Screening of Mediterranean Plant-Derived Extracts for Antioxidant Effect in Cell-Free and Human Cell Line Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Plant Species, Extract Preparation, and Phytochemical Characterization
2.2. Fenton Reaction
2.3. Cell Cultures
2.4. Cell Viability Assay
2.5. Intracellular ROS Measurement
2.6. Statistical Analysis
3. Results
3.1. Phytochemical Characterization
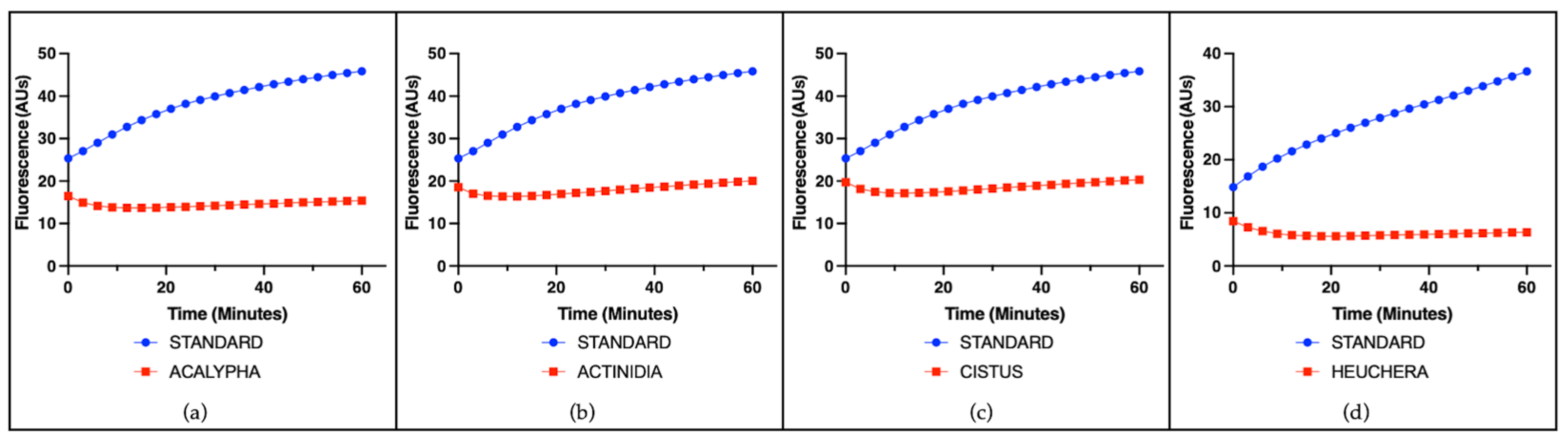
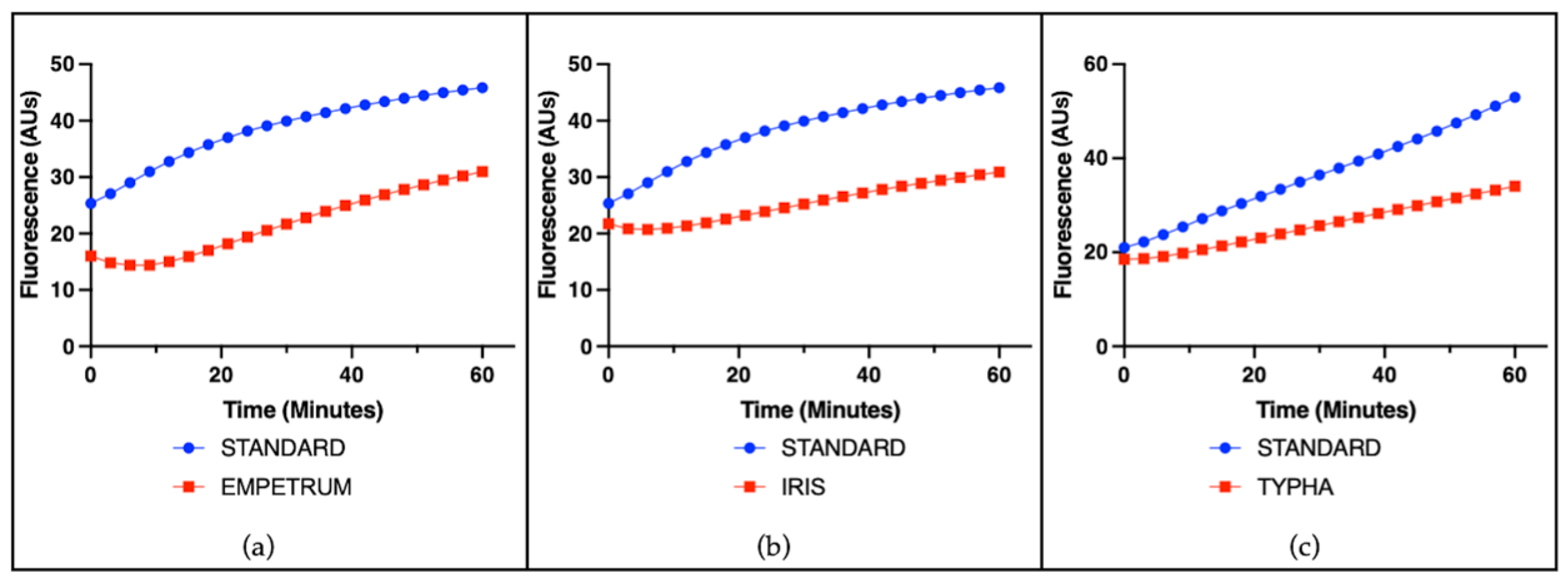
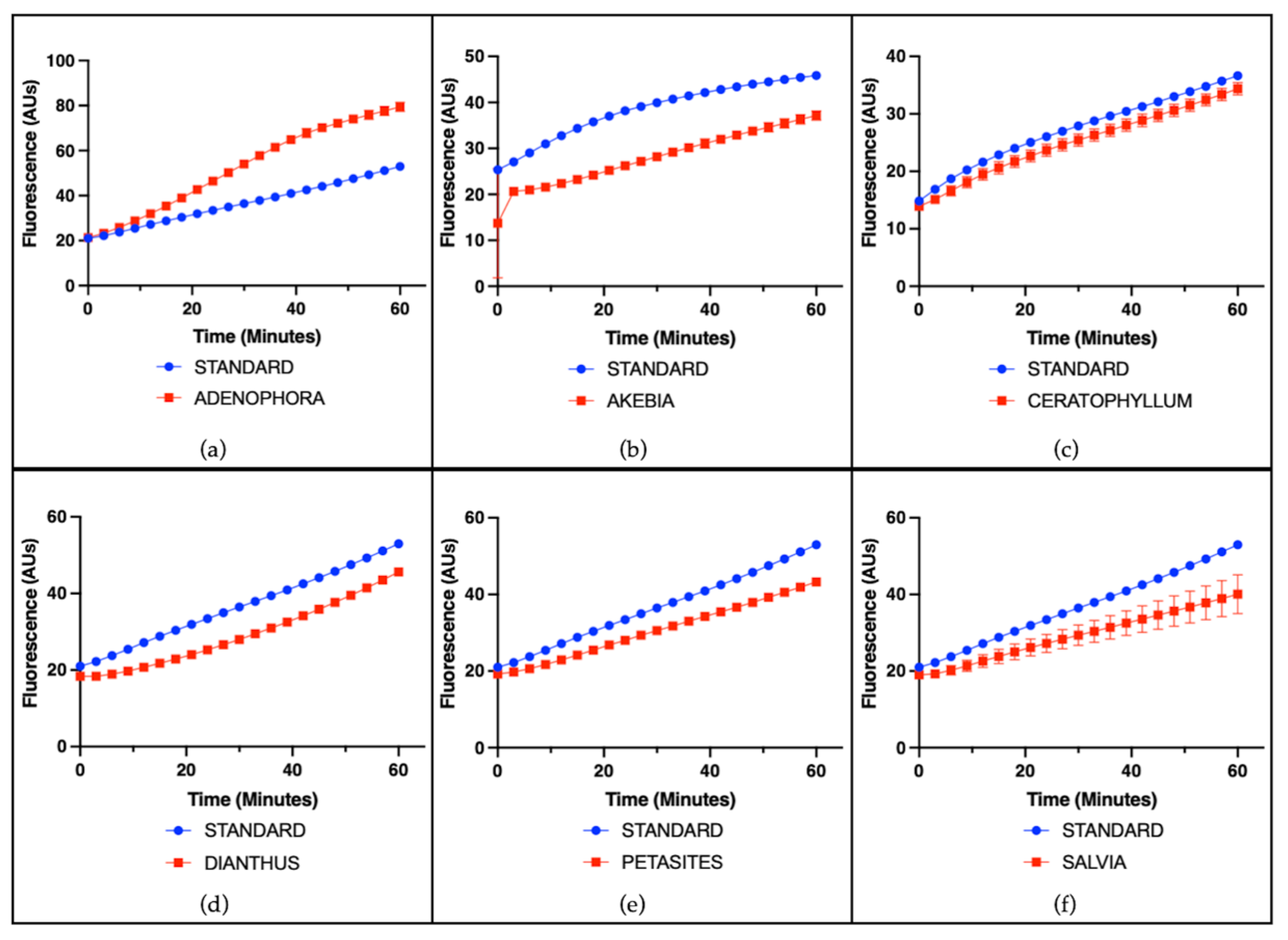
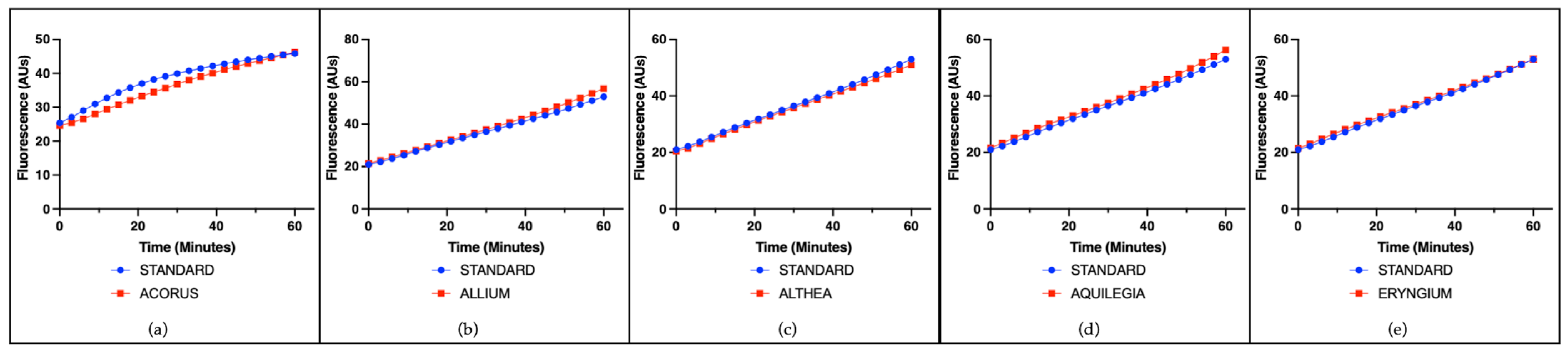
3.2. Cell-Free Radical-Scavenging Activity
3.3. Effect of Plant Extract on Cell Viability
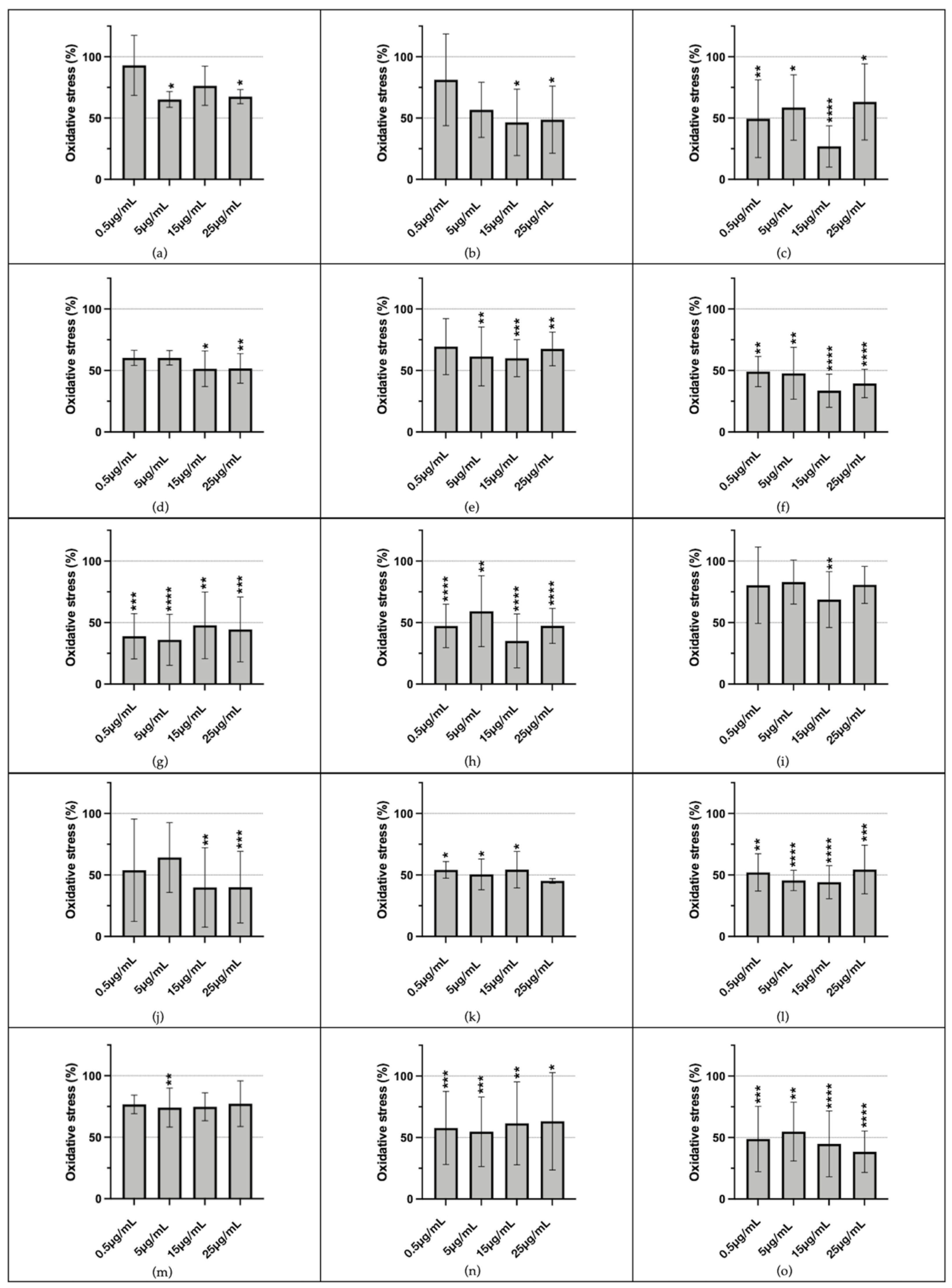
3.4. Evaluation of Antioxidant Activity
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| CAT | catalase |
| GPx | glutathione peroxidase |
| ACALYPHA | Acalypha virginica Linnaeus |
| ACORUS | Acorus calamus Linnaeus |
| ACTINIDIA | Actinidia deliciosa (A.Chev.) C.F. Liang & A.R. Ferguson |
| ADENOPHORA | Adenophora liliifolia (L.) Gaudin |
| AKEBIA | Akebia quinata (Thunberg ex Houttuyn) Decaisne |
| ALLIUM | Allium lusitanicum (L.) Mertens |
| ALTHAEA | Althaea officinalis Linnaeus |
| AQUILEGIA | Aquilegia atrata Wilhelm Daniel Joseph Koch |
| CERATOPHYLLUM | Ceratophyllum demersum Linnaeus |
| CISTUS | Cistus monspeliensis Linnaeus |
| DIANTHUS | Dianthus superbus Linnaeus subsp. superbus |
| EMPETRUM | Empetrum hermaphroditum Hagerup |
| ERYNGIUM | Eryngium maritimum Linnaeus |
| HEUCHERA | Heuchera sanguinea Pursh |
| IRIS | Iris pseudacorus Linnaeus (synonim Limniris pseudacorus) |
| PETASITES | Petasites paradoxus (Retzius) Baumgarten |
| SALVIA | Salvia pratensis Linnaeus |
| SUCCISA | Succisa pratensis Moench |
| TYPHA | Typha laxmannii Lepech |
| THP-1 | human monocytic leukemia cells |
| HUVECs | human umbilical vein endothelial cells |
| HIECs | human primary small intestinal epithelial cells |
| FBS | fetal bovine serum |
| AnnV | Annexin V |
| TBHP | tert-butyl hydroperoxide |
| UPLC | ultra-pressure liquid chromatography |
| ESI | electro-spray ionization |
| HRMS | High-resolution mass spectrometry |
| COPD | chronic obstructive pulmonary disease |
References
- Ahmed, S.A.; Alahmadi, Y.M.; Abdou, Y.A. The Impact of Serum Levels of Reactive Oxygen and Nitrogen Species on the Disease Severity of COVID-19. Int. J. Mol. Sci. 2023, 24, 8973. [Google Scholar] [CrossRef]
- Ghosh, S.; Roy, P.; Prasad, S.; Mugesh, G. A GPx-Mimetic Copper Vanadate Nanozyme Mediates the Release of Nitric Oxide from S-Nitrosothiols. Faraday Discuss. 2022, 234, 284–303. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Ghalami, R.Z.; Kamran, M.; Van Breusegem, F.; Karpiński, S. To Be or Not to Be? Are Reactive Oxygen Species, Antioxidants, and Stress Signalling Universal Determinants of Life or Death? Cells 2022, 11, 4105. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Tsesmelis, K.; Maity-Kumar, G.; Croner, D.; Sprissler, J.; Tsesmelis, M.; Hein, T.; Baumann, B.; Wirth, T. Accelerated Aging in Mice with Astrocytic Redox Imbalance as a Consequence of SOD2 Deletion. Aging Cell 2023, 22. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ye, C.; Lin, Z.; Huang, L.; Li, D. Recent Progress of Near-Infrared Fluorescent Probes in the Determination of Reactive Oxygen Species for Disease Diagnosis. Talanta 2024, 268. [Google Scholar] [CrossRef]
- Lam, C.R.I.; Tan, M.J.; Tan, S.H.; Tang, M.B.Y.; Cheung, P.C.F.; Tan, N.S. TAK1 Regulates SCF Expression to Modulate PKBα Activity That Protects Keratinocytes from ROS-Induced Apoptosis. Cell Death Differ. 2011, 18, 1120–1129. [Google Scholar] [CrossRef]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z. ROS-Scavenging Materials for Skin Wound Healing: Advancements and Applications. Front. Bioeng. Biotechnol. 2023, 11, 1304835. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Pan, K.; Li, J.; Chen, Q. The Function and Mechanism of Ferroptosis in Cancer. Apoptosis 2020, 25, 786–798. [Google Scholar] [CrossRef]
- Evavold, C.L.; Hafner-Bratkovič, I.; Devant, P.; D’Andrea, J.M.; Ngwa, E.M.; Boršić, E.; Doench, J.G.; LaFleur, M.W.; Sharpe, A.H.; Thiagarajah, J.R.; et al. Control of Gasdermin D Oligomerization and Pyroptosis by the Ragulator-Rag-mTORC1 Pathway. Cell 2021, 184, 4495–4511.e19. [Google Scholar] [CrossRef]
- Zhang, L.; Song, A.; Yang, Q.C.; Li, S.J.; Wang, S.; Wan, S.C.; Sun, J.; Kwok, R.T.K.; Lam, J.W.Y.; Deng, H.; et al. Integration of AIEgens into Covalent Organic Frameworks for Pyroptosis and Ferroptosis Primed Cancer Immunotherapy. Nat. Commun. 2023, 14, 5355. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B.; Kitts, D.D. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Dubois-deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Weinberg Sibony, R.; Segev, O.; Dor, S.; Raz, I. Overview of Oxidative Stress and Inflammation in Diabetes. J. Diabetes 2024, 16, e70014. [Google Scholar] [CrossRef]
- Houldsworth, A. Role of Oxidative Stress in Neurodegenerative Disorders: A Review of Reactive Oxygen Species and Prevention by Antioxidants. Brain Commun. 2024, 6. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Heard, C.M.; Garcia-Pardo, J. Bioactive Molecules from Plants: Discovery and Pharmaceutical Applications. Pharmaceutics 2022, 14, 2116. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative Stress: The Role of Antioxidant Phytochemicals in the Prevention and Treatment of Diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef]
- World Health Organization Healthy Diet. 2020. Available online: https://www.Who.Int/News-Room/Fact-Sheets/Detail/Healthy-Diet (accessed on 28 August 2025).
- Giammona, A.; Commisso, M.; Bonanomi, M.; Remedia, S.; Avesani, L.; Porro, D.; Gaglio, D.; Bertoli, G.; Lo Dico, A. A Novel Strategy for Glioblastoma Treatment by Natural Bioactive Molecules Showed a Highly Effective Anti-Cancer Potential. Nutrients 2024, 16, 2389. [Google Scholar] [CrossRef]
- De Soricellis, G.; Rinaldi, F.; Tengattini, S.; Temporini, C.; Negri, S.; Capelli, D.; Montanari, R.; Cena, H.; Salerno, S.; Massolini, G.; et al. Development of an Analytical Platform for the Affinity Screening of Natural Extracts by SEC-MS towards PPARα and PPARγ Receptors. Anal. Chim. Acta 2024, 1309, 342666. [Google Scholar] [CrossRef]
- Zorzi, G.; Gambini, S.; Negri, S.; Guzzo, F.; Commisso, M. Untargeted Metabolomics Analysis of the Orchid Species Oncidium Sotoanum Reveals the Presence of Rare Bioactive C-Diglycosylated Chrysin Derivatives. Plants 2023, 12, 655. [Google Scholar] [CrossRef]
- Commisso, M.; Negri, S.; Bianconi, M.; Gambini, S.; Avesani, S.; Ceoldo, S.; Avesani, L.; Guzzo, F. Untargeted and Targeted Metabolomics and Tryptophan Decarboxylase in Vivo Characterization Provide Novel Insight on the Development of Kiwifruits (Actinidia deliciosa). Int. J. Mol. Sci. 2019, 20, 897. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and Where to Find Them: A Meta-Analytic Approach to Investigate Their Chemistry, Biosynthesis, Distribution, and Effect on Human Health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and Their Role in Oxidative Stress, Inflammation, and Human Diseases. Chem.-Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Luo, J.; Zhao, C.; Zhao, J.; Ye, J.; He, K.; Zou, J. Oleanane Triterpenoids with C-14 Carboxyl Group from Astilbe Grandis Inhibited LPS-Induced Macrophages Activation by Suppressing the NF-κB Signaling Pathway. Front. Pharmacol. 2024, 15, 1413876. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological Activities and Distribution of Plant Saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Sampol, B.; Bota, J.; Riera, D.; Medrano, H.; Flexas, J. Analysis of the Virus-induced Inhibition of Photosynthesis in Malmsey Grapevines. New Phytol. 2003, 160, 403–412. [Google Scholar] [CrossRef]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Reviews: Current topics Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Zhou, L.; Jian, T.; Wan, Y.; Huang, R.; Fang, H.; Wang, Y.; Liang, C.; Ding, X.; Chen, J. Luteolin Alleviates Oxidative Stress in Chronic Obstructive Pulmonary Disease Induced by Cigarette Smoke via Modulation of the TRPV1 and CYP2A13/NRF2 Signaling Pathways. Int. J. Mol. Sci. 2023, 25, 369. [Google Scholar] [CrossRef]
- Dou, B.; Zhu, Y.; Sun, M.; Wang, L.; Tang, Y.; Tian, S.; Wang, F. Mechanisms of Flavonoids and Their Derivatives in Endothelial Dysfunction Induced by Oxidative Stress in Diabetes. Molecules 2024, 29, 3265. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Patanè, G.T.; Barreca, D.; Straface, E.; Gambardella, L.; Bozzuto, G.; Caruso, D.; Falliti, G.; Dossena, S.; et al. AAPH-Induced Oxidative Damage Reduced Anion Exchanger 1 (SLC4A1/AE1) Activity in Human Red Blood Cells: Protective Effect of an Anthocyanin-Rich Extract. Front. Physiol. 2023, 14, 1303815. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Zafar, F.; Asif, H.M.; Shaheen, G.; Ghauri, A.O.; Rajpoot, S.R.; Tasleem, M.W.; Shamim, T.; Hadi, F.; Noor, R.; Ali, T.; et al. A Comprehensive Review on Medicinal Plants Possessing Antioxidant Potential. Clin. Exp. Pharmacol. Physiol. 2023, 50, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Mosmann T Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E.; Youness, E.R.; Mohammed, N.A.; Morsy, S.M.Y.; Omara, E.A.; Sleem, A.A. Citric Acid Effects on Brain and Liver Oxidative Stress in Lipopolysaccharide-Treated Mice. J. Med. Food 2014, 17, 588–598. [Google Scholar] [CrossRef]
- May, N.; de Sousa Alves Neri, J.L.; Clunas, H.; Shi, J.; Parkes, E.; Dongol, A.; Wang, Z.; Jimenez Naranjo, C.; Yu, Y.; Huang, X.F.; et al. Investigating the Therapeutic Potential of Plants and Plant-Based Medicines: Relevance to Antioxidant and Neuroprotective Effects. Nutrients 2023, 15, 3912. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
| Species | Characterizing Metabolites |
|---|---|
| ACALYPHA | gallic acid derivatives, hydrolysable tannins |
| ACORUS | C-glycosylated flavonoids |
| ACTINIDIA | proanthocyanidins, flavonols, ursane/taraxastane triterpenoids |
| ADENOPHORA | flavonoids, pyrrolidine alkaloids |
| AKEBIA | flavonols, oleanane triterpenoids |
| ALLIUM | steroidal saponins |
| ALTHAEA | norisoprenoids sulfoglycosides, hypoleatin derivatives |
| AQUILEGIA | swertisin derivatives, cycloartane triterpenoids |
| CERATOPHYLLUM | hydroxycinnamates, flavonoids |
| CISTUS | ellagitannins, flavonols/methoxylated flavonols, labdane diterpenoids |
| DIANTHUS | flavonoids, oleanane triterpenoids |
| EMPETRUM | quercetin derivatives, flavanones, dihydrochalcones |
| ERYNGIUM | flavonols, triterpenoids |
| HEUCHERA | gallotannins |
| IRIS | xanthones, flavones |
| PETASITES | hydroxycinnamate esters, otonecine-type pyrrolizidine alkaloids |
| SALVIA | rosmarinic acid and related phenylethanoid/phenylpropanoid esters |
| SUCCISA | iridoids, flavones |
| TYPHA | proanthocyanidins, flavonols |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argentino, G.; Di Leo, E.G.; Stranieri, C.; Negri, S.; Commisso, M.; Guzzo, F.; Fratta Pasini, A.M.; Castagna, A.; Friso, S. Screening of Mediterranean Plant-Derived Extracts for Antioxidant Effect in Cell-Free and Human Cell Line Models. Antioxidants 2025, 14, 1217. https://doi.org/10.3390/antiox14101217
Argentino G, Di Leo EG, Stranieri C, Negri S, Commisso M, Guzzo F, Fratta Pasini AM, Castagna A, Friso S. Screening of Mediterranean Plant-Derived Extracts for Antioxidant Effect in Cell-Free and Human Cell Line Models. Antioxidants. 2025; 14(10):1217. https://doi.org/10.3390/antiox14101217
Chicago/Turabian StyleArgentino, Giuseppe, Edoardo Giuseppe Di Leo, Chiara Stranieri, Stefano Negri, Mauro Commisso, Flavia Guzzo, Anna Maria Fratta Pasini, Annalisa Castagna, and Simonetta Friso. 2025. "Screening of Mediterranean Plant-Derived Extracts for Antioxidant Effect in Cell-Free and Human Cell Line Models" Antioxidants 14, no. 10: 1217. https://doi.org/10.3390/antiox14101217
APA StyleArgentino, G., Di Leo, E. G., Stranieri, C., Negri, S., Commisso, M., Guzzo, F., Fratta Pasini, A. M., Castagna, A., & Friso, S. (2025). Screening of Mediterranean Plant-Derived Extracts for Antioxidant Effect in Cell-Free and Human Cell Line Models. Antioxidants, 14(10), 1217. https://doi.org/10.3390/antiox14101217










