugouvieux, V.; Jarno, N.; Ezan, E.; Garin, J.; Junot, C.; Bourguignon, J. Investigating the Plant Response to Cadmium Exposure by Proteomic and Metabolomic Approaches. Proteomics 2011, 11, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-Epibrassinolide and Salicylic Acid Regulates Pigment Contents, Antioxidative Defense Responses, and Gene Expression in Brassica juncea L. Seedlings under Pb Stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Yadav, P.; Sharma, A.; Thukral, A.K.; Kumar, V.; Kohli, S.K.; Bhardwaj, R. Castasterone and Citric Acid Treatment Restores Photosynthetic Attributes in Brassica juncea L. under Cd (II) Toxicity. Ecotoxicol. Environ. Saf. 2017, 145, 466–475. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef] [PubMed]
- Mira, L.; Tereza Fernandez, M.; Santos, M.; Rocha, R.; Helena Florêncio, M.; Jennings, K.R. Interactions of Flavonoids with Iron and Copper Ions: A Mechanism for Their Antioxidant Activity. Free Radic. Res. 2002, 36, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and Applications of Flavonoid Metal Complexes. RSC Adv. 2015, 5, 45853–45877. [Google Scholar] [CrossRef]
- Anjitha, K.S.; Sameena, P.P.; Puthur, J.T. Functional Aspects of Plant Secondary Metabolites in Metal Stress Tolerance and Their Importance in Pharmacology. Plant Stress 2021, 2, 100038. [Google Scholar] [CrossRef]
- Kaur, P.; Bali, S.; Sharma, A.; Vig, A.P.; Bhardwaj, R. Effect of Earthworms on Growth, Photosynthetic Efficiency and Metal Uptake in Brassica juncea L. Plants Grown in Cadmium-Polluted Soils. Environ. Sci. Pollut. Res. 2017, 24, 13452–13465. [Google Scholar] [CrossRef]
- Kaur, P.; Bali, S.; Sharma, A.; Vig, A.P.; Bhardwaj, R. Role of Earthworms in Phytoremediation of Cadmium (Cd) by Modulating the Antioxidative Potential of Brassica juncea L. Appl. Soil Ecol. 2018, 124, 306–316. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Kumar, V.; Kaur, P.; Bhardwaj, R. Synergistic Effect of 24-Epibrassinolide and Salicylic Acid on Photosynthetic Efficiency and Gene Expression in Brassica juncea L. under Pb Stress. Turk. J. Biol. 2017, 41, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.-M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of Bioactive Compounds and Gene Expression Alterations in Hairy Root Cultures of Chinese Cabbage Elicited by Copper Oxide Nanoparticles. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 134, 95–106. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Cichoński, J.; Michalik, P.; Chrzanowski, G. Effect of Heavy Metal Stress on Phenolic Compounds Accumulation in Winter Wheat Plants. Molecules 2022, 28, 241. [Google Scholar] [CrossRef] [PubMed]
- Handa, N.; Kohli, S.K.; Sharma, A.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Selenium Ameliorates Chromium Toxicity through Modifications in Pigment System, Antioxidative Capacity, Osmotic System, and Metal Chelators in Brassica juncea Seedlings. S. Afr. J. Bot. 2018, 119, 1–10. [Google Scholar] [CrossRef]
- Kısa, D.; Elmastaş, M.; Öztürk, L.; Kayır, Ö. Responses of the Phenolic Compounds of Zea mays under Heavy Metal Stress. Appl. Biol. Chem. 2016, 59, 813–820. [Google Scholar] [CrossRef]
- Smirnov, O.E.; Kosyan, A.M.; Kosyk, O.I.; Taran, N.Y. Response of Phenolic Metabolism Induced by Aluminium Toxicity in Fagopyrum Esculentum Moench. Plants. Ukr. Biochem. J. 2015, 87, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Q.; Lu, H.; Li, J.; Yang, D.; Liu, J.; Yan, C. Phenolic Metabolism and Related Heavy Metal Tolerance Mechanism in Kandelia obovata under Cd and Zn Stress. Ecotoxicol. Environ. Saf. 2019, 169, 134–143. [Google Scholar] [CrossRef]
- Leng, X.; Jia, H.; Sun, X.; Shangguan, L.; Mu, Q.; Wang, B.; Fang, J. Comparative Transcriptome Analysis of Grapevine in Response to Copper Stress. Sci. Rep. 2015, 5, 17749. [Google Scholar] [CrossRef]
- Ling, J.; Tan, J.; Chen, H.; Yang, Z.; Luo, Q.; Jia, J. Physiology, Transcriptome and Root Exudates Analysis of Response to Aluminum Stress in Pinus massoniana. Forests 2023, 14, 1410. [Google Scholar] [CrossRef]
- Lu, H.; Zhu, F.; Xu, H.; Liu, J.; Wu, Y. Insights of Mechanism into Enhanced Removal of Cr (VI) by Ginkgo Biloba Leaves Synthesized Bimetallic Nano-Zero-Valent Iron/Copper. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132094. [Google Scholar] [CrossRef]
- Zafari, S.; Sharifi, M.; Chashmi, N.A.; Mur, L.A.J. Modulation of Pb-Induced Stress in Prosopis Shoots through an Interconnected Network of Signaling Molecules, Phenolic Compounds and Amino Acids. Plant Physiol. Biochem. 2016, 99, 11–20. [Google Scholar] [CrossRef]
- Correia, B.; Hancock, R.D.; Amaral, J.; Gomez-Cadenas, A.; Valledor, L.; Pinto, G. Combined Drought and Heat Activates Protective Responses in Eucalyptus Globulus That Are Not Activated When Subjected to Drought or Heat Stress Alone. Front. Plant Sci. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; de Oliveira, J.M.P.F.; Marum, L.; Pereira, V.; Almeida, T.; Nunes, S.; Araujo, M.; Moutinho-Pereira, J.; Correia, C.M.; Santos, C. Pinus elliottii and P. elliottii x P. caribaea Hybrid Differently Cope with Combined Drought and Heat Episodes. Ind. Crops Prod. 2022, 176, 114428. [Google Scholar] [CrossRef]
- Valente, S.; Machado, B.; Pinto, D.C.G.A.; Santos, C.; Silva, A.M.S.; Dias, M.C. Modulation of Phenolic and Lipophilic Compounds of Olive Fruits in Response to Combined Drought and Heat. Food Chem. 2020, 329, 127191. [Google Scholar] [CrossRef]
- Peres, F.; Martins, L.L.; Mourato, M.; Vitorino, C.; Antunes, P.; Ferreira-Dias, S. Phenolic Compounds of ‘Galega Vulgar’ and ‘Cobrançosa’ Olive Oils along Early Ripening Stages. Food Chem. 2016, 211, 51–58. [Google Scholar] [CrossRef]
- Martinelli, F.; Remorini, D.; Saia, S.; Massai, R.; Tonutti, P. Metabolic Profiling of Ripe Olive Fruit in Response to Moderate Water Stress. Sci. Hortic. 2013, 159, 52–58. [Google Scholar] [CrossRef]
- Cizmarova, B.; Hubkova, B.; Birkova, A. Quercetin as an Effective Antioxidant against Superoxide Radical. Funct. Food Sci. 2023, 3, 15–25. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I. Review of the Flavonoids Quercetin, Hesperetin, and Naringenin. Dietary Sources, Bioactivities, Bioavailability, and Epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Saviranta, N.M.M.; Veeroos, L.; Granlund, L.J.; Hassinen, V.H.; Kaarniranta, K.; Karjalainen, R.O. Plant Flavonol Quercetin and Isoflavone Biochanin A Differentially Induce Protection against Oxidative Stress and Inflammation in ARPE-19 Cells. Food Res. Int. 2011, 44, 109–113. [Google Scholar] [CrossRef]
- Wang, F.; Ren, X.; Zhang, F.; Qi, M.; Zhao, H.; Chen, X.; Ye, Y.; Yang, J.; Li, S.; Zhang, Y. A R2R3-Type MYB Transcription Factor Gene from Soybean, GmMYB12, Is Involved in Flavonoids Accumulation and Abiotic Stress Tolerance in Transgenic Arabidopsis. Plant Biotechnol. Rep. 2019, 13, 219–233. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Hudecova, L.; Lauro, P.; Simunková, M.; Barbierikova, Z.; Malcek, M.; Alwasel, S.H.; Alhazza, I.M.; Rhodes, C.J.; Valko, M. The Effect of Luteolin on DNA Damage Mediated by a Copper Catalyzed Fenton Reaction. J. Inorg. Biochem. 2022, 226, 111635. [Google Scholar] [CrossRef] [PubMed]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanadian, M.; Talebi, M.; Ghanati, F. Melatonin and Calcium Modulate the Production of Rosmarinic Acid, Luteolin, and Apigenin in Dracocephalum kotschyi under Salinity Stress. Phytochemistry 2020, 177, 112422. [Google Scholar] [CrossRef]
- Borges Bubols, G.; da Rocha Vianna, D.; Medina-Remon, A.; von Poser, G.; Maria Lamuela-Raventos, R.; Lucia Eifler-Lima, V.; Cristina Garcia, S. The Antioxidant Activity of Coumarins and Flavonoids. Mini Rev. Med. Chem. 2013, 13, 318–334. [Google Scholar]
- Dędek, K.; Rosicka-Kaczmarek, J.; Nebesny, E.; Kowalska, G. Characteristics and Biological Properties of Ferulic Acid. Biotechnol. Food Sci. 2019, 83, 71–85. [Google Scholar]
- Kaurinovic, B.; Vastag, D. Flavonoids and Phenolic Acids as Potential Natural Antioxidants; IntechOpen: London, UK, 2019; ISBN 1789239206. [Google Scholar]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.; Jabeen, N.; Ashraf, K.; Sultan, K.; Farhan, M.; Hussain, M.I.; Deng, G.; Alsudays, I.M.; Saleh, M.A.; Tariq, S. Exploring the Role of Caffeic Acid in Mitigating Abiotic Stresses in Plants: A Review. Plant Stress 2024, 12, 100487. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).