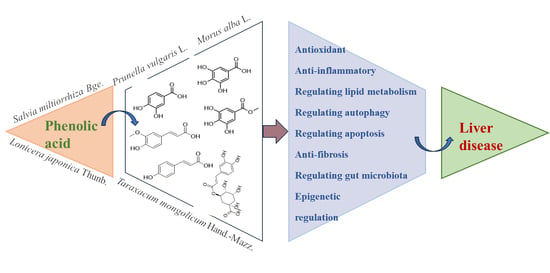
The Protective Effect of Phenolic Acids on Liver Disease: A Review of Possible Mechanisms
Abstract
1. Introduction
2. Biological Characteristics of Phenolic Acids
2.1. Chemical Structure of Phenolic Acids
2.2. The Relationship Between Chemical Structure and Biological Activity
2.3. Pharmacokinetic Properties
3. Molecular Mechanisms of Phenolic Acids in Modulating Liver Diseases
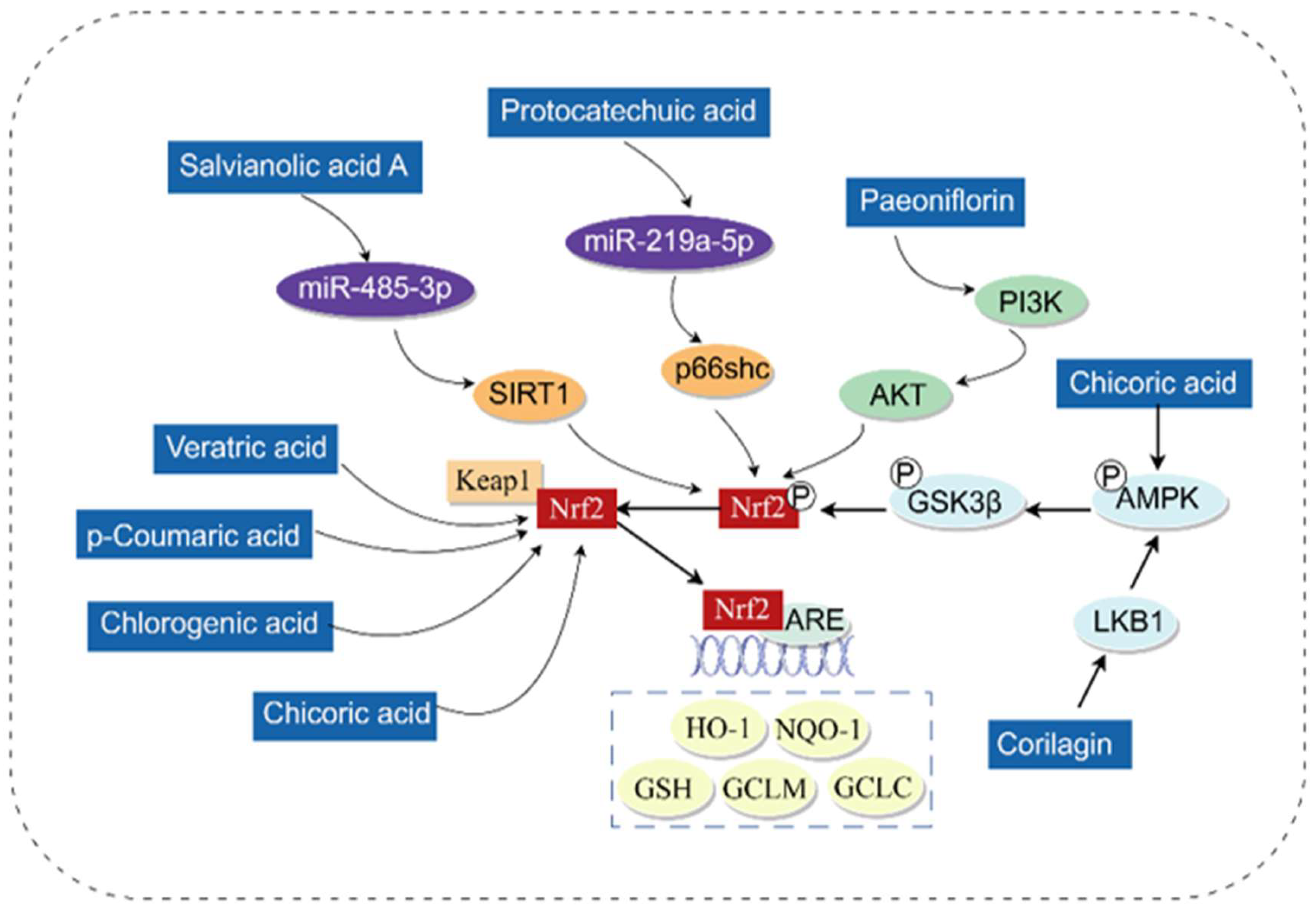
3.1. Antioxidant
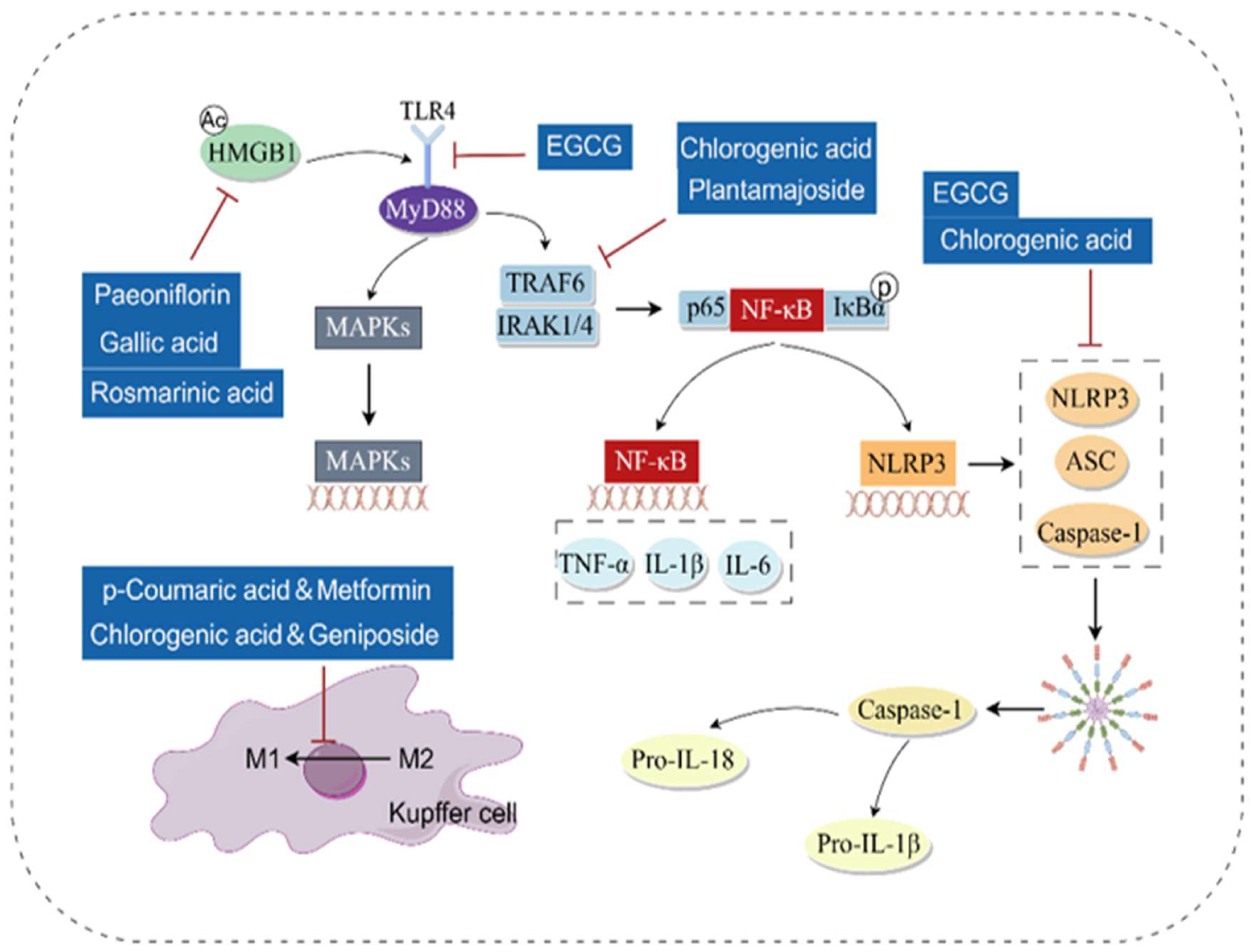
3.2. Anti-Inflammatory
3.3. Regulating Lipid Metabolism
3.4. Regulating Autophagy
3.5. Regulating Apoptosis
3.6. Anti-Fibrosis
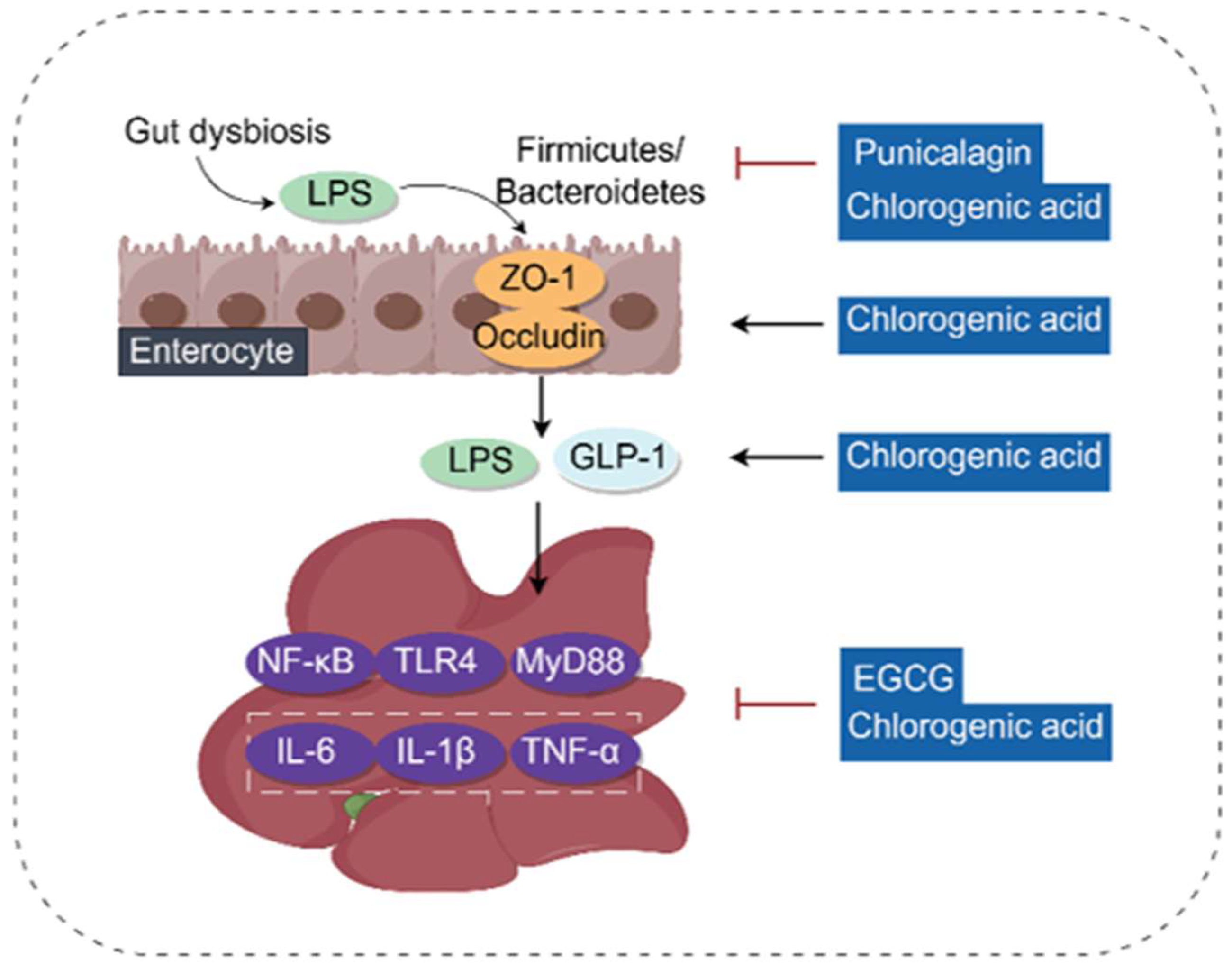
3.7. Regulating Gut Microbiota and Gut–Liver Axis
3.8. Other Mechanisms
4. Clinical Translation Prospects of Phenolic Acids
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, F.; Wong, N.K.; He, J.; Zhang, R.; Sun, R.; Xu, Y.; Liu, Y.; Li, W.; Koike, K.; et al. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J. Hepatol. 2019, 71, 212–221. [Google Scholar] [CrossRef]
- Andrade, R.J.; Chalasani, N.; Björnsson, E.S.; Suzuki, A.; Kullak-Ublick, G.A.; Watkins, P.B.; Devarbhavi, H.; Merz, M.; Lucena, M.I.; Kaplowitz, N.; et al. Drug-induced liver injury. Nat. Rev. Dis. Primers 2019, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Camini, F.C.; Costa, D.C. Silymarin: Not just another antioxidant. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190206. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, X.; Feng, W.; Xu, W.; Wu, C.; Xie, S.; Yu, S.; Fu, R. Biological synthesis of ursodeoxycholic acid. Front. Microbiol. 2023, 14, 1140662. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ji, X.; Cui, J.; Mi, Y.; Zhang, J.; Guo, Z. Synthesis, Characterization, and the Antioxidant Activity of Phenolic Acid Chitooligosaccharide Derivatives. Mar. Drugs 2022, 20, 489. [Google Scholar] [CrossRef]
- Simón, J.; Casado-Andrés, M.; Goikoetxea-Usandizaga, N.; Serrano-Maciá, M.; Martínez-Chantar, M.L. Nutraceutical Properties of Polyphenols against Liver Diseases. Nutrients 2020, 12, 3517. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Tsimidou, M.Z. Crocin bleaching assay (CBA) in structure-radical scavenging activity studies of selected phenolic compounds. J. Agric. Food Chem. 2006, 54, 9347–9356. [Google Scholar] [CrossRef]
- Kawabata, J.; Okamoto, Y.; Kodama, A.; Makimoto, T.; Kasai, T. Oxidative dimers produced from protocatechuic and gallic esters in the DPPH radical scavenging reaction. J. Agric. Food Chem. 2002, 50, 5468–5471. [Google Scholar] [CrossRef]
- Siquet, C.; Paiva-Martins, F.; Lima, J.L.; Reis, S.; Borges, F. Antioxidant profile of dihydroxy- and trihydroxyphenolic acids—A structure-activity relationship study. Free Radic. Res. 2006, 40, 433–442. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef]
- Sidoryk, K.; Jaromin, A.; Filipczak, N.; Cmoch, P.; Cybulski, M. Synthesis and Antioxidant Activity of Caffeic Acid Derivatives. Molecules 2018, 23, 2199. [Google Scholar] [CrossRef] [PubMed]
- Touaibia, M.; Jean-François, J.; Doiron, J. Caffeic Acid, a versatile pharmacophore: An overview. Mini Rev. Med. Chem. 2011, 11, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Lewis, B.A. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: Structure-activity relationship. J. Agric. Food Chem. 2002, 50, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Z.; Cao, Y.; Hung, C.H.; Du, R.; Leung, A.S.; So, P.K.; Chan, P.H.; Wong, W.L.; Leung, Y.C.; et al. Discovery of a novel class of rosmarinic acid derivatives as antibacterial agents: Synthesis, structure-activity relationship and mechanism of action. Bioorg. Chem. 2024, 146, 107318. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S. Ethnomedicinal uses of Indian spices used for cancer treatment: A treatise on structure-activity relationship and signaling pathways. Curr. Res. Food Sci. 2022, 5, 1845–1872. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Nunes, S.; Madureira, A.R.; Campos, D.; Sarmento, B.; Gomes, A.M.; Pintado, M.; Reis, F. Therapeutic and nutraceutical potential of rosmarinic acid-Cytoprotective properties and pharmacokinetic profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 1799–1806. [Google Scholar] [CrossRef]
- Azuma, K.; Ippoushi, K.; Nakayama, M.; Ito, H.; Higashio, H.; Terao, J. Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J. Agric. Food Chem. 2000, 48, 5496–5500. [Google Scholar] [CrossRef]
- Gan, R.Y.; Li, H.B.; Sui, Z.Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Sapountzaki, E.; Rova, U.; Christakopoulos, P. Ferulic Acid From Plant Biomass: A Phytochemical with Promising Antiviral Properties. Front. Nutr. 2021, 8, 777576. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Dariya, B.; Kasa, P.; Peela, S.; El-Rayes, B.F. Epigenetics in hepatocellular carcinoma. Semin. Cancer Biol. 2022, 86, 622–632. [Google Scholar] [CrossRef]
- Che, Z.; Zhou, Z.; Li, S.Q.; Gao, L.; Xiao, J.; Wong, N.K. ROS/RNS as molecular signatures of chronic liver diseases. Trends Mol. Med. 2023, 29, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Sun, J.; Zhang, W.; Yu, J.; Zhuang, C. Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free Radic. Biol. Med. 2020, 159, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Feng, C.; Jiang, H. Novel target for treating Alzheimer’s Diseases: Crosstalk between the Nrf2 pathway and autophagy. Ageing Res. Rev. 2021, 65, 101207. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ou, Z.; Chen, R.; Niu, X.; Chen, D.; Kang, R.; Tang, D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology 2016, 63, 173–184. [Google Scholar] [CrossRef]
- Suzuki, T.; Motohashi, H.; Yamamoto, M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013, 34, 340–346. [Google Scholar] [CrossRef]
- Sabitha, R.; Nishi, K.; Gunasekaran, V.P.; Agilan, B.; David, E.; Annamalai, G.; Vinothkumar, R.; Perumal, M.; Subbiah, L.; Ganeshan, M. p-Coumaric acid attenuates alcohol exposed hepatic injury through MAPKs, apoptosis and Nrf2 signaling in experimental models. Chem.-Biol. Interact. 2020, 321, 109044. [Google Scholar] [CrossRef]
- Ding, X.; Jian, T.; Li, J.; Lv, H.; Tong, B.; Li, J.; Meng, X.; Ren, B.; Chen, J. Chicoric Acid Ameliorates Nonalcoholic Fatty Liver Disease via the AMPK/Nrf2/NFκB Signaling Pathway and Restores Gut Microbiota in High-Fat-Diet-Fed Mice. Oxidative Med. Cell. Longev. 2020, 2020, 9734560. [Google Scholar] [CrossRef]
- Shi, A.; Li, T.; Zheng, Y.; Song, Y.; Wang, H.; Wang, N.; Dong, L.; Shi, H. Chlorogenic Acid Improves NAFLD by Regulating gut Microbiota and GLP-1. Front. Pharmacol. 2021, 12, 693048. [Google Scholar] [CrossRef]
- Shi, A.; Shi, H.; Wang, Y.; Liu, X.; Cheng, Y.; Li, H.; Zhao, H.; Wang, S.; Dong, L. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int. Immunopharmacol. 2018, 54, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, H.; Han, L.; Ding, L.; Shen, B.; Tian, Y.; Zhao, L.; Jin, M.; Wang, Q.; Qin, H.; et al. Chicoric acid ameliorate inflammation and oxidative stress in Lipopolysaccharide and d-galactosamine induced acute liver injury. J. Cell. Mol. Med. 2020, 24, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Hong, L.; Tian, Y.; Yin, C.; Zhu, C.; Feng, H. Corilagin alleviates acetaminophen-induced hepatotoxicity via enhancing the AMPK/GSK3β-Nrf2 signaling pathway. Cell Commun. Signal. 2019, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ma, X.; Zhu, Y.; Zhao, Y.; Wang, J.; Li, R.; Chen, C.; Wei, S.; Jiao, W.; Zhang, Y.; et al. Paeoniflorin ameliorates ANIT-induced cholestasis by activating Nrf2 through an PI3K/Akt-dependent pathway in rats. Phytother. Res. PTR 2015, 29, 1768–1775. [Google Scholar] [CrossRef]
- Fu, R.; Zhou, J.; Wang, R.; Sun, R.; Feng, D.; Wang, Z.; Zhao, Y.; Lv, L.; Tian, X.; Yao, J. Protocatechuic Acid-Mediated miR-219a-5p Activation Inhibits the p66shc Oxidant Pathway to Alleviate Alcoholic Liver Injury. Oxidative Med. Cell. Longev. 2019, 2019, 3527809. [Google Scholar] [CrossRef]
- Tang, F.; Wang, Z.; Zhou, J.; Yao, J. Salvianolic Acid A Protects against Acetaminophen-Induced Hepatotoxicity via Regulation of the miR-485-3p/SIRT1 Pathway. Antioxidants 2023, 12, 870. [Google Scholar] [CrossRef]
- Pang, C.; Zheng, Z.; Shi, L.; Sheng, Y.; Wei, H.; Wang, Z.; Ji, L. Caffeic acid prevents acetaminophen-induced liver injury by activating the Keap1-Nrf2 antioxidative defense system. Free Radic. Biol. Med. 2016, 91, 236–246. [Google Scholar] [CrossRef]
- Sanjay, S.; Girish, C.; Toi, P.C.; Bobby, Z. Gallic acid attenuates isoniazid and rifampicin-induced liver injury by improving hepatic redox homeostasis through influence on Nrf2 and NF-κB signalling cascades in Wistar Rats. J. Pharm. Pharmacol. 2021, 73, 473–486. [Google Scholar] [CrossRef]
- Luo, J.; Long, Y.; Ren, G.; Zhang, Y.; Chen, J.; Huang, R.; Yang, L. Punicalagin Reversed the Hepatic Injury of Tetrachloromethane by Antioxidation and Enhancement of Autophagy. J. Med. Food 2019, 22, 1271–1279. [Google Scholar] [CrossRef]
- Al-Khawalde, A.A.A.; Abukhalil, M.H.; Jghef, M.M.; Alfwuaires, M.A.; Alaryani, F.S.; Aladaileh, S.H.; Algefare, A.I.; Karimulla, S.; Alasmari, F.; Aldal’in, H.K.; et al. Punicalagin Protects against the Development of Methotrexate-Induced Hepatotoxicity in Mice via Activating Nrf2 Signaling and Decreasing Oxidative Stress, Inflammation, and Cell Death. Int. J. Mol. Sci. 2022, 23, 12334. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wei, M.; Hu, F.; Ouyang, H.; Huang, Z.; Lu, B.; Ji, L. Chlorogenic acid ameliorated non-alcoholic steatohepatitis via alleviating hepatic inflammation initiated by LPS/TLR4/MyD88 signaling pathway. Chem.-Biol. Interact. 2023, 376, 110461. [Google Scholar] [CrossRef]
- Wang, Y.; Jian, S.; Li, W.; Zhao, L.; Ye, G.; Shi, F.; Li, L.; Zou, Y.; Song, X.; Zhao, X.; et al. Epigallocatechin-3-gallate ameliorates liver injury secondary to Pseudomonas aeruginosa pneumonia. Int. Immunopharmacol. 2022, 112, 109239. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Guo, R.; Liao, W.; Li, J.; Cao, S. Plantamajoside alleviates acute sepsis-induced organ dysfunction through inhibiting the TRAF6/NF-κB axis. Pharm. Biol. 2023, 61, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Y.; Zhang, Y.; Yang, T.; Zhao, S.; Sun, N.; Tan, H.; Zhang, H.; Wang, C.; Fan, H. Effect of Chlorogenic Acid via Upregulating Resolvin D1 Inhibiting the NF-κB Pathway on Chronic Restraint Stress-Induced Liver Inflammation. J. Agric. Food Chem. 2022, 70, 10532–10542. [Google Scholar] [CrossRef]
- Xu, K.; Lu, G.; Feng, Q.; Chen, S.; Wang, Y. Hepatoprotective effect of protocatechuic acid against type 2 diabetes-induced liver injury. Pharm. Biol. 2023, 61, 737–745. [Google Scholar] [CrossRef]
- Gaskell, H.; Ge, X.; Nieto, N. High-Mobility Group Box-1 and Liver Disease. Hepatol. Commun. 2018, 2, 1005–1020. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, Y.Y.; Chen, W.Y.; Liao, S.L.; Chou, S.T.; Yang, C.P.; Chen, C.J. Hepatoprotective activities of rosmarinic acid against extrahepatic cholestasis in rats. Food Chem. Toxicol. 2017, 108, 214–223. [Google Scholar] [CrossRef]
- Xie, T.; Li, K.; Gong, X.; Jiang, R.; Huang, W.; Chen, X.; Tie, H.; Zhou, Q.; Wu, S.; Wan, J.; et al. Paeoniflorin protects against liver ischemia/reperfusion injury in mice via inhibiting HMGB1-TLR4 signaling pathway. Phytother. Res. PTR 2018, 32, 2247–2255. [Google Scholar] [CrossRef]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, Q.; Wang, T.; Kan, Z.; Li, X.; Hu, L.; Peng, C.Y.; Qian, F.; Wang, Y.; Granato, D. Green tea polyphenols and epigallocatechin-3-gallate protect against perfluorodecanoic acid induced liver damage and inflammation in mice by inhibiting NLRP3 inflammasome activation. Food Res. Int. 2020, 127, 108628. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, G.; Tehrani, S.S.; Panahi, G.; Bahramzadeh, A.; Meshkani, R. Combination therapy of metformin and p-coumaric acid mitigates metabolic dysfunction associated with obesity and nonalcoholic fatty liver disease in high-fat diet obese C57BL/6 mice. J. Nutr. Biochem. 2023, 118, 109369. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Jin, Y.; Wang, X.; Cai, B.; An, Z.; Hu, Y.Y.; Feng, Q. A Combination of Geniposide and Chlorogenic Acid Combination Ameliorates Nonalcoholic Steatohepatitis in Mice by Inhibiting Kupffer Cell Activation. BioMed Res. Int. 2021, 2021, 6615881. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, S.; Kong, W.; Wu, C.; Zeng, T.; Xie, S.; Chen, Q.; Kuang, S.; Zheng, R.; Wang, F.; et al. Corilagin alleviates liver fibrosis in zebrafish and mice by repressing IDO1-mediated M2 macrophage repolarization. Phytomedicine 2023, 119, 155016. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Gambino, R. Non-alcoholic steatohepatitis: Emerging molecular targets and therapeutic strategies. Nat. Rev. Drug Discov. 2016, 15, 249–274. [Google Scholar] [CrossRef]
- Röhrig, F.; Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 2016, 16, 732–749. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Afrin, S.; Mazzoni, L.; Cordero, M.D.; Mezzetti, B.; Quiles, J.L.; Battino, M. Lipid Accumulation in HepG2 Cells Is Attenuated by Strawberry Extract through AMPK Activation. Nutrients 2017, 9, 621. [Google Scholar] [CrossRef]
- Nyandwi, J.B.; Ko, Y.S.; Jin, H.; Yun, S.P.; Park, S.W.; Kim, H.J. Rosmarinic Acid Exhibits a Lipid-Lowering Effect by Modulating the Expression of Reverse Cholesterol Transporters and Lipid Metabolism in High-Fat Diet-Fed Mice. Biomolecules 2021, 11, 1470. [Google Scholar] [CrossRef]
- Ma, K.; Sheng, W.; Song, X.; Song, J.; Li, Y.; Huang, W.; Liu, Y. Chlorogenic Acid from Burdock Roots Ameliorates Oleic Acid-Induced Steatosis in HepG2 Cells through AMPK/ACC/CPT-1 Pathway. Molecules 2023, 28, 7257. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.Q.; Zeng, Y.; Xu, J.; Xu, X.L. Naringenin alleviates nonalcoholic steatohepatitis in middle-aged Apoe(-/-)mice: Role of SIRT1. Phytomedicine 2021, 81, 153412. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Yang, Q.; Tang, M.; Gao, L.; Wang, Y.; Wang, J.; Liu, Z.; Li, X.; Mao, L.; Jia, R.Z.; et al. TBC1D23 mediates Golgi-specific LKB1 signaling. Nat. Commun. 2024, 15, 1785. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qian, Q.; Ying, N.; Lai, J.; Feng, L.; Zheng, S.; Jiang, F.; Song, Q.; Chai, H.; Dou, X. Activation of the AMPK-SIRT1 pathway contributes to protective effects of Salvianolic acid A against lipotoxicity in hepatocytes and NAFLD in mice. Front. Pharmacol. 2020, 11, 560905. [Google Scholar] [CrossRef]
- Yu, M.H.; Hung, T.W.; Wang, C.C.; Wu, S.W.; Yang, T.W.; Yang, C.Y.; Tseng, T.H.; Wang, C.J. Neochlorogenic Acid Attenuates Hepatic Lipid Accumulation and Inflammation via Regulating miR-34a In Vitro. Int. J. Mol. Sci. 2021, 22, 13163. [Google Scholar] [CrossRef]
- Guo, D.; Bell, E.H.; Mischel, P.; Chakravarti, A. Targeting SREBP-1-driven lipid metabolism to treat cancer. Curr. Pharm. Des. 2014, 20, 2619–2626. [Google Scholar] [CrossRef]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; Enjoji, M.; et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef]
- Jensen-Urstad, A.P.; Semenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta 2012, 1821, 747–753. [Google Scholar] [CrossRef]
- Mohammadi, M.; Abbasalipourkabir, R.; Ziamajidi, N. Fish oil and chicoric acid combination protects better against palmitate-induced lipid accumulation via regulating AMPK-mediated SREBP-1/FAS and PPARα/UCP2 pathways. Arch. Physiol. Biochem. 2023, 129, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, R.; Lang, J.; Fu, Y.; Yang, L.; Zhao, D. Epigallocatechin-3-gallate ameliorates hepatic damages by relieve FGF21 resistance and promotion of FGF21-AMPK pathway in mice fed a high fat diet. Diabetol. Metab. Syndr. 2022, 14, 53. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, G.; Randy, A.; Kim, H.S.; Nho, C.W. Chicoric acid attenuate a nonalcoholic steatohepatitis by inhibiting key regulators of lipid metabolism, fibrosis, oxidation, and inflammation in mice with methionine and choline deficiency. Mol. Nutr. Food Res. 2017, 61, 1600632. [Google Scholar] [CrossRef] [PubMed]
- Zamani-Garmsiri, F.; Ghasempour, G.; Aliabadi, M.; Hashemnia, S.M.R.; Emamgholipour, S.; Meshkani, R. Combination of metformin and chlorogenic acid attenuates hepatic steatosis and inflammation in high-fat diet fed mice. IUBMB Life 2021, 73, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, X.; Yu, Y.; Wu, Y.; Xie, L.; Chang, C. Lonicerae Japonicae Flos extract and chlorogenic acid attenuates high-fat-diet- induced prediabetes via CTRPs-AdipoRs-AMPK/PPARα axes. Front. Nutr. 2022, 9, 1007679. [Google Scholar] [CrossRef]
- Yuan, Z.; Lu, X.; Lei, F.; Sun, H.; Jiang, J.; Xing, D.; Du, L. Novel Effect of p-Coumaric Acid on Hepatic Lipolysis: Inhibition of Hepatic Lipid-Droplets. Molecules 2023, 28, 4641. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, L.; Wu, T.; Fang, X.; Zhao, L. Echinacoside ameliorates alcohol-induced oxidative stress and hepatic steatosis by affecting SREBP1c/FASN pathway via PPARα. Food Chem. Toxicol. 2021, 148, 111956. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, Y.W.; Yang, C.F.; Zhong, Y.J.; He, H.; Zhu, F.C.; Li, L. Methyl ferulic acid attenuates ethanol-induced hepatic steatosis by regulating AMPK and FoxO1 Pathways in Rats and L-02 cells. Chem.-Biol. Interact. 2018, 291, 180–189. [Google Scholar] [CrossRef]
- Ding, H.; Ge, K.; Fan, C.; Liu, D.; Wu, C.; Li, R.; Yan, F.J. Chlorogenic Acid Attenuates Hepatic Steatosis by Suppressing ZFP30. J. Agric. Food Chem. 2024, 72, 245–258. [Google Scholar] [CrossRef]
- Luo, C.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Zhang, C.; Zhu, W.; Jin, Y. Rosmarinic acid exerts an antagonistic effect on nonalcoholic fatty liver disease by regulating the YAP1/TAZ-PPARγ/PGC-1α signaling pathway. Phytother. Res. PTR 2021, 35, 1010–1022. [Google Scholar] [CrossRef]
- Ding, C.; Zhao, Y.; Shi, X.; Zhang, N.; Zu, G.; Li, Z.; Zhou, J.; Gao, D.; Lv, L.; Tian, X.; et al. New insights into salvianolic acid A action: Regulation of the TXNIP/NLRP3 and TXNIP/ChREBP pathways ameliorates HFD-induced NAFLD in rats. Sci. Rep. 2016, 6, 28734. [Google Scholar] [CrossRef]
- Nag, S.; Manna, K.; Saha, M.; Das Saha, K. Tannic acid and vitamin E loaded PLGA nanoparticles ameliorate hepatic injury in a chronic alcoholic liver damage model via EGFR-AKT-STAT3 pathway. Nanomedicine 2020, 15, 235–257. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V. Autophagy in inflammation, infection, and immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 2018, 20, 521–527. [Google Scholar] [CrossRef]
- Itakura, E.; Kishi, C.; Inoue, K.; Mizushima, N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell 2008, 19, 5360–5372. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Cao, Y.; An, X.; Chen, J.; Yang, L. Punicalagin Protects against Diabetic Liver Injury by Upregulating Mitophagy and Antioxidant Enzyme Activities. Nutrients 2022, 14, 2782. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Yao, H.T.; Li, C.C.; Chang, C.H. Epigallocatechin-3-Gallate Reduces Hepatic Oxidative Stress and Lowers CYP-Mediated Bioactivation and Toxicity of Acetaminophen in Rats. Nutrients 2019, 11, 1862. [Google Scholar] [CrossRef]
- Zhang, R.; Chu, K.; Zhao, N.; Wu, J.; Ma, L.; Zhu, C.; Chen, X.; Wei, G.; Liao, M. Corilagin Alleviates Nonalcoholic Fatty Liver Disease in High-Fat Diet-Induced C57BL/6 Mice by Ameliorating Oxidative Stress and Restoring Autophagic Flux. Front. Pharmacol. 2019, 10, 1693. [Google Scholar] [CrossRef]
- Matsuda, N.; Sato, S.; Shiba, K.; Okatsu, K.; Saisho, K.; Gautier, C.A.; Sou, Y.S.; Saiki, S.; Kawajiri, S.; Sato, F.; et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 2010, 189, 211–221. [Google Scholar] [CrossRef]
- Geisler, S.; Holmström, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef]
- Narendra, D.; Kane, L.A.; Hauser, D.N.; Fearnley, I.M.; Youle, R.J. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 2010, 6, 1090–1106. [Google Scholar] [CrossRef]
- Cao, Y.; Ren, G.; Zhang, Y.; Qin, H.; An, X.; Long, Y.; Chen, J.; Yang, L. A new way for punicalagin to alleviate insulin resistance: Regulating gut microbiota and autophagy. Food Nutr. Res. 2021, 65, 5689. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lai, X.; Yuan, D.; Liu, Y.; Wang, J.; Liang, Y. Effects of ferulic acid, a major component of rice bran, on proliferation, apoptosis, and autophagy of HepG2 cells. Food Res. Int. 2022, 161, 111816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, Y.; Chen, J.; Qin, H.; Yang, L. A New Possible Mechanism by Which Punicalagin Protects against Liver Injury Induced by Type 2 Diabetes Mellitus: Upregulation of Autophagy via the Akt/FoxO3a Signaling Pathway. J. Agric. Food Chem. 2019, 67, 13948–13959. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Wang, Y.; Zhang, Q.; Li, J.; Zhong, P.; Xie, Z.; Ji, A.; Li, Y. Epigallocatechin-3-Gallate Alleviates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease via Inhibition of Apoptosis and Promotion of Autophagy through the ROS/MAPK Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 5599997. [Google Scholar] [CrossRef]
- Luo, L.; Lin, J.; Chen, S.; Ni, J.; Peng, H.; Shen, F.; Huang, Z. Rosmarinic acid alleviates toosendanin-induced liver injury through restoration of autophagic flux and lysosomal function by activating JAK2/STAT3/CTSC pathway. J. Ethnopharmacol. 2024, 330, 118196. [Google Scholar] [CrossRef]
- Qin, L.; Tan, J.; Lv, X.; Zhang, J. Vanillic acid alleviates liver fibrosis through inhibiting autophagy in hepatic stellate cells via the MIF/CD74 signaling pathway. Biomed. Pharmacother. 2023, 168, 115673. [Google Scholar] [CrossRef]
- Brown, S.B.; Bailey, K.; Savill, J. Actin is cleaved during constitutive apoptosis. Biochem. J. 1997, 323 Pt 1, 233–237. [Google Scholar] [CrossRef]
- Wang, K.K.; Posmantur, R.; Nath, R.; McGinnis, K.; Whitton, M.; Talanian, R.V.; Glantz, S.B.; Morrow, J.S. Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J. Biol. Chem. 1998, 273, 22490–22497. [Google Scholar] [CrossRef]
- Kothakota, S.; Azuma, T.; Reinhard, C.; Klippel, A.; Tang, J.; Chu, K.; McGarry, T.J.; Kirschner, M.W.; Koths, K.; Kwiatkowski, D.J.; et al. Caspase-3-generated fragment of gelsolin: Effector of morphological change in apoptosis. Science 1997, 278, 294–298. [Google Scholar] [CrossRef]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of caspase activation. Curr. Opin. Cell Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef]
- Marzo, I.; Brenner, C.; Zamzami, N.; Susin, S.A.; Beutner, G.; Brdiczka, D.; Rémy, R.; Xie, Z.H.; Reed, J.C.; Kroemer, G. The permeability transition pore complex: A target for apoptosis regulation by caspases and bcl-2-related proteins. J. Exp. Med. 1998, 187, 1261–1271. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, Y.; Liu, X.; Wang, X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999, 274, 11549–11556. [Google Scholar] [CrossRef] [PubMed]
- Budihardjo, I.; Oliver, H.; Lutter, M.; Luo, X.; Wang, X. Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol. 1999, 15, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, F.; Fan, G.; Liu, J.; Wang, Y.; Xue, X.; Lyu, X.; Lin, S.; Li, X. Ferulic acid ameliorates acetaminophen-induced acute liver injury by promoting AMPK-mediated protective autophagy. IUBMB Life 2022, 74, 880–895. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Hu, Q.; Chen, Y.; Zhang, W.; Qin, X.; Wei, F.; Lu, X.; Ma, X.; Zeng, J.; et al. Paeonia lactiflora Pall. ameliorates acetaminophen-induced oxidative stress and apoptosis via inhibiting the PKC-ERK pathway. J. Ethnopharmacol. 2024, 329, 118107. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; Zhao, T.; Chen, Q.; Yang, Y.; Wang, S.; Zhang, J.; Deng, G.; Sun, K.; Nan, Y.; et al. Epigallocatechin-3-gallate Mo nanoparticles (EGM NPs) efficiently treat liver injury by strongly reducing oxidative stress, inflammation and endoplasmic reticulum stress. Front. Pharmacol. 2022, 13, 1039558. [Google Scholar] [CrossRef]
- Foroutanfar, A.; Mehri, S.; Kamyar, M.; Tandisehpanah, Z.; Hosseinzadeh, H. Protective effect of punicalagin, the main polyphenol compound of pomegranate, against acrylamide-induced neurotoxicity and hepatotoxicity in rats. Phytother. Res. PTR 2020, 34, 3262–3272. [Google Scholar] [CrossRef]
- Li, M.; Liu, P.; Xue, Y.; Liang, Y.; Shi, J.; Han, X.; Zhang, J.; Chu, X.; Chu, L. Tannic acid attenuates hepatic oxidative stress, apoptosis and inflammation by activating the Keap1-Nrf2/ARE signaling pathway in arsenic trioxide-toxicated rats. Oncol. Rep. 2020, 44, 2306–2316. [Google Scholar] [CrossRef]
- Wu, X.; Wang, J.; Li, B.; Gong, M.; Cao, C.; Song, L.; Qin, L.; Wang, Y.; Zhang, Y.; Li, Y. Chlorogenic acid, rutin, and quercetin from Lysimachia christinae alleviate triptolide-induced multi-organ injury in vivo by modulating immunity and AKT/mTOR signal pathway to inhibit ferroptosis and apoptosis. Toxicol. Appl. Pharmacol. 2023, 467, 116479. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, S.; Tang, H.; Zhang, X.; Tao, R.; Yan, Z.; Shi, J.; Guo, W.; Zhang, S. Veratric acid alleviates liver ischemia/reperfusion injury by activating the Nrf2 signaling pathway. Int. Immunopharmacol. 2021, 101, 108294. [Google Scholar] [CrossRef]
- Zhou, H.Q.; Liu, W.; Wang, J.; Huang, Y.Q.; Li, P.Y.; Zhu, Y.; Wang, J.B.; Ma, X.; Li, R.S.; Wei, S.Z.; et al. Paeoniflorin attenuates ANIT-induced cholestasis by inhibiting apoptosis in vivo via mitochondria-dependent pathway. Biomed. Pharmacother. 2017, 89, 696–704. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Khomich, O.; Ivanov, A.V.; Bartosch, B. Metabolic Hallmarks of Hepatic Stellate Cells in Liver Fibrosis. Cells 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Budi, E.H.; Schaub, J.R.; Decaris, M.; Turner, S.; Derynck, R. TGF-β as a driver of fibrosis: Physiological roles and therapeutic opportunities. J. Pathol. 2021, 254, 358–373. [Google Scholar] [CrossRef]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef]
- Dooley, S.; Hamzavi, J.; Breitkopf, K.; Wiercinska, E.; Said, H.M.; Lorenzen, J.; Ten Dijke, P.; Gressner, A.M. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology 2003, 125, 178–191. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Li, W.; Liu, C.; Jiang, Z.; Gu, P.; Li, J.; Wang, W.; You, R.; Ba, Q.; et al. Rebalancing TGF-β/Smad7 signaling via Compound kushen injection in hepatic stellate cells protects against liver fibrosis and hepatocarcinogenesis. Clin. Transl. Med. 2021, 11, e410. [Google Scholar] [CrossRef]
- Liu, J.; Kong, D.; Qiu, J.; Xie, Y.; Lu, Z.; Zhou, C.; Liu, X.; Zhang, R.; Wang, Y. Praziquantel ameliorates CCl4-induced liver fibrosis in mice by inhibiting TGF-β/Smad signalling via up-regulating Smad7 in hepatic stellate cells. Br. J. Pharmacol. 2019, 176, 4666–4680. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Y.; Luo, X.; Shen, Z.; Sun, Z.; Shen, B.; Zhou, C.; Wang, J.; Lu, J.; Zhang, Q.; et al. Hydronidone ameliorates liver fibrosis by inhibiting activation of hepatic stellate cells via Smad7-mediated degradation of TGFβRI. Liver Int. 2023, 43, 2523–2537. [Google Scholar] [CrossRef]
- Mu, M.; Zuo, S.; Wu, R.M.; Deng, K.S.; Lu, S.; Zhu, J.J.; Zou, G.L.; Yang, J.; Cheng, M.L.; Zhao, X.K. Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF-β/Smad signaling pathway. Drug Des. Dev. Ther. 2018, 12, 4107–4115. [Google Scholar] [CrossRef]
- Yang, F.; Luo, L.; Zhu, Z.D.; Zhou, X.; Wang, Y.; Xue, J.; Zhang, J.; Cai, X.; Chen, Z.L.; Ma, Q.; et al. Chlorogenic Acid Inhibits Liver Fibrosis by Blocking the miR-21-Regulated TGF-β1/Smad7 Signaling Pathway in Vitro and in Vivo. Front. Pharmacol. 2017, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, T.; Xu, H.; Wang, T.; Gong, Q.; Li, T.; Liu, X.; Wang, J.; Wang, Y.; Xiong, L. Echinacoside Exerts Antihepatic Fibrosis Effects in High-Fat Mice Model by Modulating the ACVR2A-Smad Pathway. Mol. Nutr. Food Res. 2024, 68, e2300553. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Yang, Z.; Wang, S.; Guo, M.; Li, Q.; Zhang, Q.; Bi, X. The protective role of protocatechuic acid against chemically induced liver fibrosis in vitro and in vivo. Die Pharm. 2021, 76, 232–238. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Wang, L. Thymosinβ4 alleviates cholestatic liver fibrosis in mice through downregulating PDGF/PDGFR and TGFβ/Smad pathways. Dig. Liver Dis. 2020, 52, 324–330. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Li, Y.; Huang, Y.; Zhu, Y.; Lv, W.; Wang, R.; Gou, L.; Cheng, C.; Feng, Z.; et al. Roseotoxin B alleviates cholestatic liver fibrosis through inhibiting PDGF-B/PDGFR-β pathway in hepatic stellate cells. Cell Death Dis. 2020, 11, 458. [Google Scholar] [CrossRef]
- Ren, H.; Li, Y.; Chen, Y.; Wang, L. Endostatin attenuates PDGF-BB- or TGF-β1-induced HSCs activation via suppressing RhoA/ROCK1 signal pathways. Drug Des. Dev. Ther. 2019, 13, 285–290. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Chen, P.; Gu, Y.; Wang, S.; Wang, L.; Chen, C.; Wang, R.; Yuan, Y. Salvianolic acid B inhibits hepatic stellate cell activation and liver fibrosis by targeting PDGFRβ. Int. Immunopharmacol. 2023, 122, 110550. [Google Scholar] [CrossRef]
- Lan, T.; Li, C.; Yang, G.; Sun, Y.; Zhuang, L.; Ou, Y.; Li, H.; Wang, G.; Kisseleva, T.; Brenner, D.; et al. Sphingosine kinase 1 promotes liver fibrosis by preventing miR-19b-3p-mediated inhibition of CCR2. Hepatology 2018, 68, 1070–1086. [Google Scholar] [CrossRef] [PubMed]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.P.; Schwabe, R.F. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver—Linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, K.; Zhang, R.J.; Mei, D.; Zhang, B. Isochlorogenic Acid A Attenuates the Progression of Liver Fibrosis Through Regulating HMGB1/TLR4/NF-κB Signaling Pathway. Front. Pharmacol. 2020, 11, 582. [Google Scholar] [CrossRef]
- Miao, H.; Ouyang, H.; Guo, Q.; Wei, M.; Lu, B.; Kai, G.; Ji, L. Chlorogenic acid alleviated liver fibrosis in methionine and choline deficient diet-induced nonalcoholic steatohepatitis in mice and its mechanism. J. Nutr. Biochem. 2022, 106, 109020. [Google Scholar] [CrossRef]
- Leshem, A.; Liwinski, T.; Elinav, E. Immune-Microbiota Interplay and Colonization Resistance in Infection. Mol. Cell 2020, 78, 597–613. [Google Scholar] [CrossRef]
- Woodhouse, C.A.; Patel, V.C.; Singanayagam, A.; Shawcross, D.L. Review article: The gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment. Pharmacol. Ther. 2018, 47, 192–202. [Google Scholar] [CrossRef]
- Ning, K.; Lu, K.; Chen, Q.; Guo, Z.; Du, X.; Riaz, F.; Feng, L.; Fu, Y.; Yin, C.; Zhang, F.; et al. Epigallocatechin Gallate Protects Mice against Methionine-Choline-Deficient-Diet-Induced Nonalcoholic Steatohepatitis by Improving Gut Microbiota To Attenuate Hepatic Injury and Regulate Metabolism. ACS Omega 2020, 5, 20800–20809. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, L.; Ma, J.; Yang, Y.; Zhang, B.; Xu, J.; Dhondrup, R.; Wong, T.W.; Zhang, D. Preventive mechanisms of Chinese Tibetan medicine Triphala against nonalcoholic fatty liver disease. Phytomedicine 2024, 123, 155229. [Google Scholar] [CrossRef]
- Ding, Y.; Li, X.; Liu, Y.; Wang, S.; Cheng, D. Protection Mechanisms Underlying Oral Administration of Chlorogenic Acid against Cadmium-Induced Hepatorenal Injury Related to Regulating Intestinal Flora Balance. J. Agric. Food Chem. 2021, 69, 1675–1683. [Google Scholar] [CrossRef]
- Mu, H.N.; Zhou, Q.; Yang, R.Y.; Tang, W.Q.; Li, H.X.; Wang, S.M.; Li, J.; Chen, W.X.; Dong, J. Caffeic acid prevents non-alcoholic fatty liver disease induced by a high-fat diet through gut microbiota modulation in mice. Food Res. Int. 2021, 143, 110240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, J.; Chen, L.; He, J.; Qu, D.; Zhang, Z.; Liu, Y.; Li, X.; Liu, J.; Li, J.; et al. Gut Microbiota Participates in Polystyrene Microplastics-Induced Hepatic Injuries by Modulating the Gut-Liver Axis. ACS Nano 2023, 17, 15125–15145. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A. Anti-hypertensive Effect of Cereal Antioxidant Ferulic Acid and Its Mechanism of Action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Iwasa, K.; Nagai, T.; Kobayashi, S.; Nakamura, H.; Yamada, M. Pharmacokinetics, Safety and Tolerability of Melissa officinalis Extract which Contained Rosmarinic Acid in Healthy Individuals: A Randomized Controlled Trial. PLoS ONE 2015, 10, e0126422. [Google Scholar] [CrossRef]
- Lee, D.S.; Woo, J.Y.; Ahn, C.B.; Je, J.Y. Chitosan-hydroxycinnamic acid conjugates: Preparation, antioxidant and antimicrobial activity. Food Chem. 2014, 148, 97–104. [Google Scholar] [CrossRef]
- Park, S.Y.; Ahn, G.; Um, J.H.; Han, E.J.; Ahn, C.B.; Yoon, N.Y.; Je, J.Y. Hepatoprotective effect of chitosan-caffeic acid conjugate against ethanol-treated mice. Exp. Toxicol. Pathol. 2017, 69, 618–624. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, L.; Xu, Z.; Sun, T.; Gong, M.; Liu, Y.; Zhang, D. Self-Propelled Ultrasmall AuNPs-Tannic Acid Hybrid Nanozyme with ROS-Scavenging and Anti-Inflammatory Activity for Drug-Induced Liver Injury Alleviation. Small 2023, 19, e2206408. [Google Scholar] [CrossRef]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Samadi, M.; Qorbani, M.; Merat, S.; Adibi, H.; Poustchi, H.; Hekmatdoost, A. Effects of supplementation with main coffee components including caffeine and/or chlorogenic acid on hepatic, metabolic, and inflammatory indices in patients with non-alcoholic fatty liver disease and type 2 diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Nutr. J. 2021, 20, 35. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, Z.; Brown, E.A.; Ghazaryan, A.; Welm, A.L. PDX models for functional precision oncology and discovery science. Nat. Rev. Cancer 2025, 25, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Micek, A.; Kozela, M.; Stefler, D.; Bobak, M.; Pajak, A. Dietary polyphenol intake and risk of type 2 diabetes in the Polish arm of the Health, Alcohol and Psychosocial factors in Eastern Europe (HAPIEE) study. Br. J. Nutr. 2017, 118, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Stepaniak, U.; Micek, A.; Kozela, M.; Stefler, D.; Bobak, M.; Pajak, A. Dietary polyphenol intake and risk of hypertension in the Polish arm of the HAPIEE study. Eur. J. Nutr. 2018, 57, 1535–1544. [Google Scholar] [CrossRef]
- Grosso, G.; Stepaniak, U.; Micek, A.; Stefler, D.; Bobak, M.; Pająk, A. Dietary polyphenols are inversely associated with metabolic syndrome in Polish adults of the HAPIEE study. Eur. J. Nutr. 2017, 56, 1409–1420. [Google Scholar] [CrossRef]
- Rahimlou, M.; Baghdadi, G.; Khodi, A.; Rahimi, Z.; Saki, N.; Banaei Jahromi, N.; Cheraghian, B.; Tavasolian, R.; Hosseini, S.A. Polyphenol consumption and Nonalcoholic fatty liver disease risk in adults. Sci. Rep. 2024, 14, 6752. [Google Scholar] [CrossRef]
- Castellino, G.; Nikolic, D.; Magán-Fernández, A.; Malfa, G.A.; Chianetta, R.; Patti, A.M.; Amato, A.; Montalto, G.; Toth, P.P.; Banach, M.; et al. Altilix® Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2580. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Yu, C.M.; Wang, Y.; Ren, S.C.; Liu, Z.L.; Zhu, C.L.; Liu, Q.; Li, H.R.; Sun, C.Y.; Sun, X.Y.; Xie, J.; et al. Caffeic acid modulates activation of neutrophils and attenuates sepsis-induced organ injury by inhibiting 5-LOX/LTB4 pathway. Int. Immunopharmacol. 2023, 125, 111143. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Ajala-Lawal, R.A.; Adeyiga, A.B. Caffeic acid abrogates 1,3-dichloro-2-propanol-induced hepatotoxicity by upregulating nuclear erythroid-related factor 2 and downregulating nuclear factor-kappa B. Hum. Exp. Toxicol. 2019, 38, 1092–1101. [Google Scholar] [CrossRef]
- Esmaeilzadeh, M.; Heidarian, E.; Shaghaghi, M.; Roshanmehr, H.; Najafi, M.; Moradi, A.; Nouri, A. Gallic acid mitigates diclofenac-induced liver toxicity by modulating oxidative stress and suppressing IL-1β gene expression in male rats. Pharm. Biol. 2020, 58, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhao, D.; Pan, Y.; Chen, B.; Cao, Y.; Han, S.; Lian, F.; Zhang, Y.; Yan, X. Gallic Acid Attenuates Sepsis-Induced Liver Injury through C/EBPβ-Dependent MAPK Signaling Pathway. Mol. Nutr. Food Res. 2024, 68, e2400123. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.K.; Hafez, D.M. Gallic acid and metformin co-administration reduce oxidative stress, apoptosis and inflammation via Fas/caspase-3 and NF-κB signaling pathways in thioacetamide-induced acute hepatic encephalopathy in rats. BMC Complement. Med. Ther. 2023, 23, 265. [Google Scholar] [CrossRef] [PubMed]
- Adeyanju, A.A.; Asejeje, F.O.; Molehin, O.R.; Owoeye, O.; Olatoye, E.O.; Ekpo, E.N. Protective role of protocatechuic acid in carbon tetrachloride-induced oxidative stress via modulation of proinflammatory cytokines levels in brain and liver of Wistar rats. J. Basic Clin. Physiol. Pharmacol. 2021, 33, 143–154. [Google Scholar] [CrossRef]
- Abdelrahman, R.S.; El-Tanbouly, G.S. Protocatechuic acid protects against thioacetamide-induced chronic liver injury and encephalopathy in mice via modulating mTOR, p53 and the IL-6/ IL-17/ IL-23 immunoinflammatory pathway. Toxicol. Appl. Pharmacol. 2022, 440, 115931. [Google Scholar] [CrossRef]
- Habib, S.A.; Suddek, G.M.; Abdel Rahim, M.; Abdelrahman, R.S. The protective effect of protocatechuic acid on hepatotoxicity induced by cisplatin in mice. Life Sci. 2021, 277, 119485. [Google Scholar] [CrossRef]
- Tan, J.; Hu, R.; Gong, J.; Fang, C.; Li, Y.; Liu, M.; He, Z.; Hou, D.X.; Zhang, H.; He, J.; et al. Protection against Metabolic Associated Fatty Liver Disease by Protocatechuic Acid. Gut Microbes 2023, 15, 2238959. [Google Scholar] [CrossRef]
- Li, N.; Du, X.; Qu, T.; Ren, H.; Lu, W.; Cui, X.; Hu, J.; Chen, Z.; Tao, H. Pharmacodynamic material basis and pharmacological mechanisms of Cortex Mori against diabetes mellitus. J. Ethnopharmacol. 2024, 324, 117781. [Google Scholar] [CrossRef]
- Alamri, E.S.; El Rabey, H.A.; Alzahrani, O.R.; Almutairi, F.M.; Attia, E.S.; Bayomy, H.M.; Albalwi, R.A.; Rezk, S.M. Enhancement of the Protective Activity of Vanillic Acid against Tetrachloro-Carbon (CCl4) Hepatotoxicity in Male Rats by the Synthesis of Silver Nanoparticles (AgNPs). Molecules 2022, 27, 8308. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Chariyakornkul, A.; Jarukamjorn, K.; Wongpoomchai, R. Protective Role of Vanillic Acid against Diethylnitrosamine- and 1,2-Dimethylhydrazine-Induced Hepatocarcinogenesis in Rats. Molecules 2021, 26, 2718. [Google Scholar] [CrossRef]
- Gheena, S.; Ezhilarasan, D.; Shree Harini, K.; Rajeshkumar, S. Syringic acid and silymarin concurrent administration inhibits sodium valproate-induced liver injury in rats. Environ. Toxicol. 2022, 37, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Wang, F.; Wong, N.K.; Lv, Y.; Li, X.; Li, M.; Tipoe, G.L.; So, K.F.; Xu, A.; Chen, S.; et al. Divergent Roles of Kupffer Cell TLR2/3 Signaling in Alcoholic Liver Disease and the Protective Role of EGCG. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Mostafa-Hedeab, G.; Ewaiss Hassan, M.; Halawa, T.F.; Ahmed Wani, F. Epigallocatechin gallate ameliorates tetrahydrochloride-induced liver toxicity in rats via inhibition of TGFβ / p-ERK/p-Smad1/2 signaling, antioxidant, anti-inflammatory activity. Saudi Pharm. J. SPJ 2022, 30, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Wu, Q.; Li, M.; Chen, D.; Su, J.; Zuo, L.; Zhu, B.; Li, Y. Epigallocatechin-3-gallate Induced HepG2 Cells Apoptosis through ROSmediated AKT /JNK and p53 Signaling Pathway. Curr. Cancer Drug Targets 2023, 23, 447–460. [Google Scholar] [CrossRef]
- Fatima, S.; Suhail, N.; Alrashed, M.; Wasi, S.; Aljaser, F.S.; AlSubki, R.A.; Alsharidah, A.S.; Banu, N. Epigallocatechin gallate and coenzyme Q10 attenuate cisplatin-induced hepatotoxicity in rats via targeting mitochondrial stress and apoptosis. J. Biochem. Mol. Toxicol. 2021, 35, e22701. [Google Scholar] [CrossRef]
- Dey, P.; Olmstead, B.D.; Sasaki, G.Y.; Vodovotz, Y.; Yu, Z.; Bruno, R.S. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. J. Nutr. Biochem. 2020, 84, 108455. [Google Scholar] [CrossRef]
- Yang, C.; Wu, A.; Tan, L.; Tang, D.; Chen, W.; Lai, X.; Gu, K.; Chen, J.; Chen, D.; Tang, Q. Epigallocatechin-3-Gallate Alleviates Liver Oxidative Damage Caused by Iron Overload in Mice through Inhibiting Ferroptosis. Nutrients 2023, 15, 1993. [Google Scholar] [CrossRef]
- Yuan, W.; Li, S.; Yang, Y.N.; Gao, H.; Liu, C. Epigallocatechin-3-gallate ameliorates inflammatory injury caused by sepsis by regulating the lncRNA PVT1/miR-16-5p/TLR4 axis. Cytokine 2023, 162, 155994. [Google Scholar] [CrossRef]
- Huang, J.; Li, W.; Liao, W.; Hao, Q.; Tang, D.; Wang, D.; Wang, Y.; Ge, G. Green tea polyphenol epigallocatechin-3-gallate alleviates nonalcoholic fatty liver disease and ameliorates intestinal immunity in mice fed a high-fat diet. Food Funct. 2020, 11, 9924–9935. [Google Scholar] [CrossRef]
- Kang, Q.; Tong, Y.; Gowd, V.; Wang, M.; Chen, F.; Cheng, K.W. Oral administration of EGCG solution equivalent to daily achievable dosages of regular tea drinkers effectively suppresses miR483-3p induced metastasis of hepatocellular carcinoma cells in mice. Food Funct. 2021, 12, 3381–3392. [Google Scholar] [CrossRef]
- Sojoodi, M.; Wei, L.; Erstad, D.J.; Yamada, S.; Fujii, T.; Hirschfield, H.; Kim, R.S.; Lauwers, G.Y.; Lanuti, M.; Hoshida, Y.; et al. Epigallocatechin Gallate Induces Hepatic Stellate Cell Senescence and Attenuates Development of Hepatocellular Carcinoma. Cancer Prev. Res. 2020, 13, 497–508. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, J.; Cai, Z.; An, H.; Li, Y.; Peng, Y.; Chen, N.; Luo, A.; Tao, H.; Li, K. Epigallocatechin gallate induces chemopreventive effects on rats with diethylnitrosamine-induced liver cancer via inhibition of cell division cycle 25A. Mol. Med. Rep. 2020, 22, 3873–3885. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chang, Y.J.; Wei, P.L.; Hung, C.S.; Wang, W. Methyl gallate, gallic acid-derived compound, inhibit cell proliferation through increasing ROS production and apoptosis in hepatocellular carcinoma cells. PLoS ONE 2021, 16, e0248521. [Google Scholar] [CrossRef]
- Liao, M.; Zhang, R.; Wang, Y.; Mao, Z.; Wu, J.; Guo, H.; Zhang, K.; Jing, Y.; Zhang, C.; Song, H.; et al. Corilagin prevents non-alcoholic fatty liver disease via improving lipid metabolism and glucose homeostasis in high fat diet-fed mice. Front. Nutr. 2022, 9, 983450. [Google Scholar] [CrossRef]
- Yan, F.; Cheng, D.; Wang, H.; Gao, M.; Zhang, J.; Cheng, H.; Wang, C.; Zhang, H.; Xiong, H. Corilagin Ameliorates Con A-Induced Hepatic Injury by Restricting M1 Macrophage Polarization. Front. Immunol. 2021, 12, 807509. [Google Scholar] [CrossRef]
- Bogahawaththa, S.; Kawamura, T.; Bandaranayake, U.; Hirakawa, T.; Yamada, G.; Ishino, H.; Hirohashi, T.; Kawaguchi, S.I.; Wijesundera, K.K.; Wijayagunawardane, M.P.B.; et al. Identification and mechanistic investigation of ellagitannins from Osbeckia octandra that attenuate liver fibrosis via the TGF-β/SMAD signaling pathway. Biosci. Biotechnol. Biochem. 2023, 87, 1295–1309. [Google Scholar] [CrossRef]
- Feng, X.H.; Xu, H.Y.; Wang, J.Y.; Duan, S.; Wang, Y.C.; Ma, C.M. In vivo hepatoprotective activity and the underlying mechanism of chebulinic acid from Terminalia chebula fruit. Phytomedicine 2021, 83, 153479. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Zhang, B.; Li, Q.; Tu, J.; Zhou, B. Effect of punicalagin on multiple targets in streptozotocin/high-fat diet-induced diabetic mice. Food Funct. 2020, 11, 10617–10634. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Terrón, G.; Martínez, R.; Morcuende, D.; Caballero, V.; Estévez, M. Pomegranate supplementation alleviates dyslipidemia and the onset of non-alcoholic fatty liver disease in Wistar rats by shifting microbiota and producing urolithin-like microbial metabolites. Food Funct. 2024, 15, 7348–7363. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Long, Y.; Zhang, R.; Zhang, Y.; You, Z.; Yang, L. Punicalagin Ameliorates Diabetic Liver Injury by Inhibiting Pyroptosis and Promoting Autophagy via Modulation of the FoxO1/TXNIP Signaling Pathway. Mol. Nutr. Food Res. 2024, 68, e2300912. [Google Scholar] [CrossRef]
- Du, P.; Zhang, X.; Luo, K.; Li, Y.; Fu, C.; Xiao, J.; Xiao, Q. Curculigoside mitigates hepatic ischemia/reperfusion-induced oxidative stress, inflammation, and apoptosis via activation of the Nrf-2/HO-1 pathway. Hum. Exp. Toxicol. 2022, 41, 9603271221087146. [Google Scholar] [CrossRef] [PubMed]
- Gawish, R.A.; Samy, E.M.; Aziz, M.M. Ferulic acid protects against gamma-radiation induced liver injury via regulating JAK/STAT/Nrf2 pathways. Arch. Biochem. Biophys. 2024, 753, 109895. [Google Scholar] [CrossRef] [PubMed]
- Daryagasht, M.; Moosavi, M.; Khorsandi, L.; Azadnasab, R.; Khodayar, M.J. Hepatoprotective and anti-hyperglycemic effects of ferulic acid in arsenic-exposed mice. Food Chem. Toxicol. 2023, 178, 113924. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jiang, L.; Jiang, D.; Liu, B.; Jin, S. The protective effects of salvianolic acid A against hepatic ischemia-reperfusion injury via inhibiting expression of toll-like receptor 4 in rats. Arch. Med. Sci. AMS 2019, 15, 1599–1607. [Google Scholar] [CrossRef]
- Bal, S.S.; Leishangthem, G.D.; Sethi, R.S.; Singh, A. P-coumaric acid ameliorates fipronil induced liver injury in mice through attenuation of structural changes, oxidative stress and inflammation. Pestic. Biochem. Physiol. 2022, 180, 104997. [Google Scholar] [CrossRef]
- Cui, K.; Zhang, L.; La, X.; Wu, H.; Yang, R.; Li, H.; Li, Z. Ferulic Acid and P-Coumaric Acid Synergistically Attenuate Non-Alcoholic Fatty Liver Disease through HDAC1/PPARG-Mediated Free Fatty Acid Uptake. Int. J. Mol. Sci. 2022, 23, 15297. [Google Scholar] [CrossRef]
- Mehdi, S.; Ahmad, F.U.; Lodhi, A.H.; Khurshid, U.; Khalid, A.A.; Sidiq, S.S.; Hussain, L.; Baig, M.S. Protective Effects of p-CA Against Acute Liver Damage Induced by LPS/D-GalN in Wistar Albino Rats. Drug Des. Dev. Ther. 2022, 16, 3327–3342. [Google Scholar] [CrossRef]
- Jia, K.; Zhang, Y.; Luo, R.; Liu, R.; Li, Y.; Wu, J.; Xie, K.; Liu, J.; Li, S.; Zhou, F.; et al. Acteoside ameliorates hepatic ischemia-reperfusion injury via reversing the senescent fate of liver sinusoidal endothelial cells and restoring compromised sinusoidal networks. Int. J. Biol. Sci. 2023, 19, 4967–4988. [Google Scholar] [CrossRef]
- Yao, Y.; Li, R.; Liu, D.; Long, L.; He, N. Rosmarinic acid alleviates acetaminophen-induced hepatotoxicity by targeting Nrf2 and NEK7-NLRP3 signaling pathway. Ecotoxicol. Environ. Saf. 2022, 241, 113773. [Google Scholar] [CrossRef]
- Guo, C.; Shangguan, Y.; Zhang, M.; Ruan, Y.; Xue, G.; Ma, J.; Yang, J.; Qiu, L. Rosmarinic acid alleviates ethanol-induced lipid accumulation by repressing fatty acid biosynthesis. Food Funct. 2020, 11, 2094–2106. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Z.; Yue, Z.; Ding, L.; Zhou, Y.; Huang, Z.; Huang, H. Rosmarinic Acid Ameliorates H2O2-Induced Oxidative Stress in L02 Cells Through MAPK and Nrf2 Pathways. Rejuvenation Res. 2019, 22, 289–298. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Wang, C.; Shi, X.; Li, K. Rosmarinic acid inhibits proliferation and invasion of hepatocellular carcinoma cells SMMC 7721 via PI3K/AKT/mTOR signal pathway. Biomed. Pharmacother. 2019, 120, 109443. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Y.; Yan, H.Z.; Xia, Z.R.; Wen, W.; Liu, D.Y.; Wan, L.H. Rosmarinic acid ameliorates acetaminophen-induced acute liver injury in mice via RACK1/TNF-α mediated antioxidant effect. Pharm. Biol. 2021, 59, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Shang, J.; Zeng, H.; Wang, X.; Fang, M.; Xu, L.; Liu, X.; Wu, K.; Gong, Z.; Yang, Q. Hepatoprotective Effects of Rosmarinic Acid on Ovalbumin-Induced Intestinal Food Allergy Mouse Model. Molecules 2023, 28, 788. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yoo, G.; Randy, A.; Son, Y.J.; Hong, C.R.; Kim, S.M.; Nho, C.W. Lemon Balm and Its Constituent, Rosmarinic Acid, Alleviate Liver Damage in an Animal Model of Nonalcoholic Steatohepatitis. Nutrients 2020, 12, 1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Gong, Y.; Pei, Z.; Jiang, L.; Xia, L.; Wu, Y. Paeoniflorin ameliorates diabetic liver injury by targeting the TXNIP-mediated NLRP3 inflammasome in db/db mice. Int. Immunopharmacol. 2022, 109, 108792. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, N.; Kong, L.; Chu, S.; Zhang, T.; Yan, G.; Ma, D.; Dai, J.; Ma, Z. Paeoniflorin alleviates liver injury in hypercholesterolemic rats through the ROCK/AMPK pathway. Front. Pharmacol. 2022, 13, 968717. [Google Scholar] [CrossRef]
- Deng, X.; Li, Y.; Li, X.; Zhang, Z.; Dai, S.; Wu, H.; Zhang, F.; Hu, Q.; Chen, Y.; Zeng, J.; et al. Paeoniflorin Protects against Acetaminophen-Induced Liver Injury in Mice via JNK Signaling Pathway. Molecules 2022, 27, 8534. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, X.; Kuang, G.; Jiang, R.; Guo, X.; Wu, S.; Wan, J.; Yin, L. Paeoniflorin modulates oxidative stress, inflammation and hepatic stellate cells activation to alleviate CCl4-induced hepatic fibrosis by upregulation of heme oxygenase-1 in mice. J. Pharm. Pharmacol. 2021, 73, 338–346. [Google Scholar] [CrossRef]
- Lan, T.; Li, P.; Zhang, S.J.; Liu, S.Y.; Zeng, X.X.; Chai, F.; Tong, Y.H.; Mao, Z.J.; Wang, S.W. Paeoniflorin promotes PPARγ expression to suppress HSCs activation by inhibiting EZH2-mediated histone H3K27 trimethylation. Phytomedicine 2024, 128, 155477. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, D.; Jiang, C.; Jin, Q.; Zhang, J. Paeoniflorin inhibits hepatocellular carcinoma growth by reducing PD-L1 expression. Biomed. Pharmacother. 2023, 166, 115317. [Google Scholar] [CrossRef]
- Pei, X.; Tang, S.; Jiang, H.; Zhang, W.; Xu, G.; Zuo, Z.; Ren, Z.; Chen, C.; Shen, Y.; Li, C.; et al. Paeoniflorin recued hepatotoxicity under zinc oxide nanoparticles exposure via regulation on gut-liver axis and reversal of pyroptosis. Sci. Total Environ. 2023, 904, 166885. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Zhao, S.; Zhao, Y.; Chen, Y.; Zhang, J.; Wang, C.; Sun, N.; Fan, H. Paeoniflorin ameliorates lipopolysaccharide-induced acute liver injury by inhibiting oxidative stress and inflammation via SIRT1/FOXO1a/SOD2 signaling in rats. Phytother. Res. PTR 2022, 36, 2558–2571. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Zhang, Y.; Zhang, J.; Li, X.; Yan, Q.; Han, J.; Hu, F. Echinacoside exerts anti-tumor activity via the miR-503-3p/TGF-β1/Smad aixs in liver cancer. Cancer Cell Int. 2021, 21, 304. [Google Scholar] [CrossRef]






| No | Component | Dose | Model | Mechanism | Pharmacological Activity | Reference |
|---|---|---|---|---|---|---|
| 1 | P-coumaric acid | 10–100 µM | ALD | ↑Nrf2, ↓MAPKs | Inhibit cell apoptosis, anti-inflammatory, antioxidant | [28] |
| 2 | Chicoric acid | 15, 30 mg/kg | HFD | ↑AMPK, ↑Nrf2, ↓NFκB | Anti-inflammatory, antioxidant, | [29] |
| 3 | chicoric acid | 50 mg/kg | ALF | ↑AMPK, ↑Nrf2, ↓NF-κB | Anti-inflammatory, antioxidant | [32] |
| 4 | Protocatechuic acid | 10, 20 mg/kg 10 μM | ALD | ↑miR-219a-5p, ↓p66shc, ↓ROS | Antioxidant | [35] |
| 5 | Salvianolic acid A | 15, 30 mg/kg | APAP | ↓miR-485-3p, ↑SIRT1 | Anti-inflammatory, antioxidant | [36] |
| 6 | Caffeic acid | 10, 30 mg/kg | APAP | ↓Keap1, ↑Nrf2, ↑HO-1, ↑NQO1 | Anti-inflammatory, antioxidant | [37] |
| 7 | Gallic acid | 50, 100, 150 mg/kg | Isoniazid and rifampicin | ↑Nrf2, ↓NF-κB | Antioxidant | [38] |
| 8 | Pomegranate extract | 20, 40, 80 mg/kg | CCl4 | ↓AKT/FoxO3a, ↑Nrf2, ↑Beclin-1, ↓LC3-I/II | Promote autophagy, antioxidant | [39] |
| 9 | Pomegranate extract | 25, 50 mg/kg | MTX | ↑BCL-2, ↑Nrf2 | Anti-inflammatory, antioxidant | [40] |
| 10 | Epigallocatechin gallate 3-gallate | 20, 40, 80 mg/kg | Acute pneumonia | ↓TLR4-myD88-NF-κB, ↑PXR, ↑AhR, ↑CAR | Anti-inflammatory, antioxidant | [43] |
| 11 | Plantago asiatica saponins | 50, 100 mg/kg | CLP | ↓TRAF6, ↓NF-κB | Inhibit cell apoptosis, Anti-inflammatory | [44] |
| 12 | Protocatechuic acid | 15, 30 mg/kg | IR/D | ↓NF-κB, ↓IL-1β, ↑Wnt1, ↑β-catenin, ↑GLUT4 | Antioxidant, anti-inflammatory | [46] |
| 13 | Epigallocatechin gallate 3-gallate | 0.32% EGCG (w/v) | Perfluorodecanoic acid | ↓NLRP3 | Anti-inflammatory | [52] |
| 14 | P-coumaric acid | 200 mg/kg | NAFLD | ↓NF-κB, ↑BAT, ↑sWAT | Inhibit cell apoptosis, anti-inflammatory, promote thermogenesis, | [53] |
| 15 | Corilagin | 20 mg/kg 2.5, 5, 10, 20, 40 mM | NAFLD | ↑LC3A/B II, ↓p62 | Promote autophagy, antioxidant | [55] |
| 16 | Rosmarinic acid | 50, 100 mg/kg | HFD | ↑p-AMPK, ↑CPT1A ↑ABCG5, ↑ABCG8, ↑CYP7A1, | Inhibit lipid accumulation | [60] |
| 17 | Salvianolic acid A | 20, 40 mg/kg | NAFLD | ↑SIRT1, ↑AMPK, ↑LC3-II | Inhibit cell apoptosis, promote autophagy | [64] |
| 18 | Chicoric acid | 100, 200 mM | Palmitate | ↑PPARa/UCP2, ↓SREBP-1/FAS | Regulating lipid metabolism | [69] |
| 19 | Epigallocatechin gallate 3-gallate | 100 mg/kg | NAFLD | ↓FGF21, ↑FGFR/AMPK, ↑Nrf2 | Antioxidant | [70] |
| 20 | P-coumaric acid | 100 mg/kg 10 µg/mL | Hyperlipidemia | ↑PPARα/γ, ↑AMPK, ↑MSS4 | Regulating fat metabolism | [75] |
| 21 | Purple cone chrysanthemum glycoside | 100 mg/kg | ↑PPAR-α, ↓SREBP-1c/ FASN | Antioxidant, inhibits hepatic steatosis | [76] | |
| 22 | Rosmarinic acid | 100, 200 mg/kg 20, 40, 80 μmol/L | NAFLD | ↓YAP1, ↓TAZ, ↑PPARγ, ↑PGC-1α | Antioxidant | [79] |
| 23 | Pomegranate extract | 20 mg/kg | Diabetes | ↑Bnip3, ↑Pink1, ↑Parkin | Promote mitochondrial autophagy, antioxidant, | [85] |
| 24 | Epigallocatechin gallate 3-gallate | 0.6% EGCG 511 mg/kg | APAP | ↑LC3B II/I, ↓BAX/BCL-2 | Promote cell autophagy, inhibit cell apoptosis | [87] |
| 25 | Pomegranate extract | 20 mg/kg | HFD | ↓IKKβ/NF-κB | Regulating gut microbiota homeostasis, promoting liver autophagy, anti-inflammatory | [92] |
| 26 | Ferulic acid | 0, 40, 60, 80, 100, 120, 140, 160 μg/mL | ↓Caspase-3, ↑Beclin-1, ↓LC3-I/II | Promote cell apoptosis, autophagy | [93] | |
| 27 | Pomegranate extract | 20 mg/b.w | HFD | AKT/FoxO3a | [94] | |
| 28 | Epigallocatechin gallate 3-gallate | 50 mg/kg 50 μM | NAFLD | ↓ROS, ↓p-JNK, ↓p-p38, ↓BAX/BCL-2, ↓Bad/Bcl-xl, ↑Beclin-1, ↑LC3 | Inhibit cell apoptosis, promote autophagy | [95] |
| 29 | Rosmarinic acid | 40, 80 mg/kg 5, 10, 20 μM | Toosendanin | ↑JAK2/STAT3/CTSC | Promote autophagy | [96] |
| 30 | Vanillic acid | 5, 20 mg/kg 5, 20 μM | Liver fibrosis | ↓MIF, ↓CD74, ↓LC3B, ↓α-SMA | Inhibition of autophagy | [97] |
| 31 | Ferulic acid | 25, 50, 100 mg/kg | APAP | ↑HNF4a, ↑Foxa2, ↑ALB, ↑BAX/BCL2, ↑Caspase3, ↑p-AMPK | Antioxidant, anti-apoptotic, promoting autophagy | [106] |
| 32 | Epigallocatechin gallate 3-gallate | 0.5, 1, 2 mg/kg 1, 2, 5, 10 μg/mL | APAP | ↓NOS2, ↓MPO, ↓JNK, ↓BAX, ↑BCL-2 | Anti-inflammatory, antioxidant, inhibition of cell apoptosis | [108] |
| 33 | Pomegranate extract | 10, 20, 40 mg/kg | ACR | ↑GSH, ↓MDA, ↓BAX/BCI-2, ↓Caspase3 | Antioxidant, anti-apoptotic | [109] |
| 34 | Tannins | 20, 40 mg/kg | ATO | ↑Keap1-Nrf2/ARE, ↑BCL-2, ↓BAX, ↓Caspase-3 | Anti-inflammatory, antioxidant, inhibition of cell apoptosis | [110] |
| 35 | Resveratrol acid | 40, 80 mg/kg | I/R | ↑Nrf2, ↑HO-1, ↑NQO-1 | Antioxidant, anti-apoptotic | [112] |
| 36 | Chlorogenic acid | 15, 30, 60 mg/kg 20, 40, 80 mg/mL | Liver fibrosis | ↓TGF-β1/Smad7 | Anti-inflammatory, | [124] |
| 37 | Purple cone chrysanthemum glycoside | 10, 20 mg /kg | Liver fibrosis | ↓TGF- β1/Smad | Anti-inflammatory, anti-liver fibrosis | [125] |
| 38 | Protocatechuic acid | 75, 150 mg/kg 1 mM, 3 mM | TAA | TGF-β, ↓p-Smad2, ↓p-ERK, ↓c-Jun | Inhibiting cell apoptosis | [126] |
| 39 | Salvianolic acid B | 25, 50 mg/kg | CCl4 | ↓PDGFRβ | Anti-inflammatory | [130] |
| 40 | Epigallocatechin gallate 3-gallate | 50 mg/kg | MCD | ↓ALT, ↓AST | Improve intestinal microbiota imbalance | [138] |
| 41 | Caffeic acid | 10, 30, 50 mg /kg | CLP | ↓NET, ↓5- LOX/LTB4 | Anti-inflammatory, antioxidant, inhibition of cell apoptosis | [159] |
| 42 | Caffeic acid | 10, 20 mg/kg | 1,3-dichloro-2-propanol | ↑Nrf2, ↓NF-kB | Anti-inflammatory, antioxidant | [160] |
| 43 | Gallic acid | 50, 100 mg/kg | Diclofenac | ↓IL-1β | Antioxidant | [161] |
| 44 | Gallic acid | 5, 20 mg /kg | CLP | C/EBP β-Dependent MAPK | Anti-inflammatory | [162] |
| 45 | Gallic acid | 100 mg/kg | TAA | ↓Fas, ↓Caspase-3, ↓NF-κB | Antioxidant, anti-inflammatory, inhibition of cell apoptosis | [163] |
| 46 | Protocatechuic acid | 10, 20 mg/kg | CCl4 | ↓NF-κB, ↓COX-2 | Anti-inflammatory, antioxidant | [164] |
| 47 | Protocatechuic acid | 100, 150 mg/kg | TAA | ↓MDA, ↓NOx, ↑GSH, ↑SOD, ↓TNF-α, ↓IL-6, ↓IL-17, ↓IL-23, ↓Caspase3, ↓p53, ↓mTOR | Anti-inflammatory, antioxidant, inhibition of cell apoptosis, inhibition of autophagy | [165] |
| 48 | Protocatechuic acid | 100, 150 mg/kg | Cisplatin | ↓Caspase3, ↓annexin-V, ↓BAX, ↓iNOS, ↓IL-6, ↓TNF-α | Anti-inflammatory, antioxidant, inhibition of cell apoptosis | [166] |
| 49 | Protocatechuic acid | 0.025%, 0.1% (w/w) PCA high-fat feed | HFD | ↑Fgf1, ↑Igfbp2, ↑Irs1, ↑Irs | Improve insulin resistance, anti-inflammatory | [167] |
| 50 | Mulberry white skin (original catechins) | 165 mg/kg 80 μM | Diabetes | ↑PPARγ, ↑PI3K/Akt, ↓GSK3β, ↓FoxO1 | Improve insulin resistance, | [168] |
| 51 | Vanillic acid | 100 mg/kg | CCl4 | ↓ALT, ↓AST, ↑GSH, ↑SOD | Antioxidant | [169] |
| 52 | Vanillic acid | 0.75, 75 mg/ kg | Liver cancer | ↑GSTA-5, ↑Nrf2, Cyclin D1, ↑Caspase-3, ↓BAX, ↑BCL-2 | Anti-proliferation, anti-apoptosis | [170] |
| 53 | Syringic acid | 40, 80 mg/kg | SV | ↓TNF-α, ↓C-JUN, ↓NF-κB, ↓COX-2, ↓IL-6 | Anti-inflammatory, antioxidant | [171] |
| 54 | Epigallocatechin gallate 3-gallate | 50 mg/kg | NIAAA | ↓TLR2, ↑TLR 3, ↑IL-10, ↓NF-κB, | Anti-inflammatory, inhibits cell apoptosis | [172] |
| 55 | Epigallocatechin gallate 3-gallate | 300 mg/kg | CCl4 | ↓TNF-α, ↓NF-κB, ↓IL-1β, ↓TGFβ, ↓p-ERK, ↓p-Smad1/2 | Antioxidant, anti-inflammatory, anti-liver fibrosis | [173] |
| 56 | Epigallocatechin gallate 3-gallate | 10, 20, 40, 80, 160 μg/mL | ↑AKT, ↑JNK, ↑p53, ↑BAX, ↓BCL-2 | Promote cell apoptosis | [174] | |
| 57 | Epigallocatechin gallate 3-gallate | 15 mg/kg | Cisplatin | ↓p53, ↓BCL-2/BAX, ↓Caspase3 | Promote cell apoptosis | [175] |
| 58 | Epigallocatechin gallate 3-gallate | 0.3% (3 g/100 g) | NAFLD | ↓LPS-TLR4-NFκB | Anti-inflammatory, improves microbiota imbalance, alleviates insulin resistance | [176] |
| 59 | Epigallocatechin gallate 3-gallate | 50 mg/kg EGCG | FeSO4 | ↑Nrf2, ↑GPX4, ↑FTH/L | Antioxidant, regulating iron metabolism | [177] |
| 60 | Epigallocatechin gallate 3-gallate | ↓PVT1, ↓TLR4, ↑miR-16-5p | Anti-inflammatory | [178] | ||
| 61 | Epigallocatechin gallate 3-gallate | 0.4% EGCG (w/w) | NAFLD | ↓ALT, ↓AST, ↓TNF-α, ↓COX-2, ↓MMP-3 | Anti-inflammatory | [179] |
| 62 | Epigallocatechin gallate 3-gallate | 0%, 0.1%, 0.5% EGCG | HCC | ↓miR483-3p | Inhibit the metastasis of HCC | [180] |
| 63 | Epigallocatechin gallate 3-gallate | 25 mg/kg 20 μmol/L | Liver cirrhosis and liver cancer | ↓Hoshida, ↓α-SMA | Inhibition of HCC tumor formation | [181] |
| 64 | Epigallocatechin gallate methyl ester | 25 mg/kg 25, 50, 75, 100, 125, 150 µg/mL | DEN | ↑p21waf1/Cip1, ↓CDC25A | Inhibition of proliferation of human liver cancer cells | [182] |
| 65 | Methyl gallate | 10, 20, 40 μg/mL | ↓Caspase3, ↑BCI-2, ↓BAX, ↑Beclin-1, ↓LC3-I/II | Inducing cell apoptosis, promoting autophagy | [183] | |
| 66 | Corilagin | 5, 10 mg/kg | NAFLD | ↓TC, ↓TG | Improve liver lipid accumulation | [184] |
| 67 | Corilagin | 12.5, 25 mg/kg 1, 5, 10, 20 mg/mL | ConA | ↓MAPK, ↓NF-kB, ↓IRF5 | Anti-inflammatory, antioxidant | [185] |
| 68 | Mugaxanthin, gallic acid (crude leaf suspension and boiled leaf extract) | 500 mg/kg | Liver fibrosis | ↓TGF-β/SMAD | Anti-fibrosis | [186] |
| 69 | Chebullinic acid | 37.5, 75, 150 mg/kg 6.5, 13, 26 µM | CCl4 | ↑MAPK/Nrf2, ↑LDH, ↑HO-1, ↑NQO1, ↓MDA, ↑SOD | Antioxidant | [187] |
| 70 | Pomegranate extract | 100, 150, 200 mg/kg 5, 10, 20 mM | HFD | ↑PI3K/AKT 28, ↓HMGB-1/TLR4/NF-κB | Reduce gluconeogenesis | [188] |
| 71 | Pomegranate extract | 0.2% (w/v) | Fructose | Inhibited lipid deposition | [189] | |
| 72 | Pomegranate extract | 20 mg/kg | HFD | ↑FoxO1, ↓TXNIP, ↓NLRP3, ↓Caspase1, ↓IL-1β, ↓GSDMD | Inhibit pyroptosis and promote autophagy | [190] |
| 73 | Curcumin saponins | 20 mg/kg | I/R | ↑Nrf-2/HO-1 | Antioxidant, anti-inflammatory, apoptotic | [191] |
| 74 | Ferulic acid | 50 mg/kg | Cesium-137 | ↓JAK/ STAT, ↑Nrf2, ↑GPX4, ↑SLC7A11 | Anti-inflammatory, inhibits iron death, antioxidant | [192] |
| 75 | Ferulic acid | 10, 30, 100 mg/kg | SA | ↑PPAR-γ, ↑GLUT2 | Anti-inflammatory, antioxidant | [193] |
| 76 | Salvianolic acid A | 10, 20 mg/kg | IR | ↓TLR4, ↑Caspase3 | Antioxidant, anti-inflammatory, inhibition of cell apoptosis | [194] |
| 77 | P-coumaric acid | 50, 100 mg/kg | Fipronil | ↓ROS, ↓TNF-α, ↓IL-1β | Antioxidant, anti-inflammatory, | [195] |
| 78 | For coumaric acid and ferulic acid | 30 mg/kg/day | HFD | ↓HDAC1/PPARG | Inhibit lipid uptake | [196] |
| 79 | P-coumaric acid | 100 mg/kg | LPS/D-GalN | ↓TNF—α, ↓IL-6, ↓IL-1 β, ↓MDA, ↑GSH | Regulating lipid metabolism, antioxidant, anti-inflammatory | [197] |
| 80 | Arctiin | 25, 50, 100 mg/kg | HIRI | ↓HMGB1-TLR3/4-IRF1 | [198] | |
| 81 | Rosmarinic acid | 20, 40, 80 mg/kg, | APAP | ↑Nrf2, ↓NEK7-NLRP3 | Anti-inflammatory, antioxidant | [199] |
| 82 | Rosmarinic acid | ↑p-AMPK, ↓SREBP-1c | Anti-inflammatory | [200] | ||
| 83 | Rosmarinic acid | 0.25, 1, 5 μM | H2O2 | ↑MAPK, ↑Nrf2 | Antioxidant | [201] |
| 84 | Rosmarinic acid | 20, 50, 100 μmol/L | SMMC-7721 | ↓PI3K/AKT/mTOR | Inhibit the proliferation and invasion of liver cancer cells | [202] |
| 85 | Rosmarinic acid | 10, 20, 40 mg/kg | APAP | ↓RACK1/TNF-a | Antioxidant | [203] |
| 86 | Rosmarinic acid | 30, 90, 270 mg/kg | OVA | ↓TNF-α, ↓IL-4, ↓IL-6, ↓mMCP-1, ↓iNOS | Antioxidant, anti-inflammatory | [204] |
| 87 | Rosmarinic acid | 10, 30 mg/kg 20, 40 µM | NASH | ↑p-AMPK, ↓SREBP-1c, ↓FAS | Antioxidant, anti-inflammatory | [205] |
| 88 | Paeoniflorin | 25, 50, 100 mg/kg | Diabetes | ↓TXNIP/NLRP3 | Anti inflammatory | [206] |
| 89 | Paeoniflorin | 20 mg/kg | HCD | ↓FAS, ↓SREBP-1c, ↑p-AMPK, ↓p-MYPT-1 | Antioxidant, anti-inflammatory | [207] |
| 90 | Paeoniflorin | 50, 100, 200 mg/kg | APAP | ↓CYP2E1/JNK | Antioxidant, anti-inflammatory | [208] |
| 91 | Paeoniflorin | 100 mg/kg | Liver fibrosis | ↑HO-1 | Antioxidant, anti-inflammatory | [209] |
| 92 | Paeoniflorin | 50, 100 mg/kg | Liver fibrosis | ↑PPARγ | Inhibit HSC activation | [210] |
| 93 | Paeoniflorin | 25, 100 mg/kg | Liver cancer | ↑SOCS3↓STAT3/PD-L1 | Inhibit the growth of liver cancer cells | [211] |
| 94 | Paeoniflorin | 75 mg/kg | ZnO NPs | ↓SIRT1, ↑mTOR, ↓TFEB, ↓NLRP3 | Anti-inflammatory, improves intestinal microbiota and metabolic disorders | [212] |
| 95 | Paeoniflorin | 15, 30, 60 mg/kg | LPS | ↑SIRT1, ↑FOXO1a, ↑SOD2, ↓NLRP3 | Anti-inflammatory, antioxidant | [213] |
| 96 | Purple cone chrysanthemum glycoside | ↑miR-503-3p, ↓TGF-β1/Smad, ↑BAX/BCL-2 | Inhibit the proliferation, invasion, migration of liver cancer cells | [214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Xiong, S.; Xiang, F.; Li, Y.; Lin, Y.; Liu, Y.; Lin, L.; Xie, J. The Protective Effect of Phenolic Acids on Liver Disease: A Review of Possible Mechanisms. Antioxidants 2025, 14, 1247. https://doi.org/10.3390/antiox14101247
Ma X, Xiong S, Xiang F, Li Y, Lin Y, Liu Y, Lin L, Xie J. The Protective Effect of Phenolic Acids on Liver Disease: A Review of Possible Mechanisms. Antioxidants. 2025; 14(10):1247. https://doi.org/10.3390/antiox14101247
Chicago/Turabian StyleMa, Xinyi, Suhui Xiong, Feng Xiang, Yamei Li, Yan Lin, Yuexin Liu, Limei Lin, and Jingchen Xie. 2025. "The Protective Effect of Phenolic Acids on Liver Disease: A Review of Possible Mechanisms" Antioxidants 14, no. 10: 1247. https://doi.org/10.3390/antiox14101247
APA StyleMa, X., Xiong, S., Xiang, F., Li, Y., Lin, Y., Liu, Y., Lin, L., & Xie, J. (2025). The Protective Effect of Phenolic Acids on Liver Disease: A Review of Possible Mechanisms. Antioxidants, 14(10), 1247. https://doi.org/10.3390/antiox14101247