Mechanistic Insights into Mancozeb-Induced Redox Imbalance and Structural Remodelling Affecting the Function of Human Red Blood Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Solutions and Chemicals for Human RBC Sample Processing
2.2. Preparation of Human RBC Samples
2.2.1. Haemolysis Measurement
2.2.2. Detection of Apoptotic RBCs
2.3. Analysis of Cell Shape by Scanning Electron Microscopy (SEM)
2.4. Detection of Extracellular Vesicle Shedding
2.5. Evaluation of Intracellular Calcium Content
2.6. Detection of Deformability Measured by Elongation Index
2.7. Measurement of Na+/K+-ATPase Activity
2.8. Assessment of Oxidative Stress Parameters
2.8.1. Detection of ROS Levels
2.8.2. Measurement of TBARS Levels
2.8.3. Measurement of Total Sulfhydryl (-SH) Groups
2.8.4. Measurement of Methemoglobin (MetHb) Content
2.8.5. Measurement of Intracellular Free Iron
2.9. Measurement of Cytosolic Syk Kinase Activity
2.10. Preparation of Erythrocyte Plasma Membrane Proteins
SDS-PAGE Preparation and Western Blotting Analysis
2.11. Analytical Cytology
2.12. Measurement of SO42− Uptake
2.13. Endogenous Antioxidant Activity Assessment
2.13.1. Catalase Activity Assay
2.13.2. Superoxide Dismutase Activity Assay
2.13.3. GSH/GSSG Ratio Measurement
2.14. Experimental Data and Statistics
3. Results
3.1. Detection of Cellular Morphology in Mancozeb-Treated RBCs
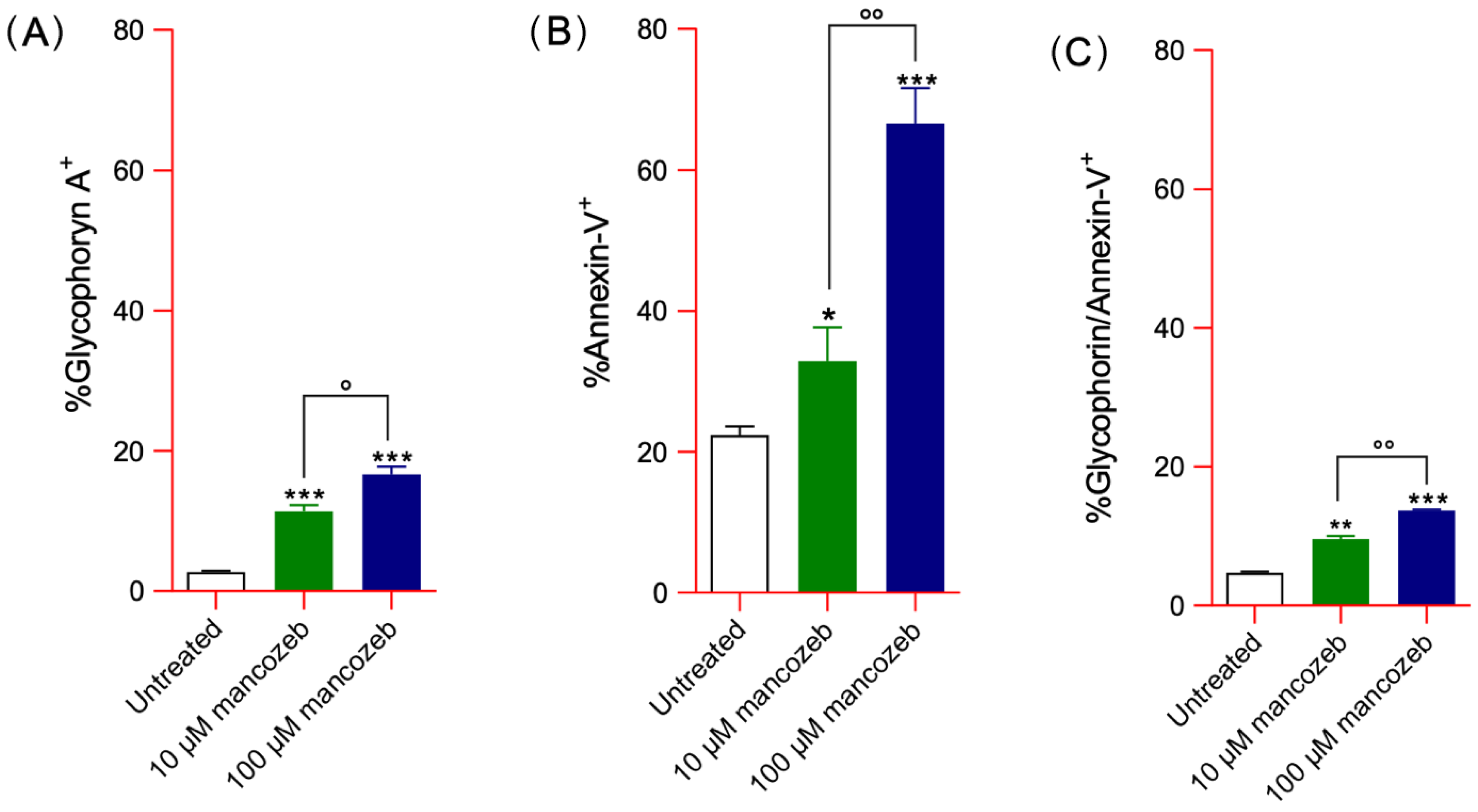
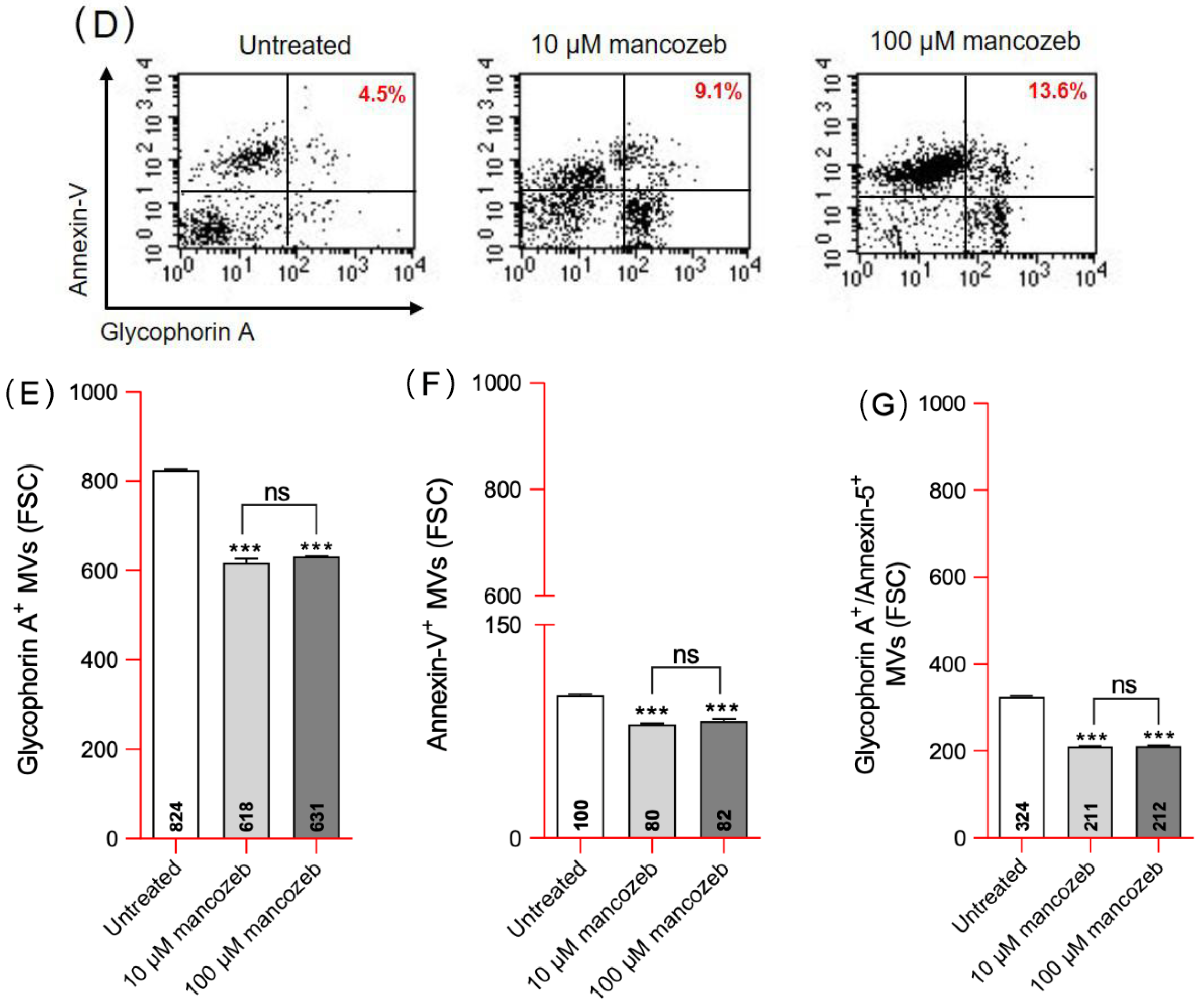
3.2. Detection of Vesicle Shedding in Mancozeb-Treated RBCs
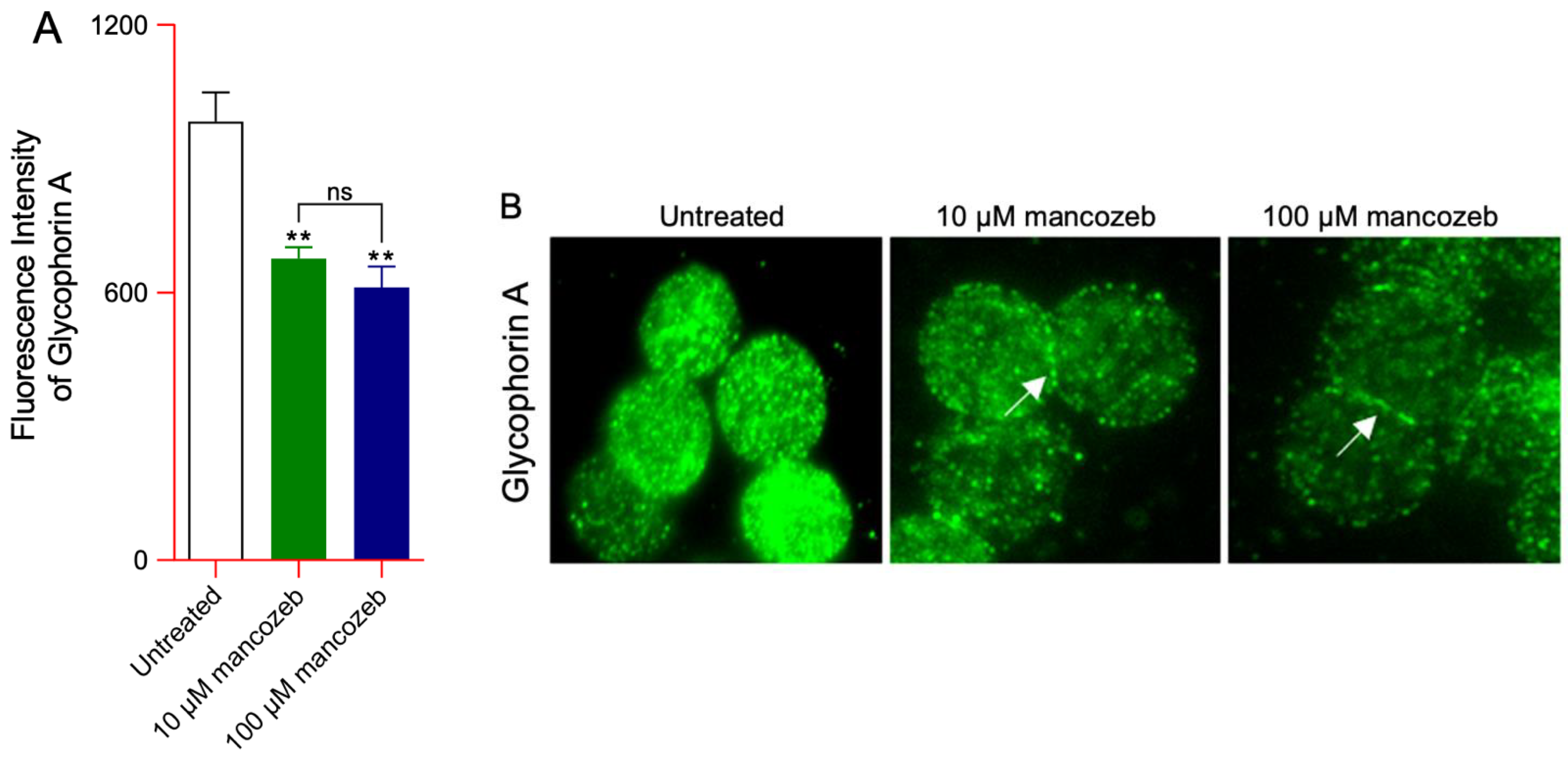
3.2.1. Detection of Glycophorin A Expression Levels and Distribution in Mancozeb-Treated RBCs
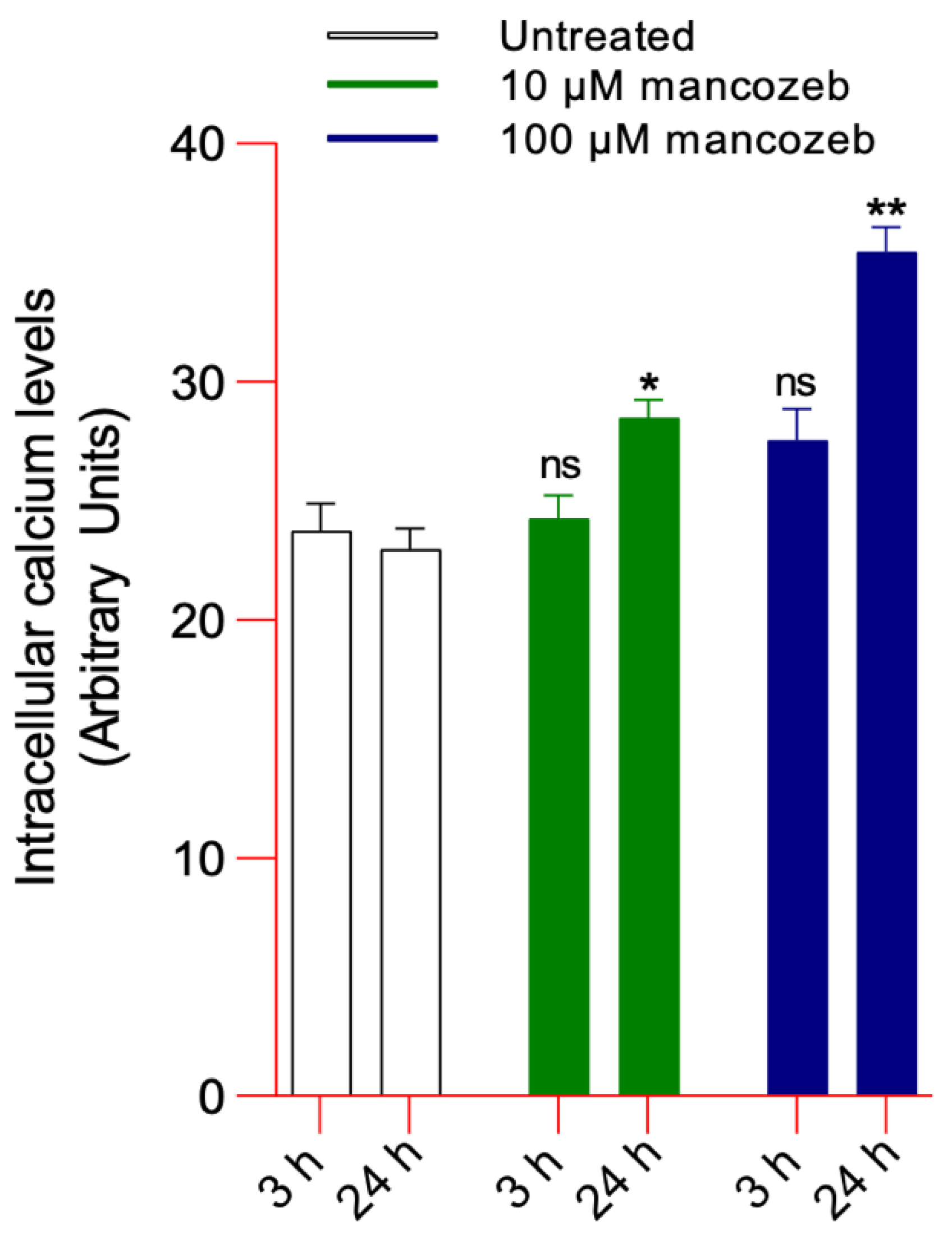
3.2.2. Detection of Intracellular Calcium Levels in Mancozeb-Treated RBCs
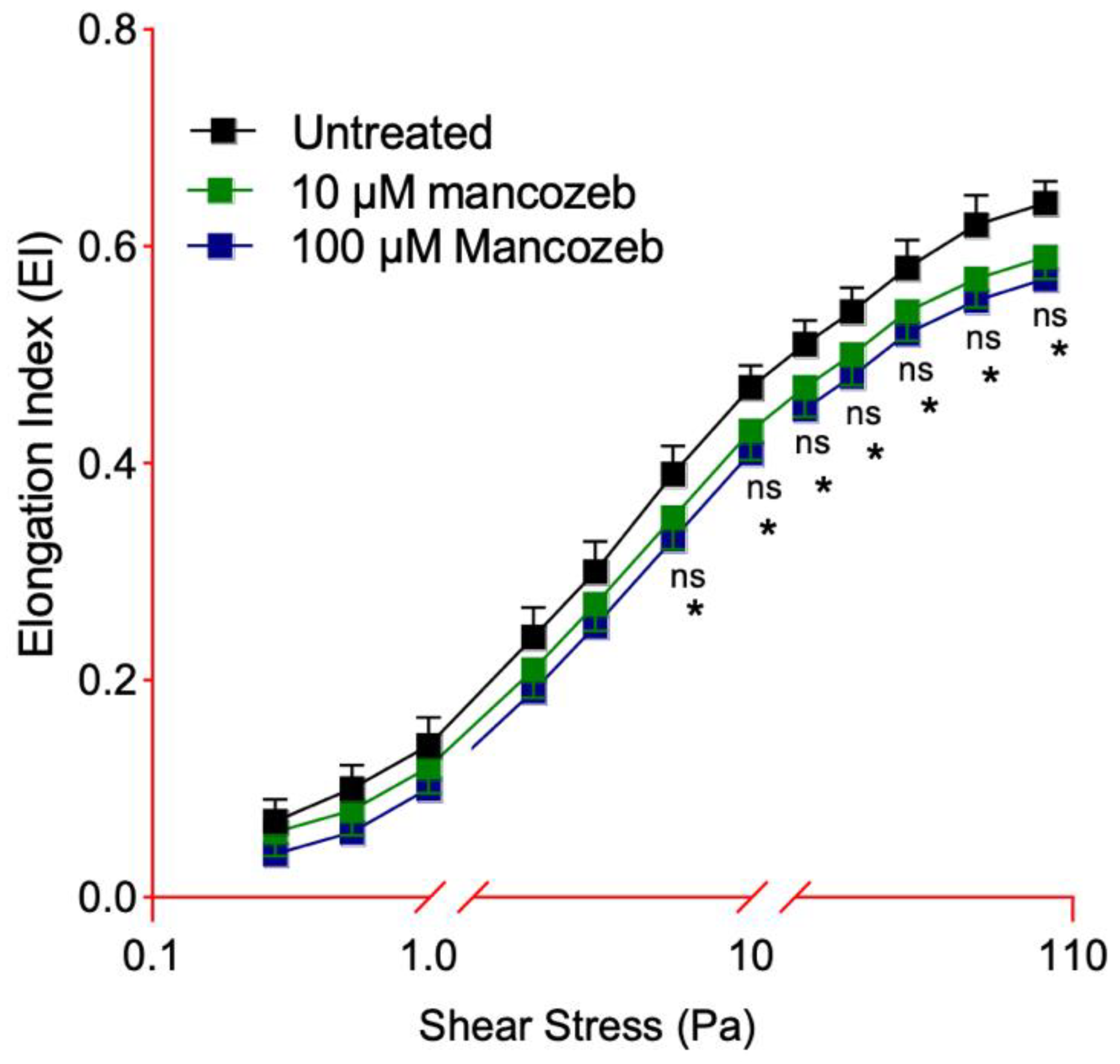
3.3. Detection of Cellular Deformability in Mancozeb-Treated RBCs
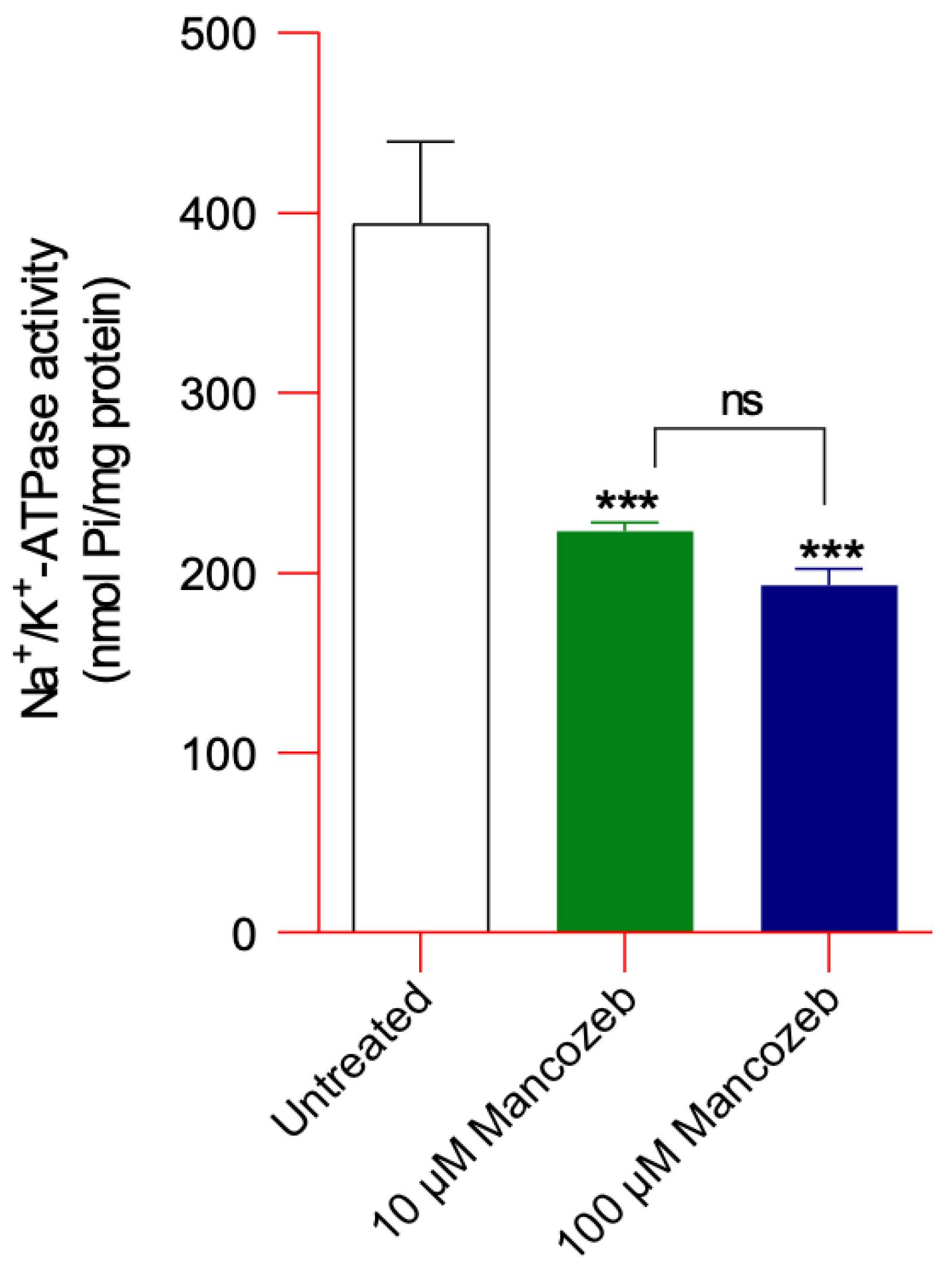
Assessment of Na+/K+ ATPase Activity in Mancozeb-Treated RBCs
3.4. Assessment of Oxidative Stress Parameters in Mancozeb-Treated RBCs
3.4.1. Evaluation of TBARS Levels
3.4.2. Evaluation of Total-SH Group Content
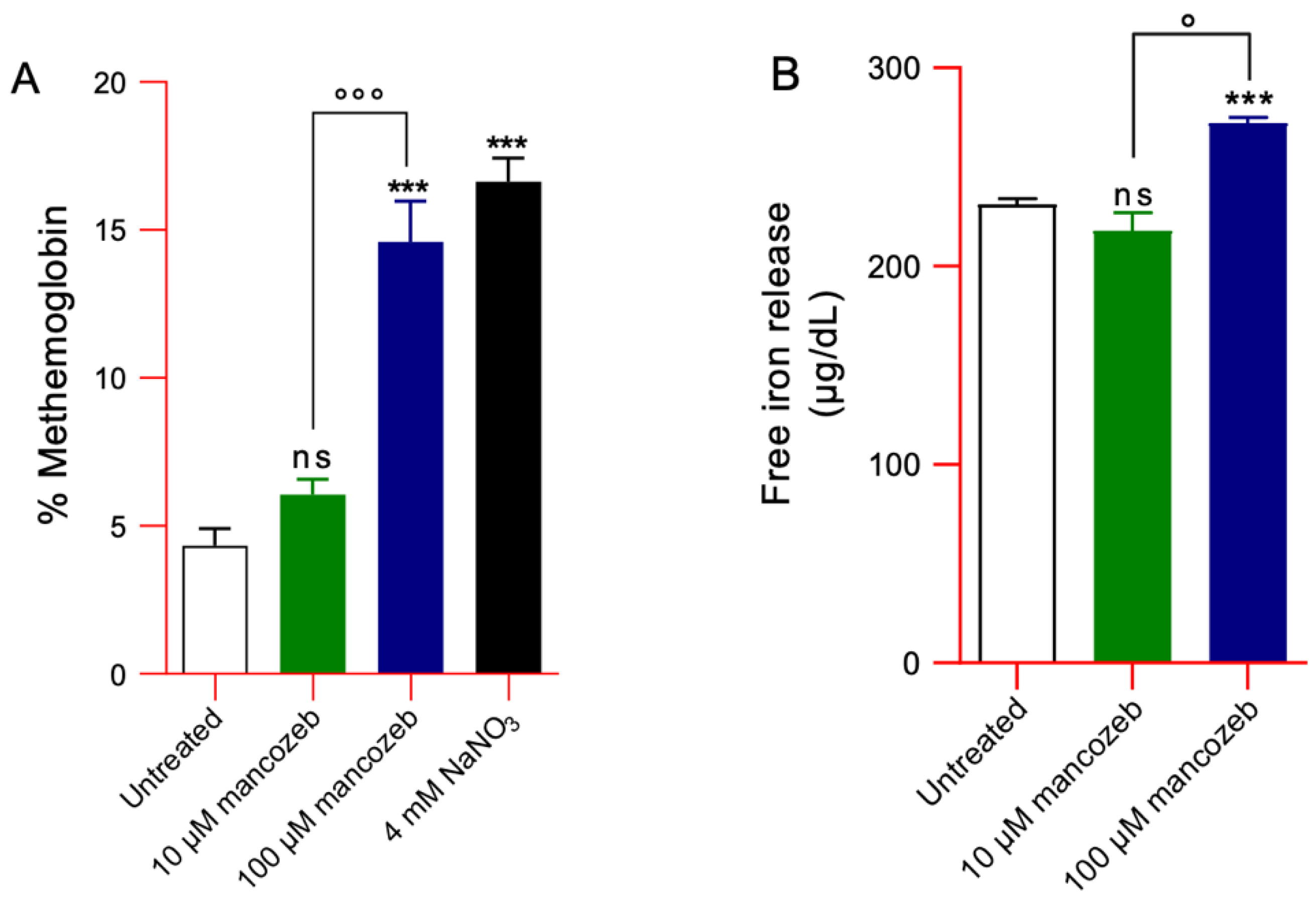
3.4.3. Detection of MetHb Levels and Intracellular Iron Release in Mancozeb-Treated RBCs
3.5. Measurement of Cytosolic Syk Kinase Activity in Mancozeb-Treated RBCs
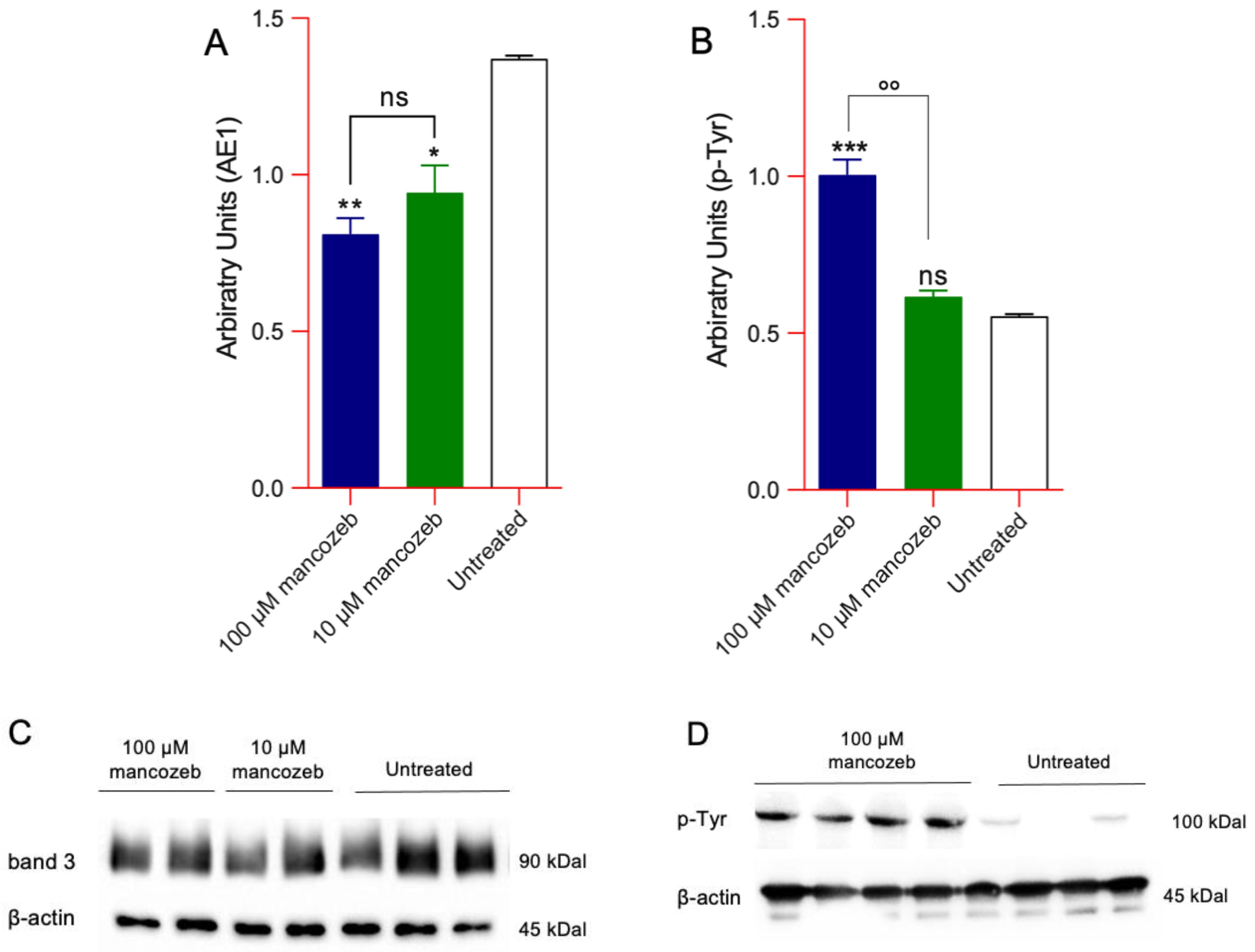
Detection of Phosphorylation and AE1 Expression Levels in Mancozeb-Treated RBCs by Western Blot
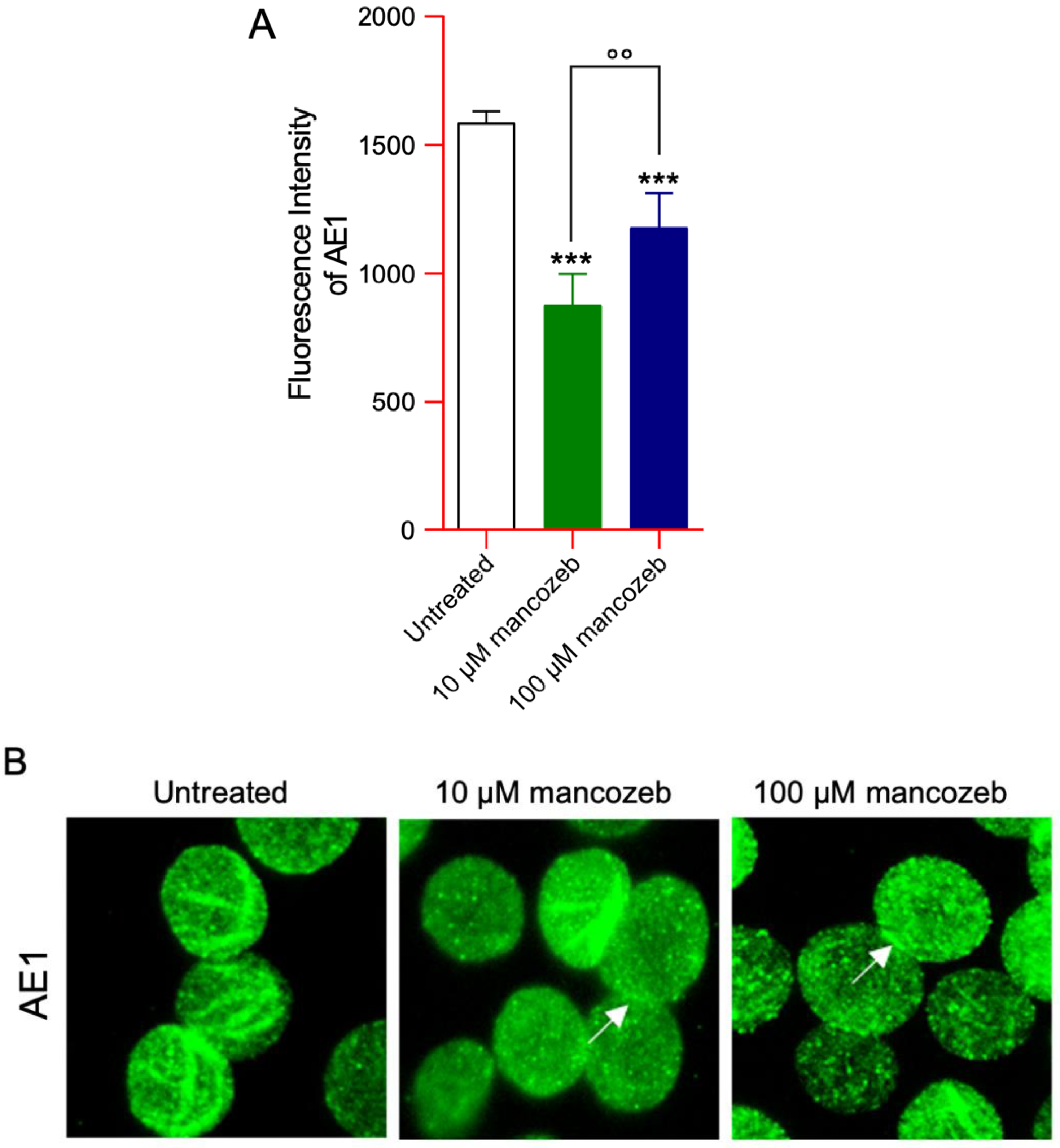
3.6. Detection of AE1 Expression Levels and Distribution in Mancozeb-Treated RBCs by Flow Cytometry
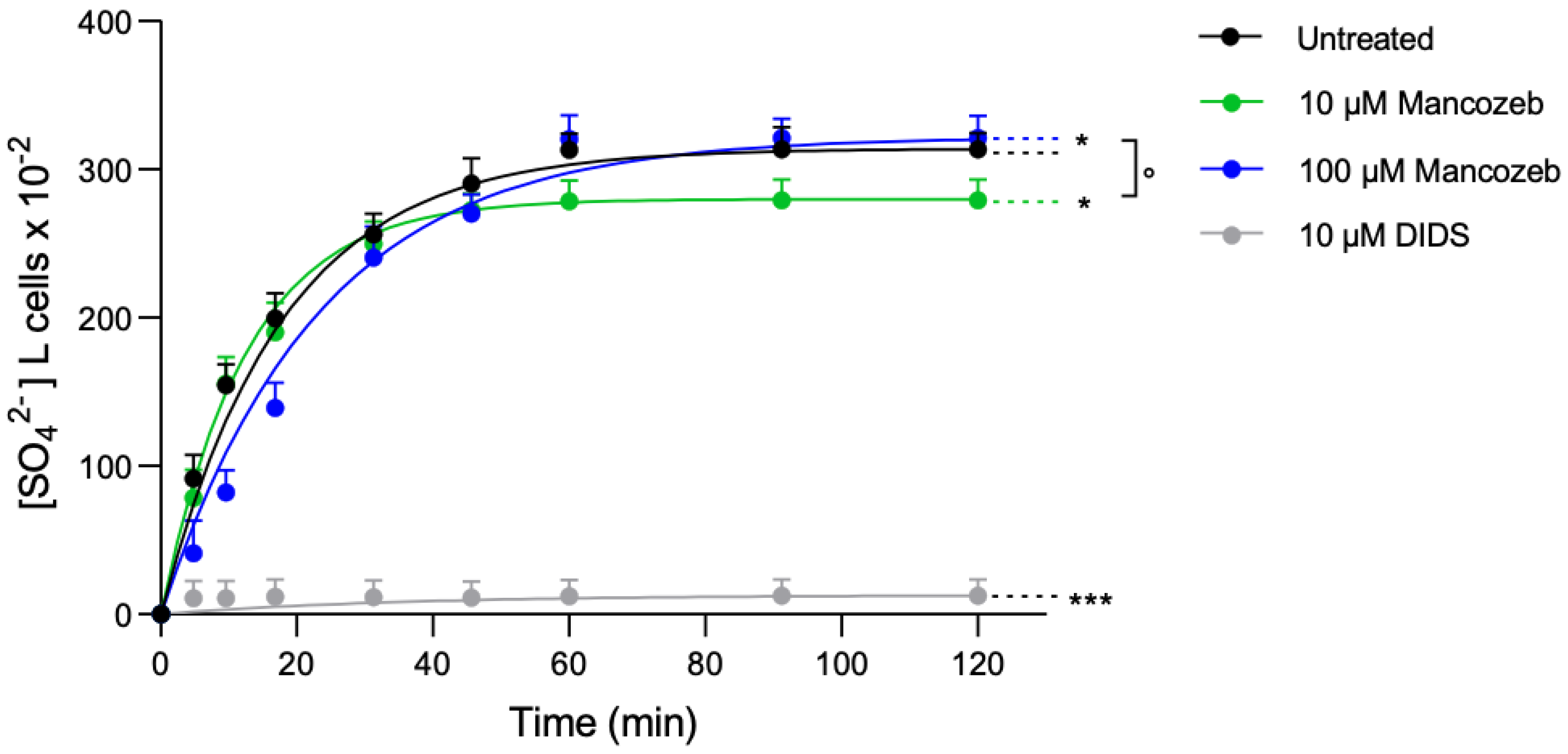
Anion Exchanger-Mediated SO42− Uptake in Mancozeb-Treated RBCs
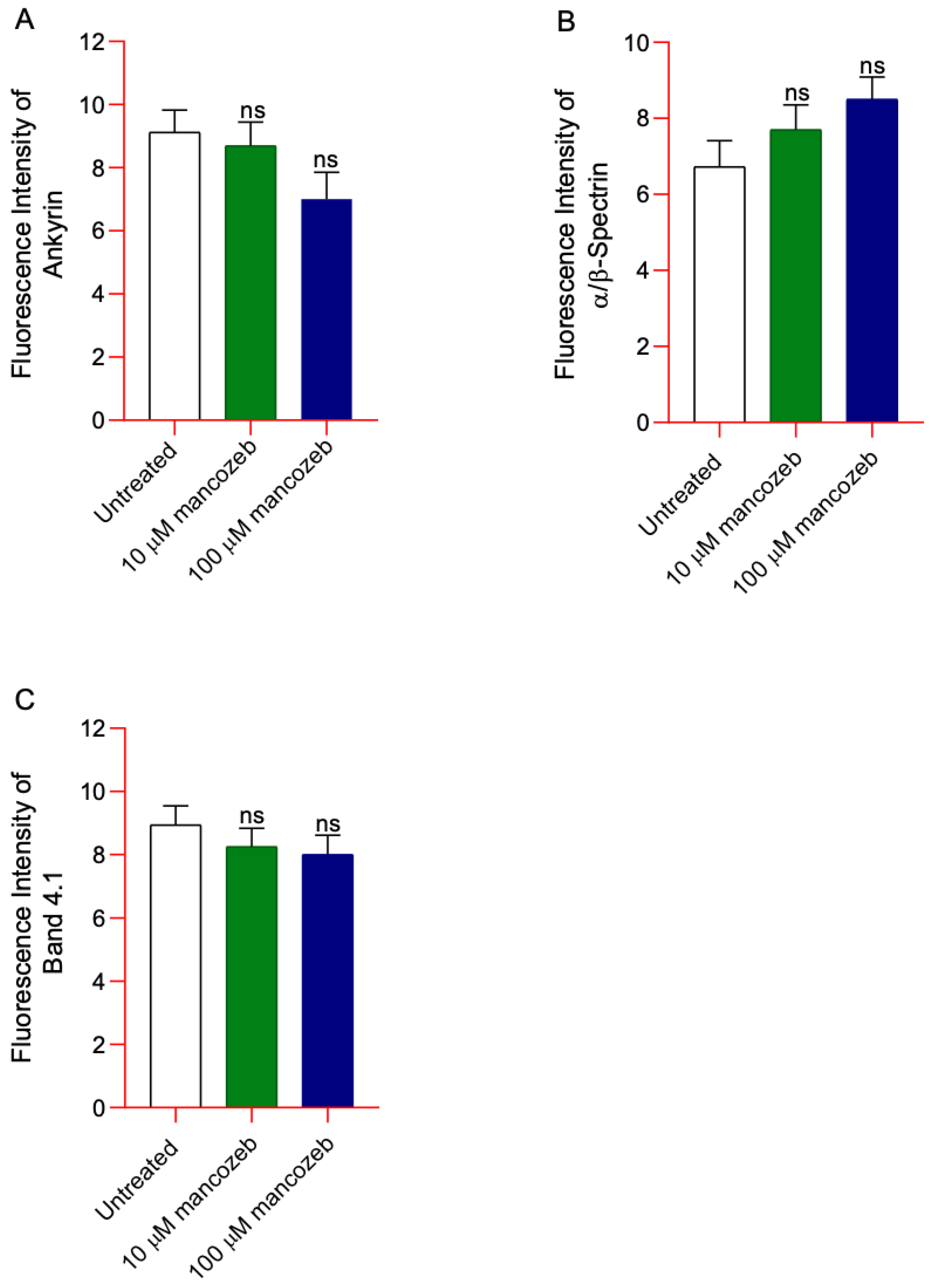
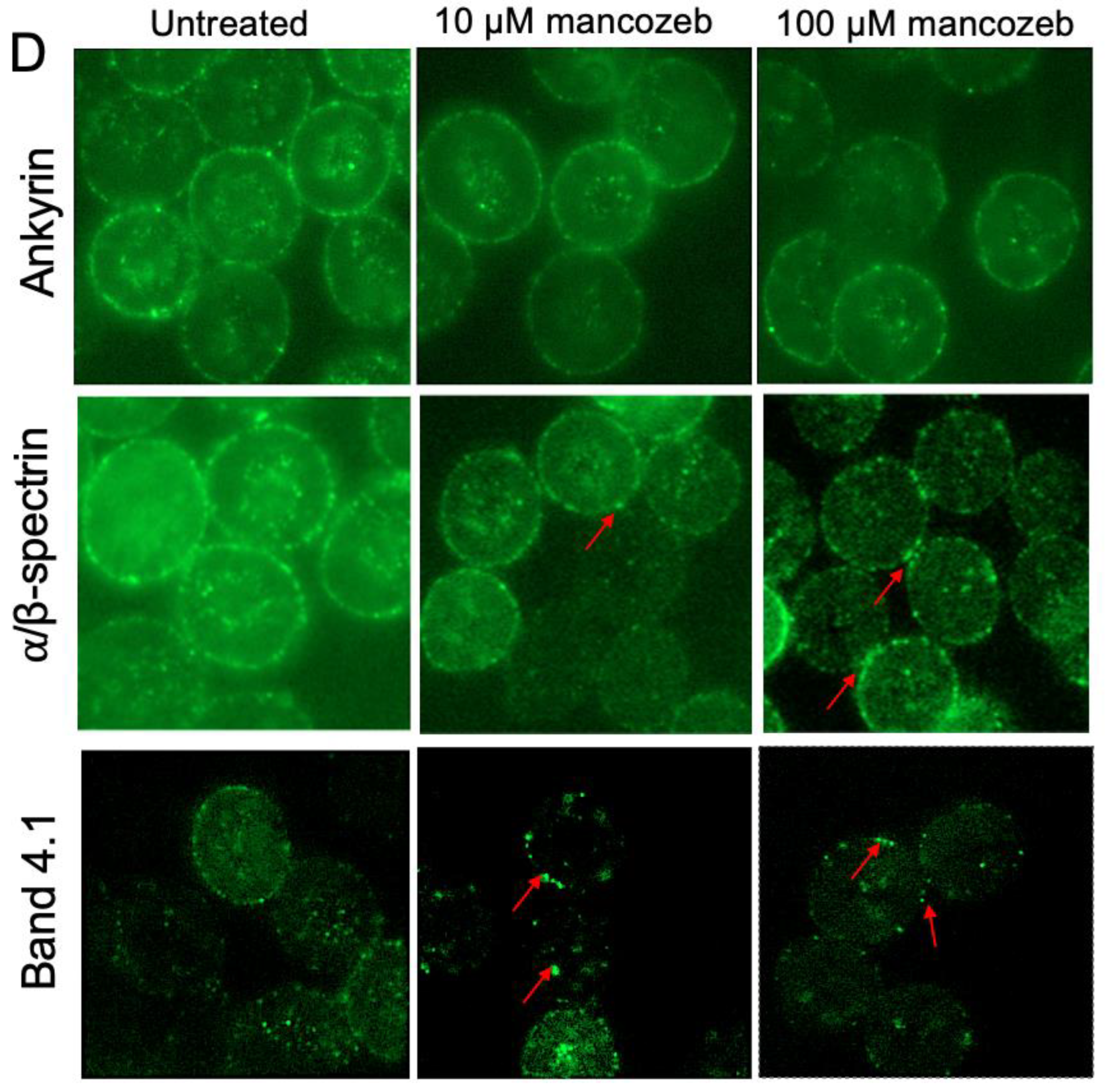
3.7. Determination of Cytoskeleton-Associated Proteins in Mancozeb-Treated RBCs
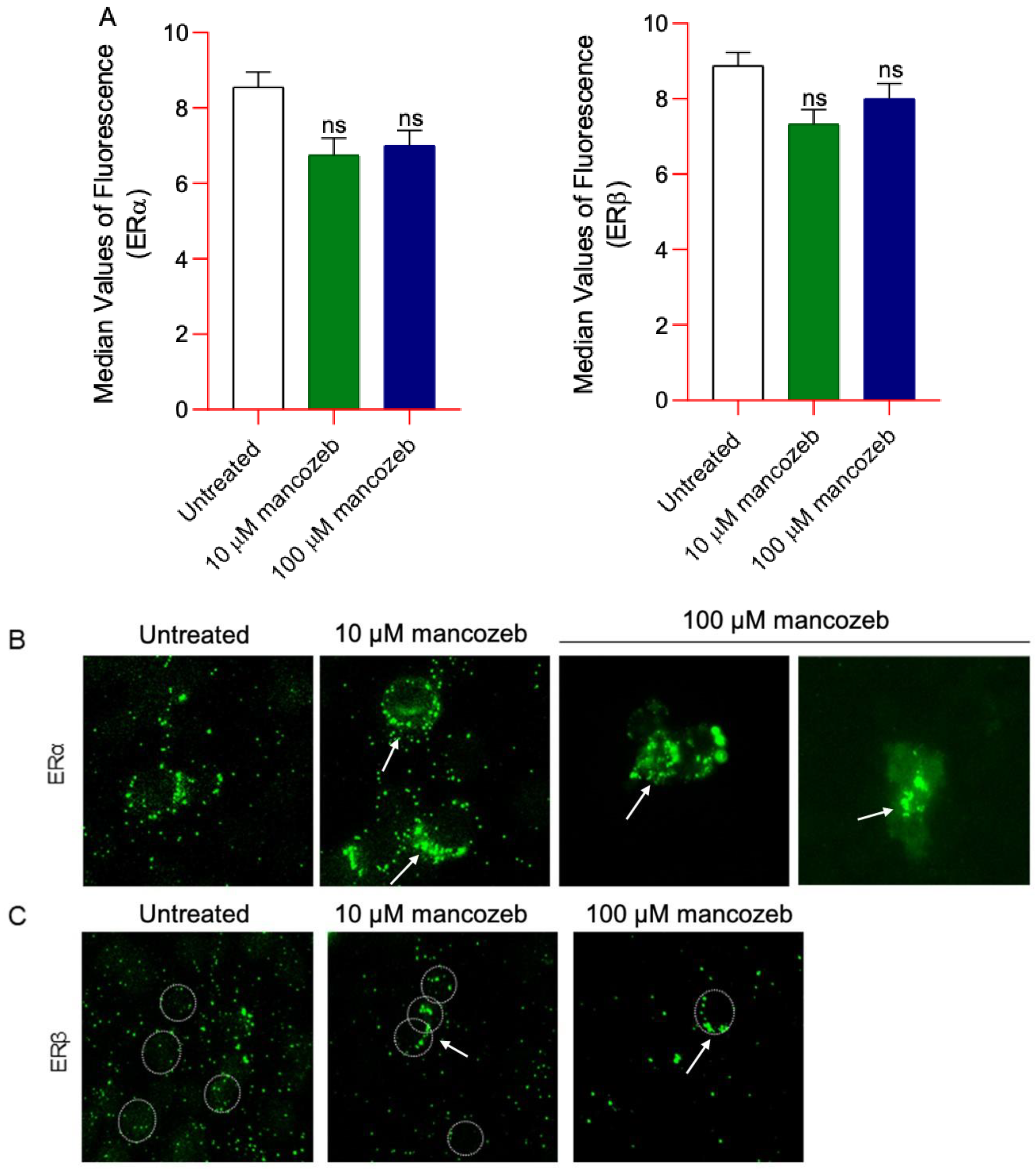
3.8. Measurement of ERα/β Content and Distribution in Mancozeb-Treated RBCs
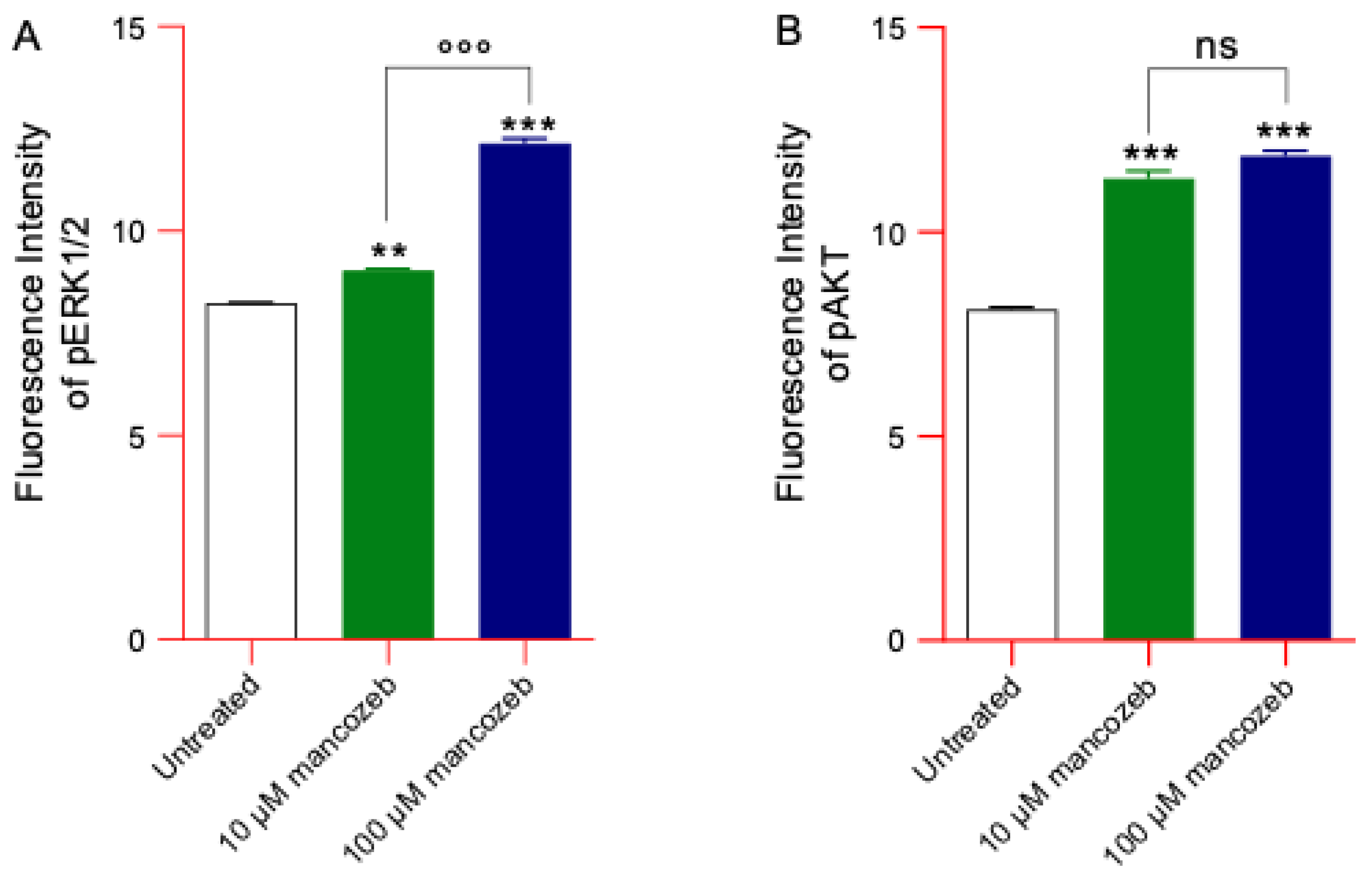
3.9. Measurement of Phosphorylated ERK and AKT Content in Mancozeb Treated-RBCs
3.10. Evaluation of the Endogenous Antioxidant Capacity in Mancozeb-Treated RBCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Market Research. Global Mancozeb Market (2025 Edition): Analysis by Form (Dry, and Liquid), by Mode of Application, by Crop Type, by Region, by Country: Market Insights and Forecast (2020–2030). Available online: https://www.marketresearch.com/Azoth-Analytics-v4068/Global-Mancozeb-Edition-Form-Dry-39514865#:~:text=For%20example%2C%20in%20countries%20like%20the%20United,protect%20crops%20from%20fungal%20diseases%20that%20could (accessed on 10 July 2025).
- Dall’Agnol, J.C.; Ferri Pezzini, M.; Suarez Uribe, N.; Joveleviths, D. Systemic effects of the pesticide mancozeb—A literature review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4113–4120. [Google Scholar] [CrossRef]
- Costa, C.; Teodoro, M.; Giambo, F.; Catania, S.; Vivarelli, S.; Fenga, C. Assessment of Mancozeb Exposure, Absorbed Dose, and Oxidative Damage in Greenhouse Farmers. Int. J. Environ. Res. Public Health 2022, 19, 10486. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.-A.; Nygren, O.; Sikström, E. Determination of mancozeb in air. Occupational exposure during its use in control of potato late blight. Chemosphere 1987, 16, 2423–2428. [Google Scholar] [CrossRef]
- Islam, J.Y.; Hoppin, J.; Mora, A.M.; Soto-Martinez, M.E.; Gamboa, L.C.; Castañeda, J.E.P.; Reich, B.; Lindh, C.; De Joode, B.V.W. Respiratory and allergic outcomes among 5-year-old children exposed to pesticides. Thorax 2023, 78, 41–49. [Google Scholar] [CrossRef]
- Mew, E.J.; Padmanathan, P.; Konradsen, F.; Eddleston, M.; Chang, S.S.; Phillips, M.R.; Gunnell, D. The global burden of fatal self-poisoning with pesticides 2006–15: Systematic review. J. Affect. Disord. 2017, 219, 93–104. [Google Scholar] [CrossRef]
- Moebus, S.; Boedeker, W. Case Fatality as an Indicator for the Human Toxicity of Pesticides-A Systematic Scoping Review on the Availability and Variability of Severity Indicators of Pesticide Poisoning. Int. J. Environ. Res. Public Health 2021, 18, 8307. [Google Scholar] [CrossRef]
- Onyeaka, H.; Ghosh, S.; Obileke, K.; Miri, T.; Odeyemi, O.A.; Nwaiwu, O.; Tamasiga, P. Preventing chemical contaminants in food: Challenges and prospects for safe and sustainable food production. Food Control 2024, 155, 110040. [Google Scholar] [CrossRef]
- European Food Safety Authority; Abdourahime, H.; Anastassiadou, M.; Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; et al. Peer review of the pesticide risk assessment of the active substance mancozeb. EFSA J. 2020, 18, e05755. [Google Scholar] [CrossRef]
- Pereira, S.I.; Figueiredo, P.I.; Barros, A.S.; Dias, M.C.; Santos, C.; Duarte, I.F.; Gil, A.M. Changes in the metabolome of lettuce leaves due to exposure to mancozeb pesticide. Food Chem. 2014, 154, 291–298. [Google Scholar] [CrossRef]
- Certel, M.; Cengiz, M.F.; Akcay, M. Kinetic and thermodynamic investigation of mancozeb degradation in tomato homogenate during thermal processing. J. Sci. Food Agric. 2012, 92, 534–541. [Google Scholar] [CrossRef]
- Skytt, Å. Drinking Water and Pesticides in Banana Growing Areas Contamination of Water Sources with Ethylenethiourea (ETU) in Matina County-Costa Rica. Bachelor’s Thesis, Lund University, Lund, Sweden, 2011. [Google Scholar]
- Srivastava, P.; Singh, A. Invivo study of effects of dithiocarbamates fungicide (mancozeb) and its metabolite ethylenethiourea (ETU) on fresh water fish Clarius batrachus. J. Biol. Earth Sci. 2013, 3, B228–B235. [Google Scholar]
- Mandic-Rajcevic, S.; Rubino, F.M.; Ariano, E.; Cottica, D.; Neri, S.; Colosio, C. Environmental and biological monitoring for the identification of main exposure determinants in vineyard mancozeb applicators. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 289–296. [Google Scholar] [CrossRef]
- Quds, R.; Sharma, M.; Mahmood, R. Cytoprotective effect of l-carnitine against mancozeb-induced oxidative damage in human erythrocytes. Pestic. Biochem. Physiol. 2025, 208, 106301. [Google Scholar] [CrossRef]
- Gullino, M.L.; Tinivella, F.; Garibaldi, A.; Kemmitt, G.M.; Bacci, L.; Sheppard, B. Mancozeb: Past, Present, and Future. Plant Dis. 2010, 94, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- van Wendel de Joode, B.; Mora, A.M.; Cordoba, L.; Cano, J.C.; Quesada, R.; Faniband, M.; Wesseling, C.; Ruepert, C.; Oberg, M.; Eskenazi, B.; et al. Aerial application of mancozeb and urinary ethylene thiourea (ETU) concentrations among pregnant women in Costa Rica: The Infants’ Environmental Health Study (ISA). Environ. Health Perspect. 2014, 122, 1321–1328. [Google Scholar] [CrossRef]
- Runkle, J.; Flocks, J.; Economos, J.; Dunlop, A.L. A systematic review of Mancozeb as a reproductive and developmental hazard. Environ. Int. 2017, 99, 29–42. [Google Scholar] [CrossRef]
- Belpoggi, F.; Soffritti, M.; Guarino, M.; Lambertini, L.; Cevolani, D.; Maltoni, C. Results of long-term experimental studies on the carcinogenicity of ethylene-bis-dithiocarbamate (Mancozeb) in rats. Ann. N. Y. Acad. Sci. 2002, 982, 123–136. [Google Scholar] [CrossRef]
- Quds, R.; Iqbal, Z.; Arif, A.; Mahmood, R. Mancozeb-induced cytotoxicity in human erythrocytes: Enhanced generation of reactive species, hemoglobin oxidation, diminished antioxidant power, membrane damage and morphological changes. Pestic. Biochem. Physiol. 2023, 193, 105453. [Google Scholar] [CrossRef]
- Perrone, P.; Spinelli, S.; Mantegna, G.; Notariale, R.; Straface, E.; Caruso, D.; Falliti, G.; Marino, A.; Manna, C.; Remigante, A.; et al. Mercury Chloride Affects Band 3 Protein-Mediated Anionic Transport in Red Blood Cells: Role of Oxidative Stress and Protective Effect of Olive Oil Polyphenols. Cells 2023, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Crupi, R.; Morabito, R.; Remigante, A.; Gugliandolo, E.; Britti, D.; Cuzzocrea, S.; Marino, A. Susceptibility of erythrocytes from different sources to xenobiotics-induced lysis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 221, 68–72. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Kobayashi, S.; Warang, P.; Madkaikar, M.; Mukherjee, M.B. Erythrocytes as a preferential target of oxidative stress in blood. Free Radic. Res. 2021, 55, 562–580. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Gambardella, L.; Bozzuto, G.; Vona, R.; Caruso, D.; Villari, V.; Cappello, T.; Maisano, M.; Dossena, S.; et al. Internalization of nano- and micro-plastics in human erythrocytes leads to oxidative stress and estrogen receptor-mediated cellular responses. Free Radic. Biol. Med. 2024, 223, 1–17. [Google Scholar] [CrossRef]
- Balaji, B.; Rajendar, B.; Ramanathan, M. Quercetin protected isolated human erythrocytes against mancozeb-induced oxidative stress. Toxicol. Ind. Health 2014, 30, 561–569. [Google Scholar] [CrossRef]
- Carter, C.M. Alterations in blood components. In Comprehensive Toxicology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 249–293. [Google Scholar]
- Ferru, E.; Giger, K.; Pantaleo, A.; Campanella, E.; Grey, J.; Ritchie, K.; Vono, R.; Turrini, F.; Low, P.S. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood 2011, 117, 5998–6006. [Google Scholar] [CrossRef]
- Diez-Silva, M.; Dao, M.; Han, J.; Lim, C.T.; Suresh, S. Shape and Biomechanical Characteristics of Human Red Blood Cells in Health and Disease. MRS Bull. 2010, 35, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Satchwell, T.J.; Toye, A.M. Band 3, an essential red blood cell hub of activity. Haematologica 2021, 106, 2792–2793. [Google Scholar] [CrossRef] [PubMed]
- Nohl, H.; Stolze, K. The effects of xenobiotics on erythrocytes. Gen. Pharmacol. 1998, 31, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I.; Igwe, M.C.; Obeagu, G.U. Oxidative stress’s impact on red blood cells: Unveiling implications for health and disease. Medicine 2024, 103, e37360. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, L.; Cortes-Maramba, N.; Dioquino, C.; Suplido, M.L.; Ho, H.; Francisco-Rivera, A.; Manglicmot-Yabes, A. Correlation between blood ethylenethiourea and thyroid gland disorders among banana plantation workers in the Philippines. Environ. Health Perspect. 2004, 112, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Costa, R.; Rizzo, V.; Remigante, A.; Nofziger, C.; La Spada, G.; Marino, A.; Paulmichl, M.; Dossena, S. Crude venom from nematocysts of Pelagia noctiluca (Cnidaria: Scyphozoa) elicits a sodium conductance in the plasma membrane of mammalian cells. Sci. Rep. 2017, 7, 41065. [Google Scholar] [CrossRef]
- Goodhead, L.K.; MacMillan, F.M. Measuring osmosis and hemolysis of red blood cells. Adv. Physiol. Educ. 2017, 41, 298–305. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Spinelli, S.; Vitale, G.; Trichilo, V.; Loddo, S.; Marino, A. High Glucose Concentrations Affect Band 3 Protein in Human Erythrocytes. Antioxidants 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Kupcho, K.; Shultz, J.; Hurst, R.; Hartnett, J.; Zhou, W.; Machleidt, T.; Grailer, J.; Worzella, T.; Riss, T.; Lazar, D.; et al. A real-time, bioluminescent annexin V assay for the assessment of apoptosis. Apoptosis 2019, 24, 184–197. [Google Scholar] [CrossRef]
- Donadello, K.; Piagnerelli, M.; Reggiori, G.; Gottin, L.; Scolletta, S.; Occhipinti, G.; Boudjeltia, K.Z.; Vincent, J.-L. Reduced red blood cell deformability over time is associated with a poor outcome in septic patients. Microvasc. Res. 2015, 101, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Sompong, W.; Cheng, H.; Adisakwattana, S. Protective Effects of Ferulic Acid on High Glucose-Induced Protein Glycation, Lipid Peroxidation, and Membrane Ion Pump Activity in Human Erythrocytes. PLoS ONE 2015, 10, e0129495. [Google Scholar] [CrossRef]
- Sweadner, K.J. Colorimetric Assays of Na,K-ATPase. Methods Mol. Biol. 2016, 1377, 89–104. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Mendanha, S.A.; Anjos, J.L.; Silva, A.H.; Alonso, A. Electron paramagnetic resonance study of lipid and protein membrane components of erythrocytes oxidized with hydrogen peroxide. Braz. J. Med. Biol. Res. 2012, 45, 473–481. [Google Scholar] [CrossRef]
- Aksenov, M.Y.; Markesbery, W.R. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci. Lett. 2001, 302, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Naoum, P.C.; Radispiel, J.; Magaly da Silva, M. Spectrometric measurement of methemoglobin without interference of chemical or enzymatic reagents. Rev. Bras. Hematol. Hemoter. 2004, 26, 19–22. [Google Scholar]
- Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Dossena, S.; Marino, A.; Morabito, R.; Remigante, A. Iron Overload-Related Oxidative Stress Leads to Hyperphosphorylation and Altered Anion Exchanger 1 (Band 3) Function in Erythrocytes from Subjects with beta-Thalassemia Minor. Int. J. Mol. Sci. 2025, 26, 1593. [Google Scholar] [CrossRef]
- Pantaleo, A.; Ferru, E.; Pau, M.C.; Khadjavi, A.; Mandili, G.; Matte, A.; Spano, A.; De Franceschi, L.; Pippia, P.; Turrini, F. Band 3 Erythrocyte Membrane Protein Acts as Redox Stress Sensor Leading to Its Phosphorylation by p (72) Syk. Oxidative Med. Cell. Longev. 2016, 2016, 6051093. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Yeung, Y.G.; Stanley, E.R. A solution for stripping antibodies from polyvinylidene fluoride immunoblots for multiple reprobing. Anal. Biochem. 2009, 389, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Romano, L.; Peritore, D.; Simone, E.; Sidoti, A.; Trischitta, F.; Romano, P. Chloride-sulphate exchange chemically measured in human erythrocyte ghosts. Cell. Mol. Biol. 1998, 44, 351–355. [Google Scholar]
- Romano, L.; Passow, H. Characterization of anion transport system in trout red blood cell. Am. J. Physiol. 1984, 246, C330–C338. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Arcuri, B.; Marino, A.; Giammanco, M.; La Spada, G. Effect of cadmium on anion exchange capability through Band 3 protein in human erythrocytes. J. Biol. Res. 2018, 91, 7203. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Cordaro, M.; Trichilo, V.; Loddo, S.; Dossena, S.; Marino, A. Impact of acute inflammation on Band 3 protein anion exchange capability in human erythrocytes. Arch. Physiol. Biochem. 2020, 128, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Straface, E.; Gambardella, L.; Russo, M.; Cafeo, G.; Caruso, D.; Falliti, G.; Dugo, P.; Dossena, S.; et al. Mechanisms underlying the anti-aging activity of bergamot (Citrus bergamia) extract in human red blood cells. Front. Physiol. 2023, 14, 1225552. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Patane, G.T.; Barreca, D.; Straface, E.; Gambardella, L.; Bozzuto, G.; Caruso, D.; Falliti, G.; Dossena, S.; et al. AAPH-induced oxidative damage reduced anion exchanger 1 (SLC4A1/AE1) activity in human red blood cells: Protective effect of an anthocyanin-rich extract. Front. Physiol. 2023, 14, 1303815. [Google Scholar] [CrossRef]
- Jessen, F.; Sjoholm, C.; Hoffmann, E.K. Identification of the anion exchange protein of Ehrlich cells: A kinetic analysis of the inhibitory effects of 4,4′-diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS) and labeling of membrane proteins with 3H-DIDS. J. Membr. Biol. 1986, 92, 195–205. [Google Scholar] [CrossRef]
- Margoliash, E.; Novogrodsky, A.; Schejter, A. Irreversible reaction of 3-amino-1:2:4-triazole and related inhibitors with the protein of catalase. Biochem. J. 1960, 74, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Huisjes, R.; Bogdanova, A.; van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; van Wijk, R. Squeezing for Life—Properties of Red Blood Cell Deformability. Front. Physiol. 2018, 9, 656. [Google Scholar] [CrossRef]
- Radosinska, J.; Vrbjar, N. Erythrocyte Deformability and Na,K-ATPase Activity in Various Pathophysiological Situations and Their Protection by Selected Nutritional Antioxidants in Humans. Int. J. Mol. Sci. 2021, 22, 11924. [Google Scholar] [CrossRef] [PubMed]
- Vikulov, A.; Osetrov, I.; Mel’nikov, A. Activity of Na, K-ATPase of Erythrocytes in Physically Active Subjects. Hum. Physiol. 2001, 27, 375–377. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Radosinska, J.; Vrbjar, N. The role of red blood cell deformability and Na,K-ATPase function in selected risk factors of cardiovascular diseases in humans: Focus on hypertension, diabetes mellitus and hypercholesterolemia. Physiol. Res. 2016, 65 (Suppl. S1), S43–S54. [Google Scholar] [CrossRef]
- Namazi, G.; Asa, P.; Sarrafzadegan, N.; Pourfarzam, M. Decreased Na+/K+-ATPase Activity and Altered Susceptibility to Peroxidation and Lipid Composition in the Erythrocytes of Metabolic Syndrome Patients with Coronary Artery Disease. Ann. Nutr. Metab. 2019, 74, 140–148. [Google Scholar] [CrossRef]
- Tikhomirova, I.; Petrochenko, E.; Muravyov, A.; Malysheva, Y.; Petrochenko, A.; Yakusevich, V.; Oslyakova, A. Microcirculation and blood rheology abnormalities in chronic heart failure. Clin. Hemorheol. Microcirc. 2017, 65, 383–391. [Google Scholar] [CrossRef]
- Costa, T.G.F.; Oliveira, M.M.; Toledo, M.M.; Santos, H.B.; Thome, R.G.; Cortes, V.F.; Santos, H.L.; Quintas, L.E.M.; Sousa, L.; Fontes, C.F.L.; et al. Effect of Fe3+ on Na,K-ATPase: Unexpected activation of ATP hydrolysis. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183868. [Google Scholar] [CrossRef]
- Daraghmeh, D.N.; Karaman, R. The Redox Process in Red Blood Cells: Balancing Oxidants and Antioxidants. Antioxidants 2024, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- McNulty, R.; Kuchi, N.; Xu, E.; Gunja, N. Food-induced methemoglobinemia: A systematic review. J. Food Sci. 2022, 87, 1423–1448. [Google Scholar] [CrossRef] [PubMed]
- Sztiller, M.; Puchala, M.; Kowalczyk, A.; Bartosz, G. The influence of ferrylhemoglobin and methemoglobin on the human erythrocyte membrane. Redox Rep. 2006, 11, 263–271. [Google Scholar] [CrossRef]
- Maruyama, T.; Hieda, M.; Mawatari, S.; Fujino, T. Rheological Abnormalities in Human Erythrocytes Subjected to Oxidative Inflammation. Front. Physiol. 2022, 13, 837926. [Google Scholar] [CrossRef]
- Kuhn, V.; Diederich, L.; Keller, T.C.S., IV; Kramer, C.M.; Luckstadt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef]
- Percy, M.J.; McFerran, N.V.; Lappin, T.R. Disorders of oxidised haemoglobin. Blood Rev. 2005, 19, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Beale, A.D.; Hayter, E.A.; Crosby, P.; Valekunja, U.K.; Edgar, R.S.; Chesham, J.E.; Maywood, E.S.; Labeed, F.H.; Reddy, A.B.; Wright, K.P., Jr.; et al. Mechanisms and physiological function of daily haemoglobin oxidation rhythms in red blood cells. EMBO J. 2023, 42, e114164. [Google Scholar] [CrossRef]
- Thiagarajan, P.; Parker, C.J.; Prchal, J.T. How Do Red Blood Cells Die? Front. Physiol. 2021, 12, 655393. [Google Scholar] [CrossRef]
- Anong, W.A.; Franco, T.; Chu, H.; Weis, T.L.; Devlin, E.E.; Bodine, D.M.; An, X.; Mohandas, N.; Low, P.S. Adducin forms a bridge between the erythrocyte membrane and its cytoskeleton and regulates membrane cohesion. Blood 2009, 114, 1904–1912. [Google Scholar] [CrossRef]
- Wu, F.; Satchwell, T.J.; Toye, A.M. Anion exchanger 1 in red blood cells and kidney: Band 3’s in a pod. Biochem. Cell Biol. 2011, 89, 106–114. [Google Scholar] [CrossRef]
- Alexy, T.; Detterich, J.; Connes, P.; Toth, K.; Nader, E.; Kenyeres, P.; Arriola-Montenegro, J.; Ulker, P.; Simmonds, M.J. Physical Properties of Blood and their Relationship to Clinical Conditions. Front. Physiol. 2022, 13, 906768. [Google Scholar] [CrossRef]
- Reithmeier, R.A.; Casey, J.R.; Kalli, A.C.; Sansom, M.S.; Alguel, Y.; Iwata, S. Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochim. Biophys. Acta 2016, 1858, 1507–1532. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Cavallaro, M.; Taormina, A.; La Spada, G.; Marino, A. Anion exchange through band 3 protein in canine leishmaniasis at different stages of disease. Pflug. Arch. 2017, 469, 713–724. [Google Scholar] [CrossRef]
- Morabito, R.; Remigante, A.; Di Pietro, M.L.; Giannetto, A.; La Spada, G.; Marino, A. SO4= uptake and catalase role in preconditioning after H2O2-induced oxidative stress in human erythrocytes. Pflug. Arch. 2017, 469, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.L. Proton fluxes associated with erythrocyte membrane anion exchange. J. Membr. Biol. 1976, 28, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Mannu, F.; Arese, P.; Cappellini, M.D.; Fiorelli, G.; Cappadoro, M.; Giribaldi, G.; Turrini, F. Role of hemichrome binding to erythrocyte membrane in the generation of band-3 alterations in beta-thalassemia intermedia erythrocytes. Blood 1995, 86, 2014–2020. [Google Scholar] [CrossRef]
- Low, P.S.; Waugh, S.M.; Zinke, K.; Drenckhahn, D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science 1985, 227, 531–533. [Google Scholar] [CrossRef]
- Jay, D.G. Role of band 3 in homeostasis and cell shape. Cell 1996, 86, 853–854. [Google Scholar] [CrossRef]
- Leal, J.K.F.; Adjobo-Hermans, M.J.W.; Bosman, G. Red Blood Cell Homeostasis: Mechanisms and Effects of Microvesicle Generation in Health and Disease. Front. Physiol. 2018, 9, 703. [Google Scholar] [CrossRef]
- Fibach, E. The Redox Balance and Membrane Shedding in RBC Production, Maturation, and Senescence. Front. Physiol. 2021, 12, 604738. [Google Scholar] [CrossRef]
- Lin, L.C.; Brown, F.L. Dynamic simulations of membranes with cytoskeletal interactions. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005, 72, 011910. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, X.; Pivkin, I.V.; Dao, M.; Karniadakis, G.E.; Suresh, S. Lipid bilayer and cytoskeletal interactions in a red blood cell. Proc. Natl. Acad. Sci. USA 2013, 110, 13356–13361. [Google Scholar] [CrossRef] [PubMed]
- Sudnitsyna, J.; Skverchinskaya, E.; Dobrylko, I.; Nikitina, E.; Gambaryan, S.; Mindukshev, I. Microvesicle formation induced by oxidative stress in human erythrocytes. Antioxidants 2020, 9, 929. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, S.; Straface, E.; Gambardella, L.; Caruso, D.; Falliti, G.; Remigante, A.; Marino, A.; Morabito, R. Aging Injury Impairs Structural Properties and Cell Signaling in Human Red Blood Cells; Acai Berry Is a Keystone. Antioxidants 2023, 12, 848. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Ciana, A.; Achilli, C.; Gaur, A.; Minetti, G. Membrane Remodelling and Vesicle Formation During Ageing of Human Red Blood Cells. Cell. Physiol. Biochem. 2017, 42, 1127–1138. [Google Scholar] [CrossRef]
- Podsiedlik, M.; Markowicz-Piasecka, M.; Sikora, J. Erythrocytes as model cells for biocompatibility assessment, cytotoxicity screening of xenobiotics and drug delivery. Chem. Biol. Interact. 2020, 332, 109305. [Google Scholar] [CrossRef]
- Hankins, H.M.; Baldridge, R.D.; Xu, P.; Graham, T.R. Role of flippases, scramblases and transfer proteins in phosphatidylserine subcellular distribution. Traffic 2015, 16, 35–47. [Google Scholar] [CrossRef]
- Salzer, U.; Zhu, R.; Luten, M.; Isobe, H.; Pastushenko, V.; Perkmann, T.; Hinterdorfer, P.; Bosman, G.J. Vesicles generated during storage of red cells are rich in the lipid raft marker stomatin. Transfusion 2008, 48, 451–462. [Google Scholar] [CrossRef]
- Wilkinson, D.K.; Turner, E.J.; Parkin, E.T.; Garner, A.E.; Harrison, P.J.; Crawford, M.; Stewart, G.W.; Hooper, N.M. Membrane raft actin deficiency and altered Ca2+-induced vesiculation in stomatin-deficient overhydrated hereditary stomatocytosis. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Couves, E.C.; Gardner, S.; Voisin, T.B.; Bickel, J.K.; Stansfeld, P.J.; Tate, E.W.; Bubeck, D. Structural basis for membrane attack complex inhibition by CD59. Nat. Commun. 2023, 14, 890. [Google Scholar] [CrossRef] [PubMed]
- Dinkla, S.; Wessels, K.; Verdurmen, W.; Tomelleri, C.; Cluitmans, J.; Fransen, J.; Fuchs, B.; Schiller, J.; Joosten, I.; Brock, R. Functional consequences of sphingomyelinase-induced changes in erythrocyte membrane structure. Cell Death Dis. 2012, 3, e410. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.B.; Ly, T.B.; Wesseling, M.C.; Hittinger, M.; Torge, A.; Devitt, A.; Perrie, Y.; Bernhardt, I. Characterization of Microvesicles Released from Human Red Blood Cells. Cell. Physiol. Biochem. 2016, 38, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Gea, M.; Zhang, C.; Tota, R.; Gilardi, G.; Di Nardo, G.; Schiliro, T. Assessment of Five Pesticides as Endocrine-Disrupting Chemicals: Effects on Estrogen Receptors and Aromatase. Int. J. Environ. Res. Public Health 2022, 19, 1959. [Google Scholar] [CrossRef]
- Simoncini, T.; Mannella, P.; Fornari, L.; Caruso, A.; Varone, G.; Genazzani, A.R. Genomic and non-genomic effects of estrogens on endothelial cells. Steroids 2004, 69, 537–542. [Google Scholar] [CrossRef]
- Skalny, A.; Aschner, M.; Paoliello, M.; Santamaria, A.; Nikitina, N.; Rejniuk, V.; Jiang, Y.; Rocha, J.; Tinkov, A. Endocrine-disrupting activity of mancozeb. Arh. Farm. 2021, 71, 491–507. [Google Scholar] [CrossRef]
- Hazegh, K.; Fang, F.; Kelly, K.; Sinchar, D.; Wang, L.; Zuchelkowski, B.E.; Ufelle, A.C.; Esparza, O.; Davizon-Castillo, P.; Page, G.P.; et al. Erythrocyte mitogen-activated protein kinases mediate hemolytic events under osmotic and oxidative stress and in hemolytic diseases. Cell. Signal. 2022, 99, 110450. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Ortona, E.; Santulli, M.; Malorni, W.; Care, A.; Pietraforte, D.; Straface, E. Functional Estrogen Receptors of Red Blood Cells. Do They Influence Intracellular Signaling? Cell. Physiol. Biochem. 2019, 53, 186–199. [Google Scholar] [CrossRef]
- Maselli, A.; Pierdominici, M.; Vitale, C.; Ortona, E. Membrane lipid rafts and estrogenic signalling: A functional role in the modulation of cell homeostasis. Apoptosis 2015, 20, 671–678. [Google Scholar] [CrossRef]
- Fraser, M.; Matuschewski, K.; Maier, A.G. Of membranes and malaria: Phospholipid asymmetry in Plasmodium falciparum-infected red blood cells. Cell. Mol. Life Sci. 2021, 78, 4545–4561. [Google Scholar] [CrossRef]
- Subbamanda, Y.D.; Bhargava, A. Intercommunication between Voltage-Gated Calcium Channels and Estrogen Receptor/Estrogen Signaling: Insights into Physiological and Pathological Conditions. Cells 2022, 11, 3850. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef] [PubMed]
- Alghareeb, S.A.; Alfhili, M.A.; Fatima, S. Molecular Mechanisms and Pathophysiological Significance of Eryptosis. Int. J. Mol. Sci. 2023, 24, 5079. [Google Scholar] [CrossRef] [PubMed]
- Leo, F.; Hutzler, B.; Ruddiman, C.A.; Isakson, B.E.; Cortese-Krott, M.M. Cellular microdomains for nitric oxide signaling in endothelium and red blood cells. Nitric Oxide 2020, 96, 44–53. [Google Scholar] [CrossRef]
- Bardyn, M.; Crettaz, D.; Rappaz, B.; Hamelin, R.; Armand, F.; Tissot, J.D.; Turcatti, G.; Prudent, M. Phosphoproteomics and morphology of stored human red blood cells treated by protein tyrosine phosphatases inhibitor. Blood Adv. 2024, 8, 1–13. [Google Scholar] [CrossRef]
- Spinelli, S.; Marino, A.; Morabito, R.; Remigante, A. Interplay Between Metabolic Pathways and Increased Oxidative Stress in Human Red Blood Cells. Cells 2024, 13, 2026. [Google Scholar] [CrossRef]
- Djordjevic, V.V.; Kostic, J.; Krivokapic, Z.; Krtinic, D.; Rankovic, M.; Petkovic, M.; Cosic, V. Decreased Activity of Erythrocyte Catalase and Glutathione Peroxidase in Patients with Schizophrenia. Medicina 2022, 58, 1491. [Google Scholar] [CrossRef] [PubMed]
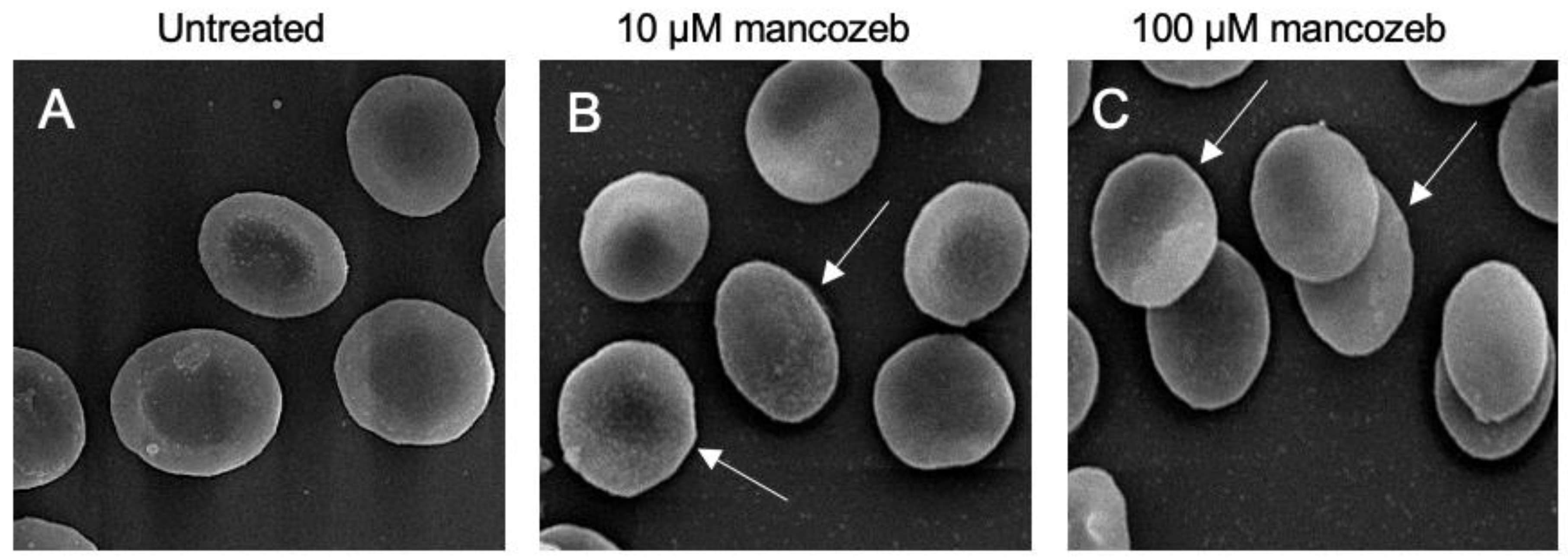



| Experimental Conditions | Biconcave Shape | Leptocytes | |
|---|---|---|---|
| % | % | RBC Volume | |
| Untreated | 84% ± 0.018 | 16% ± 0.010 | 419.5 ± 0.015 |
| 10 µM mancozeb | 59% ± 0.021 | 41% ± 0.011 *** | 412 ± 0.013 ns |
| 100 µM mancozeb | 52% ± 0.022 | 48% ± 0.09 *** | 380.5 ± 0.014 * |
| 10 µM Mancozeb Δ% | 100 µM Mancozeb Δ% | |
|---|---|---|
| Glycophorin A+ EVs | −25 ± 0.021 | −23.4 ± 0.017 |
| Annexin-V+ EVs | −20 ± 0.025 | −18 ± 0.022 |
| Glycophorin A+/Annexin-V+ EVs | −35.4 ± 0.026 | −34.5 ± 0.020 |
| Experimental Condition | Rate Constant (min−1) | Time (min) | [SO42−] Internalized After 45 min Incubation in SO42− Medium ([SO42−] L Cells × 10−2) | n |
|---|---|---|---|---|
| Untreated | 0.056 ± 0.005 | 17.71 ±1.76 | 290.62 ± 5.12 | 10 |
| 10 µM mancozeb | 0.079 ± 0.004 | 12.49 ± 2.02 * | 272.5 ± 4.31 *** | 10 |
| 100 µM mancozeb | 0.043 ± 0.005 | 23.06 ± 1.91 *,° | 270 ± 7.15 *** | 10 |
| 10 µM DIDS | 0.030 ± 0.001 | 35.18 ± 0.89 *** | 12.03 ± 0.28 *** | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinelli, S.; Straface, E.; Gambardella, L.; Bozzuto, G.; Caruso, D.; Marino, A.; Dossena, S.; Morabito, R.; Remigante, A. Mechanistic Insights into Mancozeb-Induced Redox Imbalance and Structural Remodelling Affecting the Function of Human Red Blood Cells. Antioxidants 2025, 14, 1274. https://doi.org/10.3390/antiox14111274
Spinelli S, Straface E, Gambardella L, Bozzuto G, Caruso D, Marino A, Dossena S, Morabito R, Remigante A. Mechanistic Insights into Mancozeb-Induced Redox Imbalance and Structural Remodelling Affecting the Function of Human Red Blood Cells. Antioxidants. 2025; 14(11):1274. https://doi.org/10.3390/antiox14111274
Chicago/Turabian StyleSpinelli, Sara, Elisabetta Straface, Lucrezia Gambardella, Giuseppina Bozzuto, Daniele Caruso, Angela Marino, Silvia Dossena, Rossana Morabito, and Alessia Remigante. 2025. "Mechanistic Insights into Mancozeb-Induced Redox Imbalance and Structural Remodelling Affecting the Function of Human Red Blood Cells" Antioxidants 14, no. 11: 1274. https://doi.org/10.3390/antiox14111274
APA StyleSpinelli, S., Straface, E., Gambardella, L., Bozzuto, G., Caruso, D., Marino, A., Dossena, S., Morabito, R., & Remigante, A. (2025). Mechanistic Insights into Mancozeb-Induced Redox Imbalance and Structural Remodelling Affecting the Function of Human Red Blood Cells. Antioxidants, 14(11), 1274. https://doi.org/10.3390/antiox14111274










