Resveratrol and Its Nitric Oxide–Donor Hybrid as an Emerging Therapy for Oxidative-Stress-Driven Priapism in Sickle Cell Disease
Abstract
1. Introduction
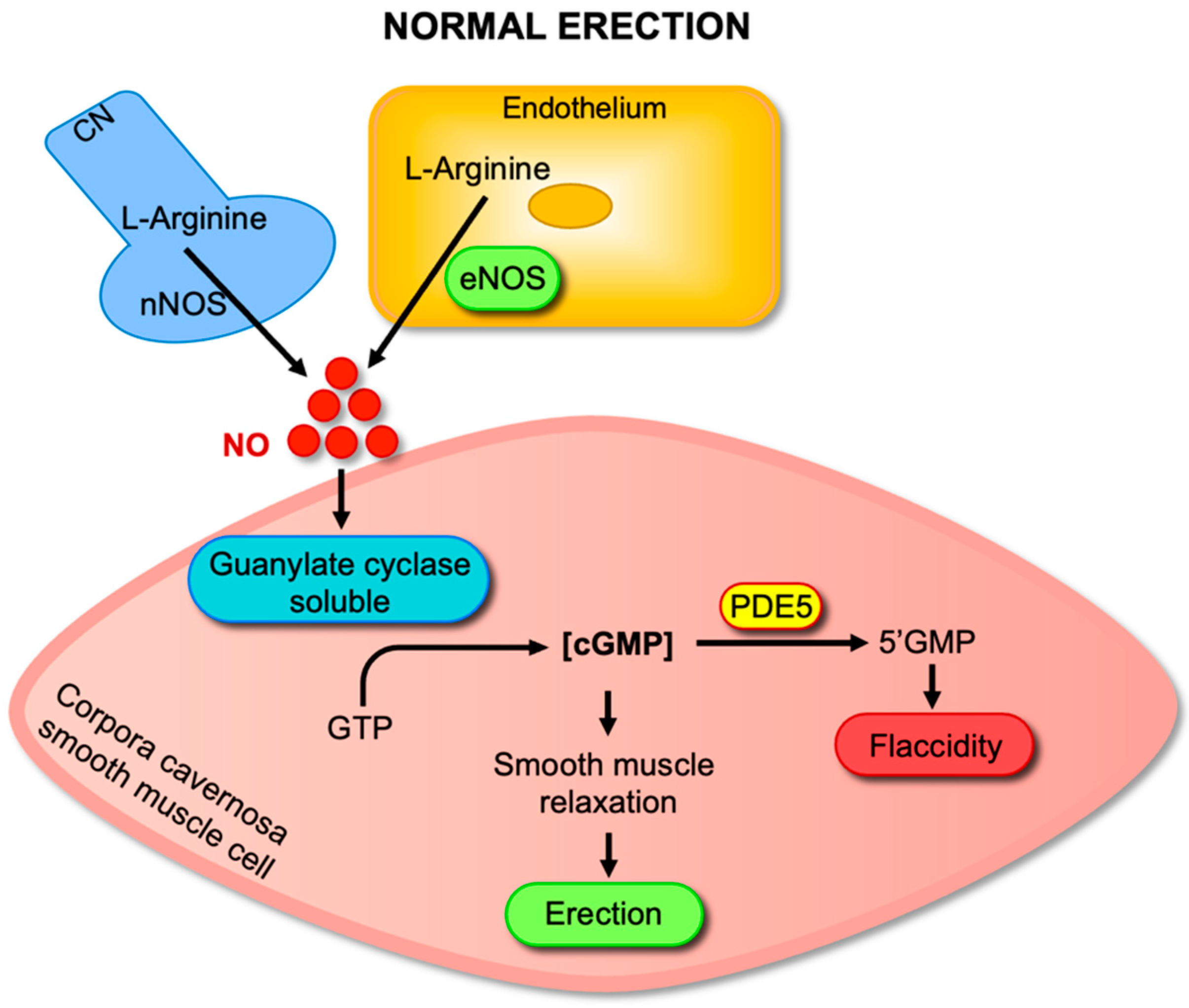
2. Molecular Mechanisms of Penile Erection
3. Pathophysiology of SCD-Associated Priapism: Role of Oxidative Stress and Nitric Oxide Dysregulation
4. Pharmacological Profile of Resveratrol
5. Evaluation of Resveratrol and Its Nitric Oxide–Donor Hybrid in SCD-Associated Priapism
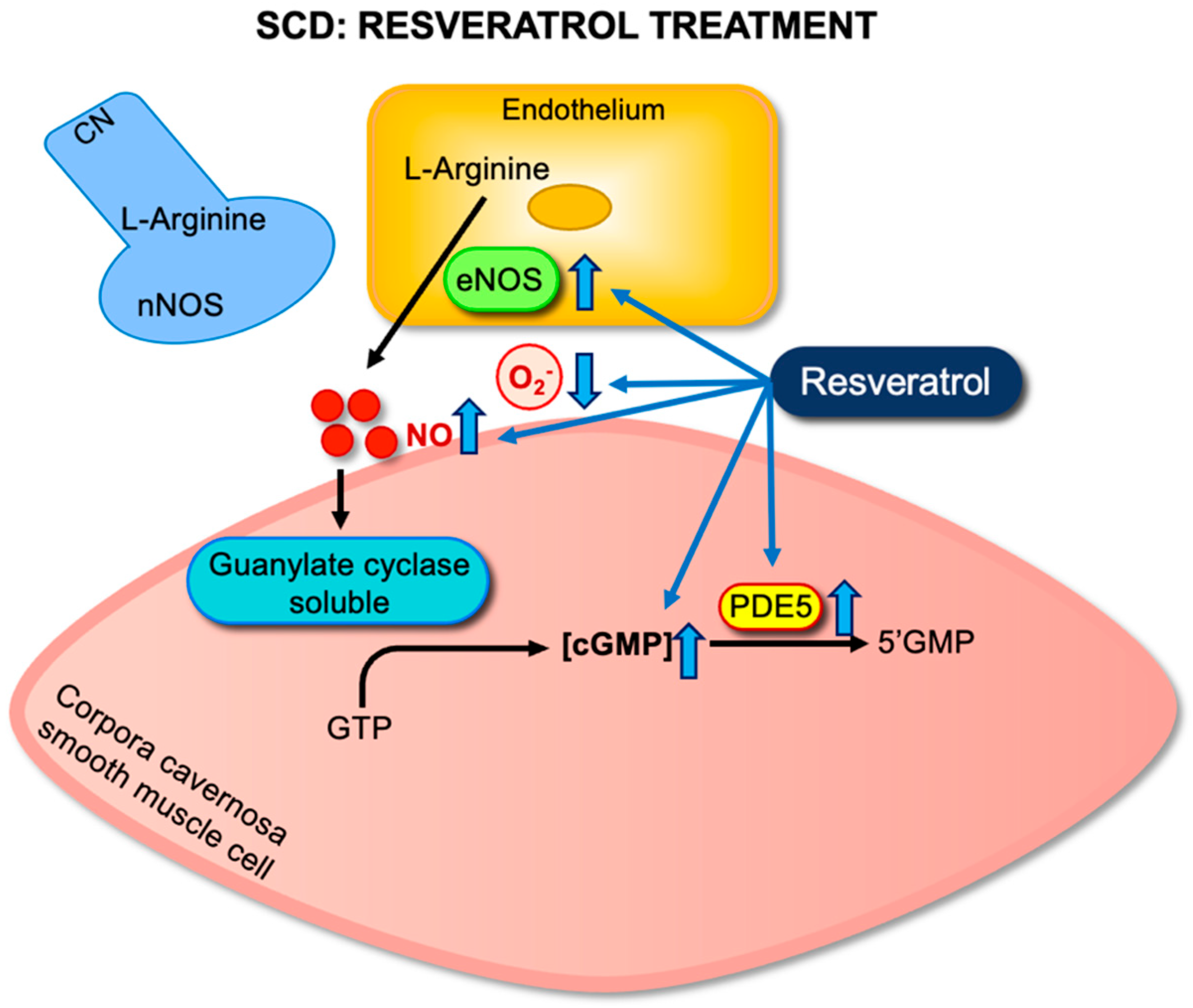
5.1. Evaluation of Resveratrol Monotherapy
5.2. Evaluation of Resveratrol–NO Donor Hybrids (RVT-FxMe)
6. Potential Therapeutic Role of Resveratrol Combined with Current Treatments
7. Clinical Evidence and Translational Challenges
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle Cell Disease. Nat. Rev. Dis. Primer 2018, 4, 18010. [Google Scholar] [CrossRef]
- Arduini, G.A.O.; Trovó de Marqui, A.B. Prevalence and Characteristics of Priapism in Sickle Cell Disease. Hemoglobin 2018, 42, 73–77. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Allen, B.K.; Brock, G.B.; Broderick, G.A.; Chou, R.; Kohler, T.S.; Mulhall, J.P.; Oristaglio, J.; Rahimi, L.L.; Rogers, Z.R.; et al. The Diagnosis and Management of Recurrent Ischemic Priapism, Priapism in Sickle Cell Patients, and Non-Ischemic Priapism: An AUA/SMSNA Guideline. J. Urol. 2022, 208, 43–52. [Google Scholar] [CrossRef]
- Silveira, T.H.R.; Pereira, D.A.; Calmasini, F.B.; Costa, F.F.; Burnett, A.L.; Silva, F.H. Sympathetic Hypoactivity Leads to Hypocontractility of the Corpus Cavernosum in Sickle Cell Mice: A Mechanism Contributing to Priapism. Int. J. Impot. Res. 2024, 1–6. [Google Scholar] [CrossRef]
- Musicki, B.; Burnett, A.L. Mechanisms Underlying Priapism in Sickle Cell Disease: Targeting and Key Innovations on the Preclinical Landscape. Expert Opin. Ther. Targets 2020, 24, 439–450. [Google Scholar] [CrossRef]
- Pereira, D.A.; Calmasini, F.B.; Costa, F.F.; Burnett, A.L.; Silva, F.H. Nitric Oxide Resistance in Priapism Associated with Sickle Cell Disease: Mechanisms, Therapeutic Challenges, and Future Directions. J. Pharmacol. Exp. Ther. 2024, 390, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Musicki, B.; Liu, T.; Sezen, S.F.; Burnett, A.L. Targeting NADPH Oxidase Decreases Oxidative Stress in the Transgenic Sickle Cell Mouse Penis. J. Sex. Med. 2012, 9, 1980–1987. [Google Scholar] [CrossRef] [PubMed]
- Bivalacqua, T.J.; Musicki, B.; Hsu, L.L.; Berkowitz, D.E.; Champion, H.C.; Burnett, A.L. Sildenafil Citrate-Restored eNOS and PDE5 Regulation in Sickle Cell Mouse Penis Prevents Priapism via Control of Oxidative/Nitrosative Stress. PLoS ONE 2013, 8, e68028. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.H.; Claudino, M.A.; Calmasini, F.B.; Alexandre, E.C.; Franco-Penteado, C.; Burnett, A.L.; Antunes, E.; Costa, F.F. Sympathetic Hyperactivity, Increased Tyrosine Hydroxylase and Exaggerated Corpus Cavernosum Relaxations Associated with Oxidative Stress Plays a Major Role in the Penis Dysfunction in Townes Sickle Cell Mouse. PLoS ONE 2016, 11, e0166291. [Google Scholar] [CrossRef]
- Pereira, P.d.S.; Pereira, D.A.; Calmasini, F.B.; Reis, L.O.; Brinkman, N.; Burnett, A.L.; Costa, F.F.; Silva, F.H. Haptoglobin Treatment Contributes to Regulating Nitric Oxide Signal and Reduces Oxidative Stress in the Penis: A Preventive Treatment for Priapism in Sickle Cell Disease. Front. Physiol. 2022, 13, 961534. [Google Scholar] [CrossRef]
- Silva, F.H.; Karakus, S.; Musicki, B.; Matsui, H.; Bivalacqua, T.J.; Dos Santos, J.L.; Costa, F.F.; Burnett, A.L. Beneficial Effect of the Nitric Oxide Donor Compound 3-(1,3-Dioxoisoindolin-2-Yl)Benzyl Nitrate on Dysregulated Phosphodiesterase 5, NADPH Oxidase, and Nitrosative Stress in the Sickle Cell Mouse Penis: Implication for Priapism Treatment. J. Pharmacol. Exp. Ther. 2016, 359, 230–237. [Google Scholar] [CrossRef]
- Musicki, B.; Karakus, S.; Akakpo, W.; Silva, F.H.; Liu, J.; Chen, H.; Zirkin, B.R.; Burnett, A.L. Testosterone Replacement in Transgenic Sickle Cell Mice Controls Priapic Activity and Upregulates PDE5 Expression and eNOS Activity in the Penis. Andrology 2018, 6, 184–191. [Google Scholar] [CrossRef]
- Musicki, B.; Karakus, S.; La Favor, J.D.; Chen, H.; Silva, F.H.; Sturny, M.; Zirkin, B.R.; Burnett, A.L. TSPO Ligand FGIN-1-27 Controls Priapism in Sickle Cell Mice via Endogenous Testosterone Production. J. Cell. Physiol. 2020, 236, 3073–3082. [Google Scholar] [CrossRef]
- Fukuhara, S.; Tsujimura, A.; Okuda, H.; Yamamoto, K.; Takao, T.; Miyagawa, Y.; Nonomura, N.; Okuyama, A. Vardenafil and Resveratrol Synergistically Enhance the Nitric Oxide/Cyclic Guanosine Monophosphate Pathway in Corpus Cavernosal Smooth Muscle Cells and Its Therapeutic Potential for Erectile Dysfunction in the Streptozotocin-Induced Diabetic Rat: Preliminary Findings. J. Sex. Med. 2011, 8, 1061–1071. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D. Resveratrol and Cardiovascular Diseases. Nutrients 2016, 8, 250. [Google Scholar] [CrossRef]
- Sener, T.E.; Tavukcu, H.H.; Atasoy, B.M.; Cevik, O.; Kaya, O.T.; Cetinel, S.; Dagli Degerli, A.; Tinay, I.; Simsek, F.; Akbal, C.; et al. Resveratrol Treatment May Preserve the Erectile Function after Radiotherapy by Restoring Antioxidant Defence Mechanisms, SIRT1 and NOS Protein Expressions. Int. J. Impot. Res. 2018, 30, 179–188. [Google Scholar] [CrossRef]
- Yazir, Y.; Şahin, T.D.; Furat Rençber, S.; Gacar, G.; Halbutoğulları, Z.S.; Utkan, T.; Aricioglu, F. Restorative Effect of Resveratrol on Expression of Endothelial and Neuronal Nitric Oxide Synthase in Cavernous Tissues of Chronic Unpredictable Mild Stress-Exposed Rats: An Impact of Inflammation. Int. J. Impot. Res. 2018, 30, 318–326. [Google Scholar] [CrossRef]
- Yu, W.; Wang, J.; Dai, Y.-T.; Wang, B.; Xu, Y.; Gao, Q.-Q.; Xu, Z.-P. Modulation of SIRT1 Expression Improves Erectile Function in Aged Rats. Asian J. Androl. 2022, 24, 666–670. [Google Scholar] [CrossRef]
- Splendore, C.O.; Silveira, T.H.R.; Pereira, D.A.; Bossarino, B.P.; de Oliveira, M.G.; Calmasini, F.B.; Burnett, A.L.; Costa, F.F.; Silva, F.H. Resveratrol Attenuates the Priapism Phenotype in Sickle Cell Mice by Restoring NO-cGMP-PDE5 Signaling and Reducing NADPH Oxidase 2 Expression. Front. Pharmacol. 2025, 16, 1551533. [Google Scholar] [CrossRef]
- Bosquesi, P.L.; Melchior, A.C.B.; Pavan, A.R.; Lanaro, C.; de Souza, C.M.; Rusinova, R.; Chelucci, R.C.; Barbieri, K.P.; Fernandes, G.F.d.S.; Carlos, I.Z.; et al. Synthesis and Evaluation of Resveratrol Derivatives as Fetal Hemoglobin Inducers. Bioorg. Chem. 2020, 100, 103948. [Google Scholar] [CrossRef]
- Pinheiro, A.K.; Pereira, D.A.; Dos Santos, J.L.; Calmasini, F.B.; Alexandre, E.C.; Reis, L.O.; Burnett, A.L.; Costa, F.F.; Silva, F.H. Resveratrol-Nitric Oxide Donor Hybrid Effect on Priapism in Sickle Cell and Nitric Oxide-Deficient Mouse. PLoS ONE 2022, 17, e0269310. [Google Scholar] [CrossRef]
- MacDonald, S.M.; Burnett, A.L. Physiology of Erection and Pathophysiology of Erectile Dysfunction. Urol. Clin. N. Am. 2021, 48, 513–525. [Google Scholar] [CrossRef]
- Andersson, K.-E. Mechanisms of Penile Erection and Basis for Pharmacological Treatment of Erectile Dysfunction. Pharmacol. Rev. 2011, 63, 811–859. [Google Scholar] [CrossRef]
- Champion, H.C.; Bivalacqua, T.J.; Takimoto, E.; Kass, D.A.; Burnett, A.L. Phosphodiesterase-5A Dysregulation in Penile Erectile Tissue Is a Mechanism of Priapism. Proc. Natl. Acad. Sci. USA 2005, 102, 1661–1666. [Google Scholar] [CrossRef]
- Lagoda, G.; Sezen, S.F.; Cabrini, M.R.; Musicki, B.; Burnett, A.L. Molecular Analysis of Erection Regulatory Factors in Sickle Cell Disease-Associated Priapism in Human Penis. J. Urol. 2013, 189, 762–768. [Google Scholar] [CrossRef]
- Lin, C.-S.; Chow, S.; Lau, A.; Tu, R.; Lue, T.F. Human PDE5A Gene Encodes Three PDE5 Isoforms from Two Alternate Promoters. Int. J. Impot. Res. 2002, 14, 15–24. [Google Scholar] [CrossRef]
- Pereira, D.A.; Pereira, D.A.; Pereira, P.d.S.; Silveira, T.H.R.; Calmasini, F.B.; Reis, L.O.; Costa, F.F.; Silva, F.H. Hydroxyurea Does Not Reverse Functional Alterations of the Nitric Oxide-cGMP Pathway Associated with Priapism Phenotype in Corpus Cavernosum from Sickle Cell Mouse. PLoS ONE 2023, 18, e0292706. [Google Scholar] [CrossRef]
- Lagoda, G.; Sezen, S.F.; Hurt, K.J.; Cabrini, M.R.; Mohanty, D.K.; Burnett, A.L. Sustained Nitric Oxide (NO)-Releasing Compound Reverses Dysregulated NO Signal Transduction in Priapism. FASEB J. 2014, 28, 76–84. [Google Scholar] [CrossRef]
- Reiter, C.D.; Wang, X.; Tanus-Santos, J.E.; Hogg, N.; Cannon, R.O.; Schechter, A.N.; Gladwin, M.T. Cell-Free Hemoglobin Limits Nitric Oxide Bioavailability in Sickle-Cell Disease. Nat. Med. 2002, 8, 1383–1389. [Google Scholar] [CrossRef]
- Iacopucci, A.P.M.; da Silva Pereira, P.; Pereira, D.A.; Calmasini, F.B.; Pittalà, V.; Reis, L.O.; Burnett, A.L.; Costa, F.F.; Silva, F.H. Intravascular Hemolysis Leads to Exaggerated Corpus Cavernosum Relaxation: Implication for Priapism in Sickle Cell Disease. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22535. [Google Scholar] [CrossRef]
- Schmidt, H.M.; Kelley, E.E.; Straub, A.C. The Impact of Xanthine Oxidase (XO) on Hemolytic Diseases. Redox Biol. 2019, 21, 101072. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Musicki, B.; Bivalacqua, T.J.; Champion, H.C.; Burnett, A.L. Sildenafil Promotes eNOS Activation and Inhibits NADPH Oxidase in the Transgenic Sickle Cell Mouse Penis. J. Sex. Med. 2014, 11, 424–430. [Google Scholar] [CrossRef]
- Fu, S.; Tar, M.T.; Melman, A.; Davies, K.P. Opiorphin Is a Master Regulator of the Hypoxic Response in Corporal Smooth Muscle Cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014, 28, 3633–3644. [Google Scholar] [CrossRef]
- Ning, C.; Wen, J.; Zhang, Y.; Dai, Y.; Wang, W.; Zhang, W.; Qi, L.; Grenz, A.; Eltzschig, H.K.; Blackburn, M.R.; et al. Excess Adenosine A2B Receptor Signaling Contributes to Priapism through HIF-1α Mediated Reduction of PDE5 Gene Expression. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014, 28, 2725–2735. [Google Scholar] [CrossRef]
- Bivalacqua, T.J.; Ross, A.E.; Strong, T.D.; Gebska, M.A.; Musicki, B.; Champion, H.C.; Burnett, A.L. Attenuated RhoA/Rho-Kinase Signaling in Penis of Transgenic Sickle Cell Mice. Urology 2010, 76, e7–e12. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant Effects of Resveratrol in the Cardiovascular System. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Leonard, S.S.; Xia, C.; Jiang, B.-H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol Scavenges Reactive Oxygen Species and Effects Radical-Induced Cellular Responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Jang, J.-H.; Li, M.-H.; Surh, Y.-J. Resveratrol Upregulates Heme Oxygenase-1 Expression via Activation of NF-E2-Related Factor 2 in PC12 Cells. Biochem. Biophys. Res. Commun. 2005, 331, 993–1000. [Google Scholar] [CrossRef]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol Protects Primary Rat Hepatocytes against Oxidative Stress Damage: Activation of the Nrf2 Transcription Factor and Augmented Activities of Antioxidant Enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef]
- Javkhedkar, A.A.; Quiroz, Y.; Rodriguez-Iturbe, B.; Vaziri, N.D.; Lokhandwala, M.F.; Banday, A.A. Resveratrol Restored Nrf2 Function, Reduced Renal Inflammation, and Mitigated Hypertension in Spontaneously Hypertensive Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R840–R846. [Google Scholar] [CrossRef]
- Guo, S.; Yao, Q.; Ke, Z.; Chen, H.; Wu, J.; Liu, C. Resveratrol Attenuates High Glucose-Induced Oxidative Stress and Cardiomyocyte Apoptosis through AMPK. Mol. Cell. Endocrinol. 2015, 412, 85–94. [Google Scholar] [CrossRef]
- Yao, Y.; Li, J.; Niu, Y.; Yu, J.-Q.; Yan, L.; Miao, Z.-H.; Zhao, X.-X.; Li, Y.-J.; Yao, W.-X.; Zheng, P.; et al. Resveratrol Inhibits Oligomeric Aβ-Induced Microglial Activation via NADPH Oxidase. Mol. Med. Rep. 2015, 12, 6133–6139. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, W.; Hu, D.; Han, X.; Wang, H.; Yang, J.; Xu, Y.; Li, Y.; Yao, W.; Chen, C. Resveratrol Efficiently Improves Pulmonary Function via Stabilizing Mast Cells in a Rat Intestinal Injury Model. Life Sci. 2017, 185, 30–37. [Google Scholar] [CrossRef]
- Csiszar, A.; Labinskyy, N.; Podlutsky, A.; Kaminski, P.M.; Wolin, M.S.; Zhang, C.; Mukhopadhyay, P.; Pacher, P.; Hu, F.; de Cabo, R.; et al. Vasoprotective Effects of Resveratrol and SIRT1: Attenuation of Cigarette Smoke-Induced Oxidative Stress and Proinflammatory Phenotypic Alterations. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H2721–H2735. [Google Scholar] [CrossRef]
- Carrizzo, A.; Puca, A.; Damato, A.; Marino, M.; Franco, E.; Pompeo, F.; Traficante, A.; Civitillo, F.; Santini, L.; Trimarco, V.; et al. Resveratrol Improves Vascular Function in Patients with Hypertension and Dyslipidemia by Modulating NO Metabolism. Hypertension 2013, 62, 359–366. [Google Scholar] [CrossRef]
- Cheang, W.S.; Wong, W.T.; Wang, L.; Cheng, C.K.; Lau, C.W.; Ma, R.C.W.; Xu, A.; Wang, N.; Huang, Y.; Tian, X.Y. Resveratrol Ameliorates Endothelial Dysfunction in Diabetic and Obese Mice through Sirtuin 1 and Peroxisome Proliferator-Activated Receptor δ. Pharmacol. Res. 2019, 139, 384–394. [Google Scholar] [CrossRef]
- Li, J.; Feng, Z.; Lu, B.; Fang, X.; Huang, D.; Wang, B. Resveratrol Alleviates High Glucose-Induced Oxidative Stress and Apoptosis in Rat Cardiac Microvascular Endothelial Cell through AMPK/Sirt1 Activation. Biochem. Biophys. Rep. 2023, 34, 101444. [Google Scholar] [CrossRef]
- Arunachalam, G.; Yao, H.; Sundar, I.K.; Caito, S.; Rahman, I. SIRT1 Regulates Oxidant- and Cigarette Smoke-Induced eNOS Acetylation in Endothelial Cells: Role of Resveratrol. Biochem. Biophys. Res. Commun. 2010, 393, 66–72. [Google Scholar] [CrossRef]
- Pan, W.; Yu, H.; Huang, S.; Zhu, P. Resveratrol Protects against TNF-α-Induced Injury in Human Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced Repression of NF-KB and P38 MAPK. PLoS ONE 2016, 11, e0147034. [Google Scholar] [CrossRef]
- Liu, C.-W.; Sung, H.-C.; Lin, S.-R.; Wu, C.-W.; Lee, C.-W.; Lee, I.-T.; Yang, Y.-F.; Yu, I.-S.; Lin, S.-W.; Chiang, M.-H.; et al. Resveratrol Attenuates ICAM-1 Expression and Monocyte Adhesiveness to TNF-α-Treated Endothelial Cells: Evidence for an Anti-Inflammatory Cascade Mediated by the miR-221/222/AMPK/P38/NF-κB Pathway. Sci. Rep. 2017, 7, 44689. [Google Scholar] [CrossRef]
- Nallasamy, P.; Kang, Z.Y.; Sun, X.; Anandh Babu, P.V.; Liu, D.; Jia, Z. Natural Compound Resveratrol Attenuates TNF-Alpha-Induced Vascular Dysfunction in Mice and Human Endothelial Cells: The Involvement of the NF-κB Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 12486. [Google Scholar] [CrossRef]
- Chen, B.; Xue, J.; Meng, X.; Slutzky, J.L.; Calvert, A.E.; Chicoine, L.G. Resveratrol Prevents Hypoxia-Induced Arginase II Expression and Proliferation of Human Pulmonary Artery Smooth Muscle Cells via Akt-Dependent Signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L317–L325. [Google Scholar] [CrossRef]
- Almajdoob, S.; Hossain, E.; Anand-Srivastava, M.B. Resveratrol Attenuates Hyperproliferation of Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats: Role of ROS and ROS-Mediated Cell Signaling. Vascul. Pharmacol. 2018, 101, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wei, S.; Zhang, X.; Chen, L.; Zhang, X.; Jiang, Y.; Sheng, M.; Chen, Y. Resveratrol Inhibits TGF-Β1-Induced Fibrotic Effects in Human Pterygium Fibroblasts. Environ. Health Prev. Med. 2023, 28, 59. [Google Scholar] [CrossRef] [PubMed]
- Idris, I.M.; Yusuf, A.A.; Ismail, I.I.; Borodo, A.M.; Hikima, M.S.; Kana, S.A.; Aliyu, T.; Musangedu, K.; Jibrilla, A.U.; Aji, S.A.; et al. A Controlled Trial for Preventing Priapism in Sickle Cell Anemia: Hydroxyurea plus Placebo vs Hydroxyurea plus Tadalafil. Blood 2025, 145, 3101–3112. [Google Scholar] [CrossRef] [PubMed]
- Niihara, Y.; Miller, S.T.; Kanter, J.; Lanzkron, S.; Smith, W.R.; Hsu, L.L.; Gordeuk, V.R.; Viswanathan, K.; Sarnaik, S.; Osunkwo, I.; et al. A Phase 3 Trial of L-Glutamine in Sickle Cell Disease. N. Engl. J. Med. 2018, 379, 226–235. [Google Scholar] [CrossRef]
- Ataga, K.I.; Kutlar, A.; Kanter, J.; Liles, D.; Cancado, R.; Friedrisch, J.; Guthrie, T.H.; Knight-Madden, J.; Alvarez, O.A.; Gordeuk, V.R.; et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 429–439. [Google Scholar] [CrossRef]
- Abboud, M.R.; Cançado, R.D.; Montalembert, M.D.; Smith, W.R.; Rimawi, H.; Voskaridou, E.; Güvenç, B.; Ataga, K.I.; Keefe, D.; Grosch, K.; et al. Crizanlizumab with or without Hydroxyurea in Patients with Sickle Cell Disease (STAND): Primary Analyses from a Placebo-Controlled, Randomised, Double-Blind, Phase 3 Trial. Lancet Haematol. 2025, 12, e248–e257. [Google Scholar] [CrossRef]
- Godos, J.; Romano, G.L.; Gozzo, L.; Laudani, S.; Paladino, N.; Dominguez Azpíroz, I.; Martínez López, N.M.; Giampieri, F.; Quiles, J.L.; Battino, M.; et al. Resveratrol and Vascular Health: Evidence from Clinical Studies and Mechanisms of Actions Related to Its Metabolites Produced by Gut Microbiota. Front. Pharmacol. 2024, 15, 1368949. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I Dose Escalation Pharmacokinetic Study in Healthy Volunteers of Resveratrol, a Potential Cancer Chemopreventive Agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- la Porte, C.; Voduc, N.; Zhang, G.; Seguin, I.; Tardiff, D.; Singhal, N.; Cameron, D.W. Steady-State Pharmacokinetics and Tolerability of Trans-Resveratrol 2000 Mg Twice Daily with Food, Quercetin and Alcohol (Ethanol) in Healthy Human Subjects. Clin. Pharmacokinet. 2010, 49, 449–454. [Google Scholar] [CrossRef]
- Chachay, V.S.; Macdonald, G.A.; Martin, J.H.; Whitehead, J.P.; O’Moore-Sullivan, T.M.; Lee, P.; Franklin, M.; Klein, K.; Taylor, P.J.; Ferguson, M.; et al. Resveratrol Does Not Benefit Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2014, 12, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. Biol. Fate Chem. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.-F.; Nunes, T.; Wright, L.; et al. Pharmacokinetic and Safety Profile of Trans-Resveratrol in a Rising Multiple-Dose Study in Healthy Volunteers. Mol. Nutr. Food Res. 2009, 53 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, L.; Calani, L.; Bocchi, L.; Delucchi, F.; Savi, M.; Ray, S.; Brighenti, F.; Stilli, D.; Del Rio, D. Bioaccumulation of Resveratrol Metabolites in Myocardial Tissue Is Dose-Time Dependent and Related to Cardiac Hemodynamics in Diabetic Rats. Nutr. Metab. Cardiovasc. Dis. NMCD 2014, 24, 408–415. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Splendore, C.O.; de Oliveira, M.G.; Costa, F.F.; Silva, F.H. Resveratrol and Its Nitric Oxide–Donor Hybrid as an Emerging Therapy for Oxidative-Stress-Driven Priapism in Sickle Cell Disease. Antioxidants 2025, 14, 1213. https://doi.org/10.3390/antiox14101213
Splendore CO, de Oliveira MG, Costa FF, Silva FH. Resveratrol and Its Nitric Oxide–Donor Hybrid as an Emerging Therapy for Oxidative-Stress-Driven Priapism in Sickle Cell Disease. Antioxidants. 2025; 14(10):1213. https://doi.org/10.3390/antiox14101213
Chicago/Turabian StyleSplendore, Carolina Oliveira, Mariana G. de Oliveira, Fernando Ferreira Costa, and Fábio Henrique Silva. 2025. "Resveratrol and Its Nitric Oxide–Donor Hybrid as an Emerging Therapy for Oxidative-Stress-Driven Priapism in Sickle Cell Disease" Antioxidants 14, no. 10: 1213. https://doi.org/10.3390/antiox14101213
APA StyleSplendore, C. O., de Oliveira, M. G., Costa, F. F., & Silva, F. H. (2025). Resveratrol and Its Nitric Oxide–Donor Hybrid as an Emerging Therapy for Oxidative-Stress-Driven Priapism in Sickle Cell Disease. Antioxidants, 14(10), 1213. https://doi.org/10.3390/antiox14101213







