Untargeted Chemical Profile, Antioxidant, and Enzyme Inhibition Activity of Physalis angulata L. from the Peruvian Amazon: A Contribution to the Validation of Its Pharmacological Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Extracts
2.3. LC Parameters and MS Parameters
2.4. LC Total Phenolic (TP)
2.5. Antioxidant Activity
2.5.1. DPPH Scavenging Activity
2.5.2. ABTS Bleaching Capacity
2.5.3. Ferric-Reducing Antioxidant Power Assay (FRAP)
2.6. Enzymatic Inhibitory Activity
2.6.1. Cholinesterase Inhibition Assay
2.6.2. α-Glucosidase Inhibition Assay
2.6.3. α-Amylase Inhibition Assay
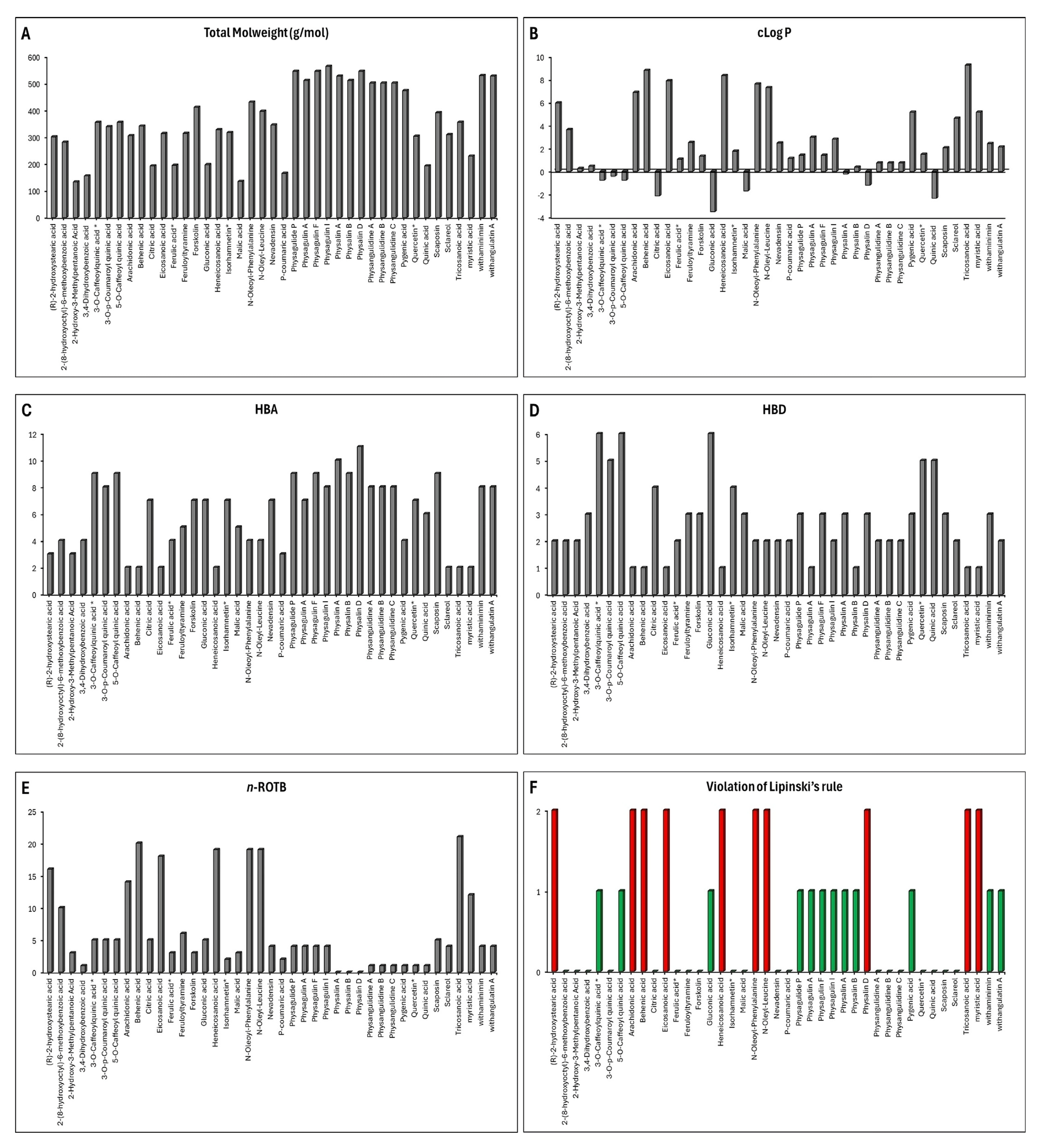
2.7. Calculation of ADME Parameters
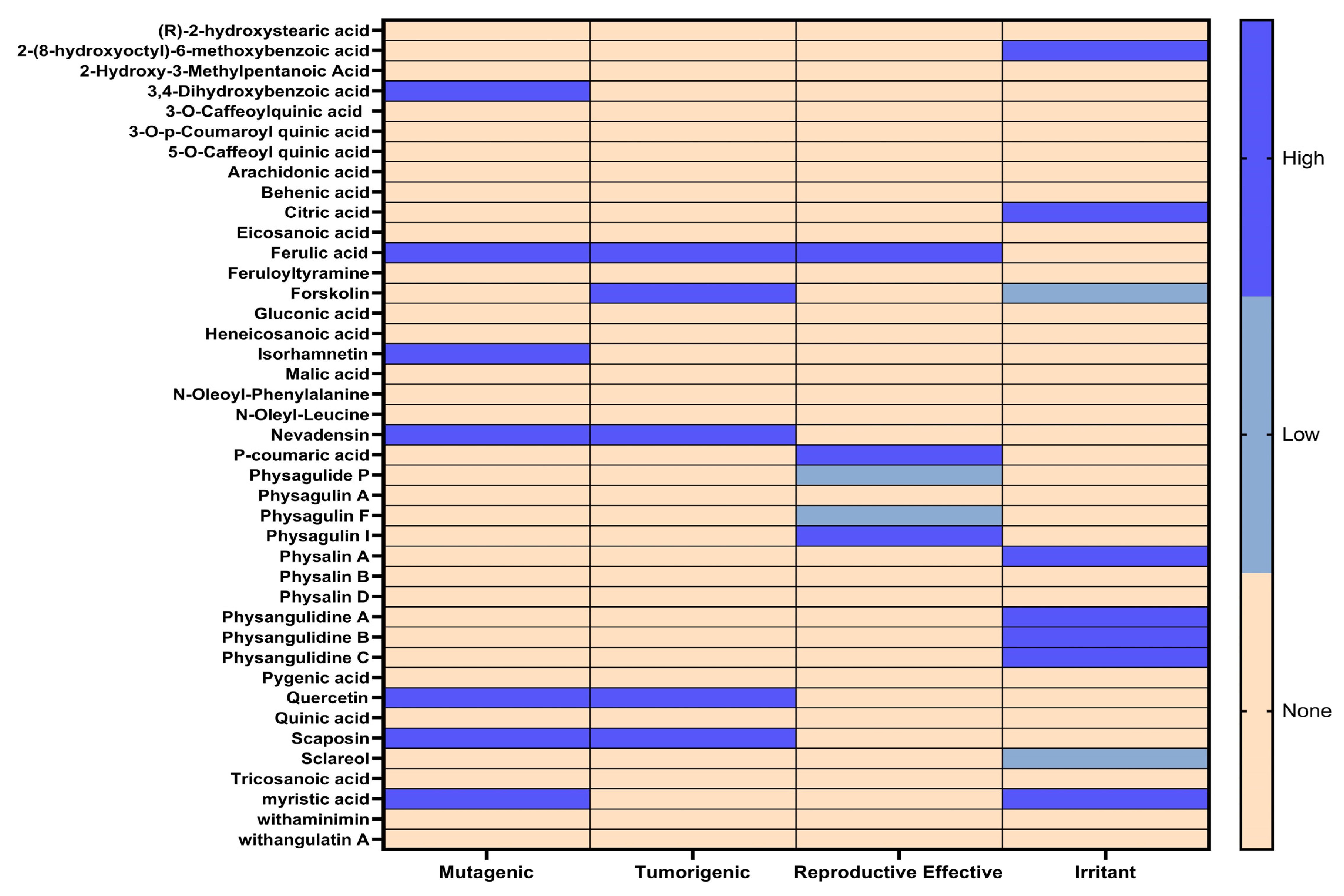
2.8. Calculation of Risk Toxicity
2.9. In Silico Analysis
2.10. Statistical Analysis
3. Results
3.1. Untargeted Chemical Profile of P. angulata Extracts
3.2. Total Phenolic Contents and Antioxidant Activity
3.3. Enzyme Inhibition Activity
3.4. ADME Prediction
3.5. Toxixity Prediction
4. Discussion
4.1. Chemical Composition
4.2. Antioxidant Properties
4.3. Enzymatic Inhibition
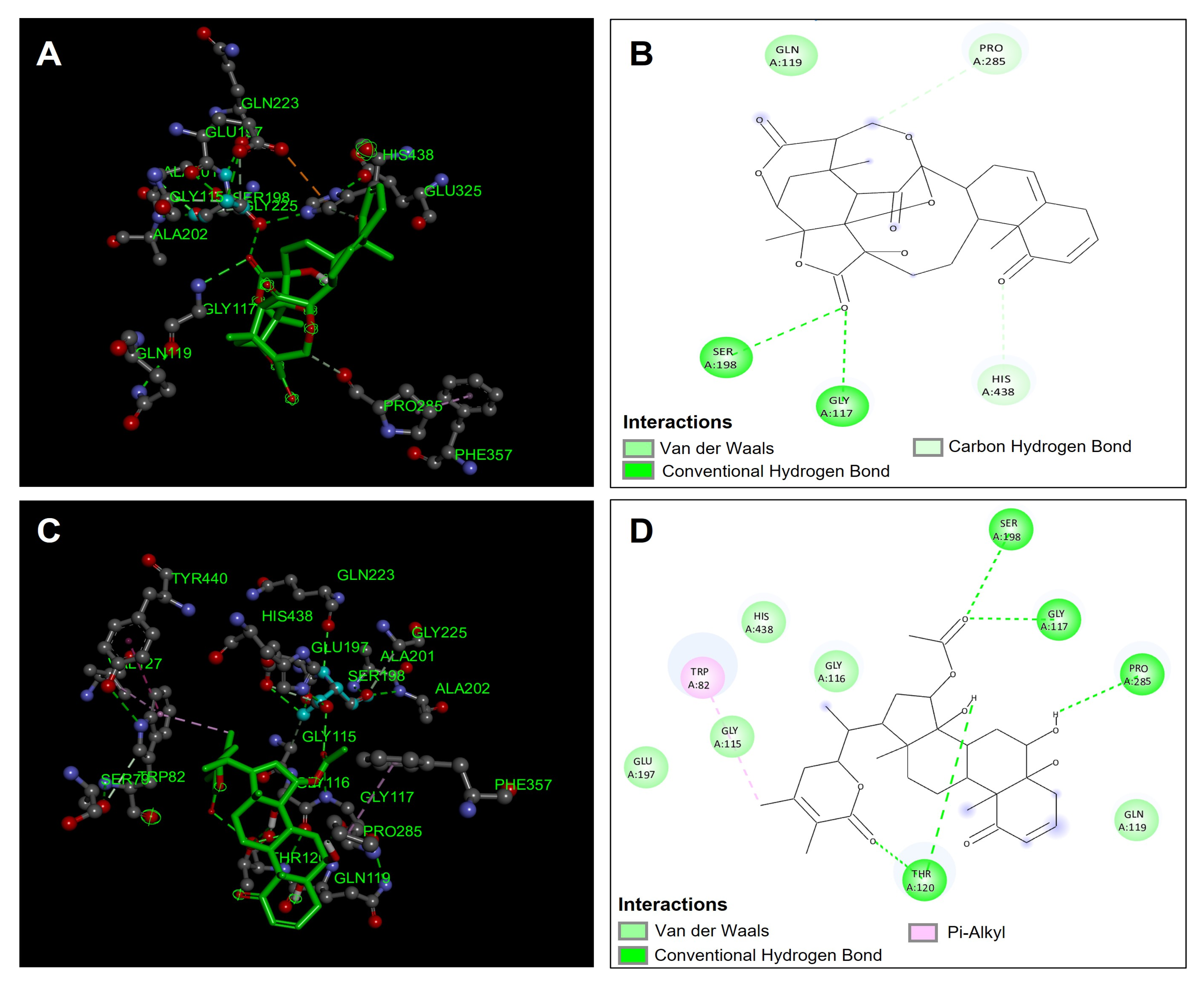
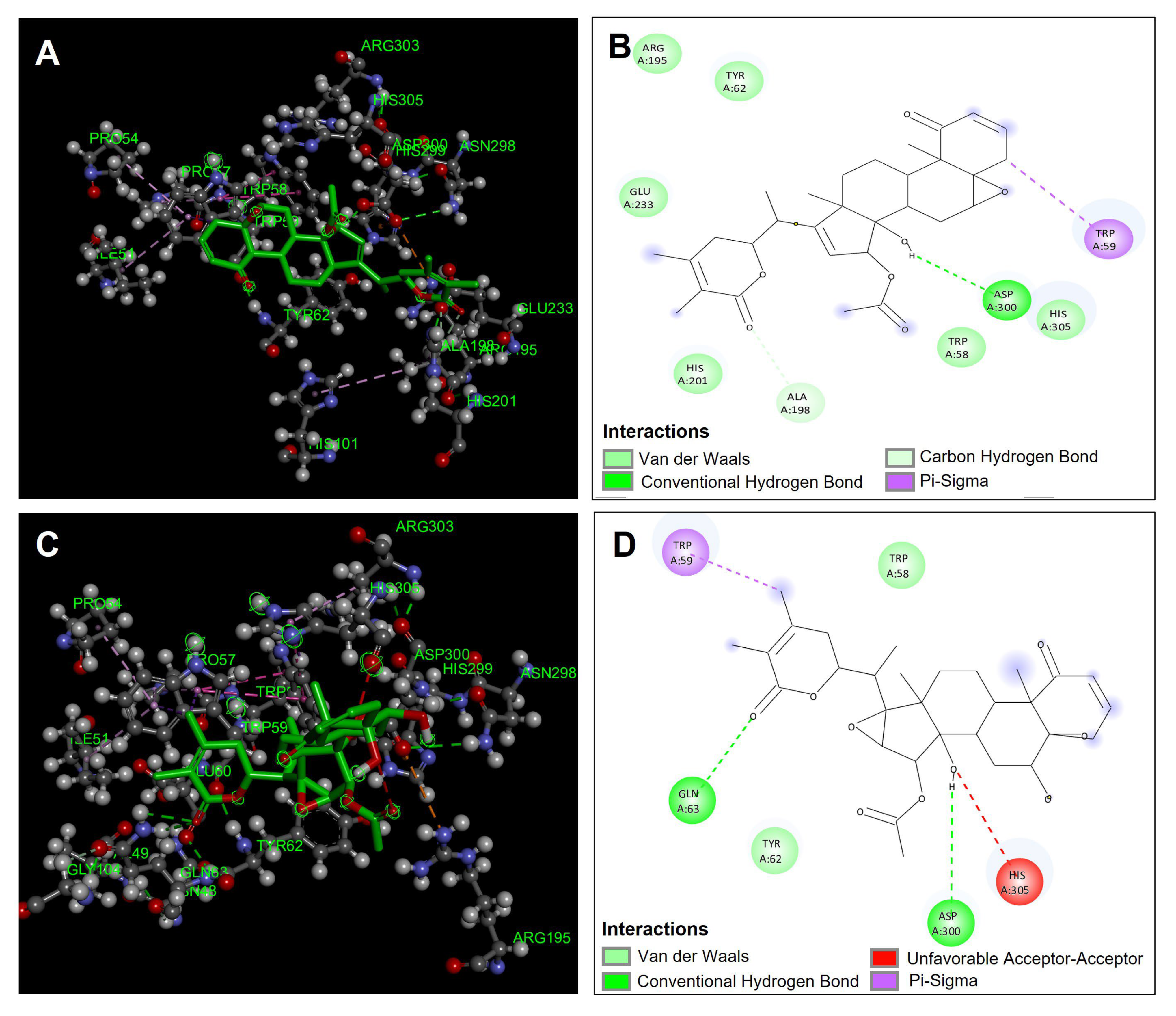
4.4. Docking Studies
4.4.1. Acetylcholinesterase (TcAChE) Molecular Docking
4.4.2. Butyrylcholinesterase (hBuChE) Molecular Docking
4.4.3. α-Amylase Molecular Docking
4.4.4. α-Glucosidase Molecular Docking
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arruda, J.C.C.; Rocha, N.C.; Santos, E.G.; Ferreira, L.G.B.; Bello, M.L.; Penido, C.; Costa, T.E.M.M.; Santos, J.A.A.; Ribeiro, I.M.; Tomassini, T.C.B.; et al. Physalin pool from Physalis angulata L. leaves and physalin D inhibit P2X7 receptor function in vitro and acute lung injury in vivo. Biomed. Pharmacother. 2021, 142, 112006. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Padilla, I.; Zamora-Tavares, M.D.P.; Ruiz-Sánchez, E.; Pérez-Alquicira, J.; Vargas-Ponce, O. Characterization of the plastome of Physalis cordata and comparative analysis of eight species of Physalis sensu stricto. PhytoKeys 2022, 210, 109–134. [Google Scholar] [CrossRef]
- Rengifo-Salgado, E.; Vargas-Arana, G. Physalis angulata L. (bolsa mullaca): A review of its traditional uses, chemistry and pharmacology. Bol. Latinoam. Caribe Plantas Med. Aromát. 2013, 12, 431–445. [Google Scholar]
- da Silva Ramos, C.A.; Soares, T.L.; Santos, N.; Pelacani, C.R. Influence of maturity stage on physical and chemical characteristics of fruit and physiological quality of seeds of Physalis angulata L. Sci. Hortic. 2021, 284, 110124. [Google Scholar] [CrossRef]
- Morales Saavedra, J.; Rodriguez, F.A.; Cabrera, D.; Sanchez, C.V.; Vargas-Ponce, O. Agromorphological characterization of wild and weedy populations of Physalis angulata in Mexico. Sci. Hortic. 2019, 246, 86–94. [Google Scholar] [CrossRef]
- Tuan, H.L.; Thao, D.T.; Dung, D.T.; Kiem, P.V.; Quang, T.H.; Hai, P.T.; Tuan, D.T.; Cuong, P.V.; Viet, L.C.; Hung, T.M. Phytochemical constituents and cytotoxic activity of Physalis angulta L. growing in Vietnam. Phytochem. Lett. 2018, 27, 193–196. [Google Scholar] [CrossRef]
- Wang, P.; Yang, X.M.; Hu, Z.X.; Li, Y.N.; Yang, J.; Hao, X.J.; Yuan, C.M.; Yi, P. UPLC-Q-Orbitrap-MS/MS-Guided isolation of bioactive withanolides from the fruits of Physalis angulata. J. Agric. Food Chem. 2023, 71, 16581–16592. [Google Scholar] [CrossRef]
- Odusina, B.O.; Onocha, P.A. A new squalene derivative from Physalis angulata L. (Solanaceae). Nat. Prod. Res. 2022, 36, 2154–2157. [Google Scholar] [CrossRef]
- Boonsombat, J.; Chawengrum, P.; Mahidol, C.; Kittakoop, P.; Ruchirawat, S.; Thongnest, S. A new 22,26-seco physalin steroid from Physalis angulata. Nat. Prod. Res. 2020, 34, 1097–1104. [Google Scholar] [CrossRef]
- Tuan Anh, H.L.; Le Ba, V.; Do, T.T.; Phan, V.K.; Pham Thi, H.Y.; Bach, L.G.; Tran, M.H.; Tran Thi, P.A.; Kim, Y.H. Bioactive compounds from Physalis angulata and their anti-inflammatory and cytotoxic activities. J. Asian Nat. Prod. Res. 2021, 23, 809–817. [Google Scholar] [CrossRef]
- Hua, C.; Xu, Z.; Tang, N.; Xu, Y.; Zhang, Y.; Li, C. Identification of P450 candidates associated with the biosynthesis of physalin-class compounds in Physalis angulata. Int. J. Mol. Sci. 2023, 24, 14077. [Google Scholar] [CrossRef]
- Meira, C.S.; Soares, J.W.C.; Dos Reis, B.P.Z.C.; Pacheco, L.V.; Santos, I.P.; Silva, D.K.C.; de Lacerda, J.C.; Daltro, S.R.T.; Guimarães, E.T.; Soares, M.B.P. Therapeutic applications of physalins: Powerful natural weapons. Front. Pharmacol. 2022, 13, 864714. [Google Scholar] [CrossRef]
- Meng, Q.; Fan, J.; Liu, Z.; Li, X.; Zhang, F.; Zhang, Y.; Sun, Y.; Li, L.; Liu, X.; Hua, E. Cytotoxic withanolides from the whole herb of Physalis angulata L. Molecules 2019, 24, 1608. [Google Scholar] [CrossRef]
- do Espírito Santo, R.F.; Lima, M.D.S.; Juiz, P.J.L.; Opretzka, L.C.F.; Nogueira, R.C.; Ribeiro, I.M.; Tomassini, T.C.B.; Soares, M.B.P.; Villarreal, C.F. Physalis angulata concentrated ethanolic extract suppresses nociception and inflammation by modulating cytokines and prostanoids pathways. Nat. Prod. Res. 2021, 35, 4675–4679. [Google Scholar] [CrossRef] [PubMed]
- Daltro, S.R.T.; Santos, I.P.; Barros, P.L.; Moreira, D.R.M.; Tomassini, T.C.B.; Ribeiro, I.M.; Ribeiro Dos Santos, R.; Meira, C.S.; Soares, M.B.P. In vitro and In Vivo Immunomodulatory Activity of Physalis angulata Concentrated Ethanolic Extract. Planta Med. 2021, 87, 160–168. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hsiao, Y.H.; Ng, K.L.; Kuo, Y.H.; Lim, Y.P.; Hsieh, W.T. Physalin A attenuates inflammation through down-regulating c-Jun NH2 kinase phosphorylation/Activator Protein 1 activation and up-regulating the antioxidant activity. Toxicol. Appl. Pharmacol. 2020, 402, 115115. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.; Wan, H.; Hou, J.; Lee, D.; Xu, J.; Guo, Y. Anti-inflammatory withanolides from the aerial parts of Physalis minima. Phytochemistry 2022, 202, 113301. [Google Scholar] [CrossRef]
- Rivera, D.E.; Ocampo, Y.C.; Castro, J.P.; Barrios, L.; Diaz, F.; Franco, L.A. A screening of plants used in Colombian traditional medicine revealed the anti-inflammatory potential of Physalis angulata calyces. Saudi J. Biol. Sci. 2019, 26, 1758–1766. [Google Scholar] [CrossRef]
- Rivera, D.; Ocampo, Y.; Franco, L.A. Physalis angulata calyces modulate macrophage polarization and alleviate chemically induced intestinal inflammation in mice. Biomedicines 2020, 8, 24. [Google Scholar] [CrossRef]
- Chairissy, M.D.; Wulandari, L.R.; Sujuti, H. Pro-apoptotic and anti-proliferative effects of Physalis angulata leaf extract on retinoblastoma cells. Int. J. Ophthalmol. 2019, 12, 1402–1407. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Zhao, Y.; Ren, C.; Gu, M.; Zhang, H.; Wu, P.; Wang, Y.; Kong, L.; Han, C. Target separation and potential anticancer activity of withanolide-based glucose transporter protein 1 inhibitors from Physalis angulata var. villosa. J. Nat. Prod. 2024, 87, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Dewi, S.; Isbagio, H.; Purwaningsih, E.H.; Kertia, N.; Setiabudy, R.; Setiati, S. A Double-blind, Randomized Controlled Trial of Ciplukan (Physalis angulata Linn) Extract on Skin Fibrosis, Inflammatory, Immunology, and Fibrosis Biomarkers in Scleroderma Patients. Acta Med. Indones. 2019, 51, 303–310. [Google Scholar] [PubMed]
- Yang, J.; Tian, J.; Yang, Y.; Zhu, Y.; Li, C.; Zhang, Y. RNAi of sterol Δ24-isomerase implicated its involvement in physalin biosynthesis in Physalis angulata L. Front. Plant Sci. 2022, 13, 850711. [Google Scholar] [CrossRef]
- Zhan, X.; Luo, X.; He, J.; Zhang, C.; Liao, X.; Xu, X.; Feng, S.; Yu, C.; Jiang, Z.; Meng, Y.; et al. Bioactive compounds induced in Physalis angulata L. by methyl-jasmonate: An investigation of compound accumulation patterns and biosynthesis-related candidate genes. Plant Mol. Biol. 2020, 103, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Palupi, K.D.; Ilyas, M.; Agusta, A. Endophytic fungi inhabiting Physalis angulata L. plant: Diversity, antioxidant, and antibacterial activities of their ethyl acetate extracts. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 823–829. [Google Scholar] [CrossRef]
- Trujillo-Pahua, V.; Vargas-Ponce, O.; Rodríguez-Zaragoza, F.A.; Ordaz-Ortiz, J.J.; Délano-Frier, J.P.; Winkler, R.; Sánchez-Hernández, C.V. Metabolic response to larval herbivory in three Physalis species. Plant Signal Behav. 2021, 16, 1962050. [Google Scholar] [CrossRef]
- Feng, S.; Zheng, K.; Jiao, K.; Cai, Y.; Chen, C.; Mao, Y.; Wang, L.; Zhan, X.; Ying, Q.; Wang, H. Complete chloroplast genomes of four Physalis species (Solanaceae): Lights into genome structure, comparative analysis, and phylogenetic relationships. BMC Plant Biol. 2020, 20, 242. [Google Scholar] [CrossRef]
- Feng, S.; Jiao, K.; Zhang, Z.; Yang, S.; Gao, Y.; Jin, Y.; Shen, C.; Lu, J.; Zhan, X.; Wang, H. Development of Chloroplast Microsatellite Markers and Evaluation of Genetic Diversity and Population Structure of Cutleaf Groundcherry (Physalis angulata L.) in China. Plants 2023, 12, 1755. [Google Scholar] [CrossRef]
- Zhan, X.; Zhang, Z.; Zhang, Y.; Gao, Y.; Jin, Y.; Shen, C.; Wang, H.; Feng, S. Complete plastome of Physalis angulata var. villosa, gene organization, comparative genomics and phylogenetic relationships among Solanaceae. Genes 2022, 13, 2291. [Google Scholar]
- Preet, R.; Gupta, R.C. Quantification of withaferin-A and withanolide-A in diploid (n = 12) and tetraploid cytotypes (n = 24) of “Rassbhary”, Physalis angulata L. Nat. Prod. Res. 2019, 33, 3157–3160. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Zhang, Y. Engineering of Saccharomyces cerevisiae for 24-methylene-cholesterol production. Biomolecules 2021, 11, 1710. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrinia, N.; Proteggente, A.; Pannalaa, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Liu, L.; Deseo, M.A.; Morris, C.; Winter, K.M.; Leach, D.N. Investigation of α-glucosidase inhibitory activity of wheat bran and germ. Food Chem. 2011, 126, 553–561. [Google Scholar] [CrossRef]
- Ali, H.; Houghton, P.J.; Soumyanath, A. Alpha-amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef]
- Ley-Martínez, J.S.; Ortega-Valencia, J.E.; García-Barradas, O.; Jiménez-Fernández, M.; Uribe-Lam, E.; Vencedor-Meraz, C.I.; Oliva-Ramírez, J. Active compounds in Zingiber officinale as possible redox inhibitors of 5-lipoxygenase using an in silico approach. Int. J. Mol. Sci. 2022, 23, 6093. [Google Scholar] [CrossRef]
- Zhao, Y.; Abraham, M.; Le, J.; Hersey, A.; Luscombe, C.; Beck, G.; Sherborne, B.; Cooper, I. Rate limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef]
- Torres-Benítez, A.; Ortega-Valencia, J.E.; Sánchez, M.; Hillmann-Eggers, M.; Gómez Serranillos, M.P.; Vargas-Arana, G.; Simirgiotis, M.J. UHPLC-MS chemical fingerprinting and antioxidant, enzyme inhibition, anti-inflammatory in silico and cytoprotective activities of Cladonia chlorophaea and C. gracilis (Cladoniaceae) from Antarctica. Antioxidants 2023, 12, 10. [Google Scholar] [CrossRef]
- Torres-Benítez, A.; Ortega-Valencia, J.E.; Sanchez, M.; Divakar, P.K.; Simirgiotis, M.J.; Gómez-Serranillos, M.P. Meta- bolomic profiling, antioxidant and enzyme inhibition properties and molecular docking analysis of Antarctic Lichens. Molecules 2022, 27, 8086. [Google Scholar] [CrossRef]
- Swargiary, A.; Daimari, M. Identification of bioactive compounds by GC-MS and α-amylase and α-glucosidase inhibitory activity of Rauvolfia tetraphylla L. and Oroxylum indicum (L.) Kurz: An in vitro and in silico approach. Clin. Phytosci. 2020, 6, 75. [Google Scholar] [CrossRef]
- Trang, H.M.V.; Duy, D.V.; Dat, V.T.; Phuong, T.V.N.; Dao, T.T. Virtual screening, oriented-synthesis and evaluation of lipase inhibitory activity of benzyl amino chalcone derivatives. MedPharmRes 2017, 1, 26–36. [Google Scholar] [CrossRef]
- Silman, I.; Harel, M.; Axelsen, P.; Raves, M.; Sussman, J.L. Three dimensional structures of acetylcholinesterase and of its complexes with anticholinesterase agents. Biochem. Soc. Trans. 1994, 22, 745–749. [Google Scholar] [CrossRef]
- BIOVIA. Available online: https://www.3ds.com/products/biovia/discovery-studio/simulations (accessed on 24 April 2024).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Huang, M.; He, J.X.; Hu, H.X.; Zhang, K.; Wang, X.N.; Zhao, B.B.; Lou, H.X.; Ren, D.M.; Shen, T. Withanolides from the genus Physalis: A review on their phytochemical and pharmacological aspects. J. Pharm. Pharmacol. 2020, 72, 649–669. [Google Scholar] [CrossRef]
- Da Silva, B.J.M.; Pereira, S.W.G.; Rodrigues, A.P.D.; Do Nascimento, J.L.M.; Silva, E.O. In vitro antileishmanial effects of Physalis angulata root extract on Leishmania infantum. J. Integr. Med. 2018, 16, 404–410. [Google Scholar] [CrossRef]
- Vieceli, P.S.; Juiz, P.J.L.; Lauria, P.S.S.; Couto, R.D.; Tomassini, T.C.B.; Ribeiro, I.M.; Soares, M.B.P.; Villarreal, C.F. Physalis angulata reduces the progression of chronic experimental periodontitis by immunomodulatory mechanisms. J. Ethnopharmacol. 2021, 273, 113986. [Google Scholar] [CrossRef]
- Taek, M.M.; Tukan, G.D.; Prajogo, B.E.W.; Agil, M. Antiplasmodial activity and phytochemical constituents of selected antimalarial plants used by native people in west Timor Indonesia. Turk. J. Pharm. Sci. 2021, 18, 80–90. [Google Scholar] [CrossRef]
- Jang, Y.S.; Wang, Z.; Lee, J.M.; Lee, J.Y.; Lim, S.S. Screening of Korean natural products for anti-adipogenesis properties and isolation of kaempferol-3-o-rutinoside as a potent anti-adipogenetic compound from Solidago virgaurea. Molecules 2016, 21, 226. [Google Scholar] [CrossRef]
- Sebastiani, B.; Falcinelli, S. Contamination of plants from Amazonia by environmental pollution. Environments 2018, 5, 33. [Google Scholar] [CrossRef]
- Siahaan, O.G.; Sibarani, R.; Lubis, S.; Purwoko, A. Herbal medicines for women and children’s health in Tipang Village, District Humbang Hasundutan, North Sumatera. Gac. Sanit. 2021, 35 (Suppl. 2), S564–S566. [Google Scholar] [CrossRef]
- Suwarsa, O.; Dharmadji, H.P.; Rohmawaty, E.; Mareta, S.; Gunawan, H.; Dwiyana, R.F.; Achdiat, P.A.; Sutedja, E.; Pangastuti, M. The Efficacy of topical formulation containing ciplukan (Physalis angulata Linn.) in modulating interleukin-17 and interferon gamma expression in mice (Mus. musculus) Psoriasis model. J. Exp. Pharmacol. 2023, 15, 367–374. [Google Scholar] [CrossRef]
- Widiatmoko, A.; Fitri, L.E.; Endharti, A.T.; Murlistyarini, S.; Brahmanti, H.; Yuniaswan, A.P.; Ekasari, D.P.; Rasyidi, F.; Nahlia, N.L.; Safitri, P.R. Inhibition effect of Physalis angulata leaf extract on viability, collagen type I, and tissue inhibitor of metalloproteinase 1 (TIMP-1) but not plasminogen activator inhibitor-1 (PAI-1) of keloid fibroblast culture. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2365–2373. [Google Scholar] [CrossRef]
- Ding, H.; Hu, Z.; Yu, L.; Ma, Z.; Ma, X.; Chen, Z.; Wang, D.; Zhao, X. Induction of quinone reductase (QR) by withanolides isolated from Physalis angulata L. var. villosa Bonati (Solanaceae). Steroids 2014, 86, 32–38. [Google Scholar] [CrossRef]
- Pinto, L.A.; Meira, C.S.; Villarreal, C.F.; Vannier-Santos, M.A.; de Souza, C.V.; Ribeiro, I.M.; Tomassini, T.C.; Galvão-Castro, B.; Soares, M.B.; Grassi, M.F. Physalin F, a seco-steroid from Physalis angulata L., has immunosuppressive activity in peripheral blood mononuclear cells from patients with HTLV1-associated myelopathy. Biomed. Pharmacother. 2016, 79, 129–134. [Google Scholar] [CrossRef]
- Jin, Z.; Mashuta, M.S.; Stolowich, N.J.; Vaisberg, A.J.; Stivers, N.S.; Bates, P.J.; Lewis, W.H.; Hammond, G.B. Physangulidines A, B, and C: Three new antiproliferative withanolides from Physalis Angulata L. Org. Lett. 2012, 14, 1230–1233. [Google Scholar] [CrossRef]
- Fang, C.; Chen, C.; Yang, Y.; Li, K.; Gao, R.; Xu, D.; Huang, Y.; Chen, Z.; Liu, Z.; Chen, S.; et al. Physalin B inhibits cell proliferation and induces apoptosis in undifferentiated human gastric cancer HGC-27 cells. Asia Pac. J. Clin. Oncol. 2022, 18, 224–231. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.; Zhao, J.; Yang, H.; Yin, F.; Ding, M.; Luo, J.; Wang, X.; Kong, L. Design and SAR of withangulatin A analogues that act as covalent TrxR inhibitors through the Michael addition reaction showing potential in cancer treatment. J. Med. Chem. 2020, 63, 11195–11214. [Google Scholar] [CrossRef]
- Okmanov, R.Y.; Makhmudova, M.M.; Bobaev, I.D.; Tashkhodjaev, B. Withanolides from Physalis angulata L. Acta Crystallogr. E Crystallogr. Commun. 2021, 77, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.Z.; Shan, S.M.; Zhang, W.; Luo, J.G.; Kong, L.Y. Withanolides from Physalis minima and their inhibitory effects on nitric oxide production. Steroids 2014, 82, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, S.; Wang, L.; Xin, M.; Xu, Y.; Wang, G.; Chen, D.; Chen, L.; Liu, S.; Zhao, F. Anti-inflammatory effects of three withanolides isolated from Physalis angulata L. in LPS-activated RAW 264.7 cells through blocking NF-κB signaling pathway. J. Ethnopharmacol. 2021, 276, 114186. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.P.; Qiu, C.Y.; Zhao, F.; Kang, N.; Chen, L.X.; Qiu, F. Physalins V-IX, 16,24-cyclo-13,14-seco withanolides from Physalis angulata and their antiproliferative and anti-inflammatory activities. Sci. Rep. 2017, 7, 4057. [Google Scholar] [CrossRef]
- Tan, Y.H.; Cai, Y.X.; Kong, M.; Li, Y.L.; Yu, Z.P.; Kong, L.Y.; Luo, J.G. Withanolides from Physalis angulata and their inhibitory effects on nitric oxide production. Chem. Biodivers. 2023, 20, e202300195. [Google Scholar] [CrossRef]
- Lem, F.F.; Yong, Y.S.; Goh, S.; Chin, S.N.; Chee, F.T. Withanolides, the hidden gem in Physalis minima: A mini review on their anti-inflammatory, anti-neuroinflammatory and anti-cancer effects. Food Chem. 2022, 377, 132002. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Zhao, J.; Wang, R.; Xia, Z.; Li, X.; Liu, Y.; Xu, Q.; Khan, I.A.; Yang, S. Anti-inflammatory and cytotoxic withanolides from Physalis minima. Phytochemistry 2018, 155, 164–170. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, T.; Yu, M.; Jia, H.; Zhang, H.; Xu, Q.; Gu, Y.; Zou, Z. Anti-inflammatory Withanolides from Physalis minima. ACS Omega 2020, 5, 12148–12153. [Google Scholar] [CrossRef]
- Lin, R.; Guan, Y.Z.; Li, R.J.; Xu, X.M.; Luo, J.G.; Kong, L.Y. 13,14-seco-withanolides from Physalis minima with potential anti-inflammatory activity. Chem. Biodivers. 2016, 13, 884–890. [Google Scholar] [CrossRef]
- Wei, S.S.; Gao, C.Y.; Li, R.J.; Kong, L.Y.; Luo, J. Withaminimas A-F, six withanolides with potential anti-inflammatory activity from Physalis minima. Chin. J. Nat. Med. 2019, 17, 469–474. [Google Scholar] [CrossRef]
- Park, H.J.; Shim, H.S.; Han, A.R.; Seo, E.K.; Kim, K.R.; Han, B.H.; Shim, I. Anti-Inflammatory effect of three isolated compounds of Physalis alkekengi var. franchetii (PAF) in lipopolysaccharide-activated raw 264.7 cells. Curr. Issues Mol. Biol. 2022, 44, 1407–1416. [Google Scholar] [PubMed]
- Xu, Y.M.; Wijeratne, E.M.K.; Brooks, A.D.; Tewary, P.; Xuan, L.J.; Wang, W.Q.; Sayers, T.J.; Gunatilaka, A.A.L. Cytotoxic and other withanolides from aeroponically grown Physalis philadelphica. Phytochemistry 2018, 152, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna Pillai, J.; Wali, A.F.; Menezes, G.A.; Rehman, M.U.; Wani, T.A.; Arafah, A.; Zargar, S.; Mir, T.M. Chemical composition analysis, cytotoxic, antimicrobial and antioxidant activities of Physalis angulata L.: A comparative study of leaves and fruit. Molecules 2022, 27, 1480. [Google Scholar] [CrossRef] [PubMed]
- Ralte, L.; Bhardwaj, U.; Singh, Y.T. Traditionally used edible Solanaceae plants of Mizoram, India have high antioxidant and antimicrobial potential for effective phytopharmaceutical and nutraceutical formulations. Heliyon 2021, 7, e07907. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.G.B.; Montenegro, J.; Abreu, J.P.; Santos, M.C.B.; Nascimento, T.P.D.; Santos, M.D.S.; Ferreira, A.G.; Cameron, L.C.; Ferreira, M.S.L.; Teodoro, A.J. Metabolite Profiling by UPLC-MSE, NMR, and Antioxidant Properties of Amazonian Fruits: Mamey Apple (Mammea Americana), Camapu (Physalis angulata), and Uxi (Endopleura Uchi). Molecules 2020, 25, 342. [Google Scholar] [CrossRef]
- Widhanti, A.; Iwansyah, A.C.; Yelliantty; Kurniawan, T.; Pramareti, G.M.J.; Indriati, A.; Hamid, H.A. Effects of foam mat-drying condition on physicochemical and antioxidant properties of instant Physalis angulata L. enriched with Moringa oleifera L. extract. An. Acad. Bras. Cienc. 2024, 96 (Suppl. 3), e20240006. [Google Scholar] [CrossRef]
- Vargas-Arana, G.; Rengifo-Salgado, E.; Simirgiotis, M. Antidiabetic potential of medicinal plants from the Peruvian Amazon: A review. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2023, 22, 277–300. [Google Scholar] [CrossRef]




| Enzymes | Grid Box Size (Å) | Grid Center Coordinate | ||||
|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | |
| Acetylcholinesterase | 30 | 30 | 30 | 3.67 | 65.99 | 64.08 |
| Butyrylcholinesterase | 30 | 30 | 30 | 134.11 | 115.17 | 38.13 |
| α-Amylase | 50 | 50 | 50 | 12.37 | 48.13 | 26.24 |
| α-Glucosidase | 40 | 40 | 40 | −20.83 | −6.56 | −5.04 |
| Peak | Retention Time (min) | UV Max | Tentative Identification | [M − H]− | Measured Mass (m/z) | Theoretical Mass (m/z) | Accuracy (ppm) | MS Ions (ppm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.45 | - | Na formiate (internal standard) | C4H2O4 | 112.9829 | 112.9856 | 3.1 | - |
| 2 | 0.82 | 273 | p-Coumaric acid | C9H8O3 | 164.04706 | 163.03978 | −2.36 | - |
| 3 | 0.98 | 227-272 | Gluconic acid | C6H12O7 | 195.05058 | 195.04993 | 3.351 | - |
| 4 | 3.43 | 229-274 | Malic acid | C4H6O5 | 133.01355 | 133.01315 | 3.008 | - |
| 5 | 4.54 | 227 | Citric acid | C6H8O7 | 191.01933 | 191.01863 | 3.684 | - |
| 6 | 4.86 | 273 | Quinic acid | C7H12O6 | 192.06287 | 191.05559 | −1.37 | - |
| 7 | 5.07 | 227-265 | 3,4-Dihydroxybenzoic acid (protocatechuic acid) | C7H6O4 | 154.02654 | 153.01927 | 2.987 | - |
| 8 | 5.16 | 196-227 | 2-(8-Hydroxyoctyl)-6-methoxybenzoic acid | C16H24O4 | 280.16649 | 279.15922 | −3.46 | - |
| 9 | 5.24 | 196-204-269 | Feruloyltyramine | C18H19NO4 | 313.13105 | 312.12377 | −1.07 | - |
| 10 | 5.54 | 227-257-312 | 3-O-Caffeoylquinic acid * | C16H17O9− | 353.08774 | 353.08671 | 2.916 | - |
| 11 | 6.24 | 227-257-312 | Forskolin | C22H34O7 | 410.22992 | 409.22264 | −1.16 | 179.78584, 160.84163, 135.04446 |
| 12 | 6.43 | 248-270-332 | Physalin B | C27H30O15 | 509.18128 | 510.18856 | 0.906 | - |
| 13 | 7.16 | 214-280-311 | Ferulic acid * | C10H10O4 | 193.05020 | 193.04954 | 3.450 | - |
| 14 | 7.25 | 214-280-312 | 3-O-p-Coumaroylquinic acid | C16H18O8 | 337.09283 | 337.09179 | 3.087 | 191.14720, 172.27388, 162.83879, 119.16192 |
| 15 | 7.52 | 227-280-309 | Scaposin | C19H18O9 | 390.09509 | 389.08781 | 0.23 | - |
| 16 | 8.12 | - | Nevadensin | C18H16O7 | 344.08961 | 343.08233 | 1.75 | - |
| 17 | 8.25 | 227-257-310 | 5-O-Caffeoylquinic acid | C16H18O9 | 353.08783 | 353.08671 | 3.175 | 191.05560, 135.04462, 133.99362 |
| 18 | 8.53 | 227-257-311 | Physalin A | C28H30O10 | 525.17576 | 526.18303 | 2.721 | 242.81560, 191.05563 |
| 19 | 8.75 | 1.3 | 2-Hydroxy-3-methylpentanoic acid | C6H12O3 | 131.07141 | 132.07868 | 0.298 | - |
| 20 | 9.43 | 250 | Physagulin I | C30H39ClO8 | 562.31525 | 563.32252 | 2.828 | - |
| 21 | 9.86 | 249-283 | Physagulin F | C30H40O9 | 543.22105 | 544.22832 | 3.558 | - |
| 22 | 10.12 | 251-304-329 | Physangulidine B | C28H36O8 | 499.23371 | 500.24099 | 0.189 | 255.02881, 151.06294 |
| 23 | 10.54 | 249-264-334 | Physalin D | C28H32O11 | 543.18735 | 544.19463 | 2.941 | - |
| 24 | 10.63 | 249-283-324 | Eicosanoic acid | C20H40O2 | 312.30296 | 311.29569 | −2.11 | - |
| 25 | 11.12 | 248-267-334 | Myristic acid | C14H28O2 | 228.20857 | 227.20133 | 2.517 | - |
| 26 | 11.53 | 255-300-351 | Physagulide P | C30H40O9 | 545.3105 | 546.31778 | 2.890 | 385.14127, 300.02771 |
| 27 | 12.01 | 253-288-311 | Withangulatin A | C30H38O8 | 525.17605 | 526.18329 | 2.811 | 284.30374, 125.87235 |
| 28 | 12.54 | - | Arachidonic acid | C20H32O2 | 304.24051 | 303.23323 | 0.34 | - |
| 29 | 12.67 | 256-348 | Withaminimin | C30H40O8 | 527.21404 | 528.22132 | 2.948 | - |
| 30 | 13.21 | 251-288-332 | Physangulidine A | C28H36O8 | 499.23371 | 500.24099 | 2.607 | 357.40594, 327.08704, 285.13358 |
| 31 | 13.26 | - | Pygenic acid | C30H48O4 | 472.35429 | 471.34701 | −2.05 | - |
| 32 | 13.63 | 252-274-281 | Physangulidine C | C28H35O8 | 499.25377 | 500.24353 | −12,455 | 298.55157, 287.13138 |
| 33 | 14.23 | 251-281 | Physagulin A | C30H38O7 | 509.23287 | 510.24015 | 2.473 | - |
| 34 | 14.76 | - | (R)-2-Hydroxystearic acid | C18H36O3 | 300.26636 | 299.25908 | −0.29 | - |
| 35 | 15.12 | - | N-Oleoyl-phenylalanine | C27H43NO3 | 429.32436 | 428.31709 | 0.36 | - |
| 36 | 15.23 | 250-281-311 | N-Oleyl-leucine | C24H45NO3 | 395.33967 | 394.33239 | −0.72 | - |
| 37 | 15.54 | 254-290-311 | Heneicosanoic acid | C21H42O2 | 326.31784 | 325.31056 | −1.98 | - |
| 38 | 16.26 | 253-292-311 | Tricosanoic acid | C23H46O2 | 354.34983 | 353.34256 | −1.63 | - |
| 39 | 16.57 | 251-267-280 | Behenic acid | C22H44O2 | 340.33361 | 339.32633 | −2.53 | 215.00941 |
| 40 | 16.52 | 252-274-281 | Physangulidine C | C18H22O5 | 499.25377 | 500.24353 | −12,455 | 298.55157, 287.13138 |
| 41 | 17.57 | 251-311 | Quercetin * | C15H10O7 | 301.03549 | 301.03428 | 4.029 | 284.31473, 151.00281 |
| 42 | 18.12 | 252-288-304 | Isorhamnetin * | C16H12O7 | 315.05096 | 315.04993 | 3.286 | 270.46667, 151.20018, 108.47343, 107.73669 |
| 43 | 19.23 | 245-285-311 | Sclareol | C20H36O2 | 308.27115 | 307.26387 | −0.09 | - |
| Extract/Assay | TPC (mg GA/g) | DPPH (μmoL Trolox/g) | ABTS (μmoL Trolox/g) | FRAP (μmoL Trolox/g) |
|---|---|---|---|---|
| Aqueous root | 88.98 ± 1.88 a | 26.71 ± 0.96 a | 52.62 ± 1.02 a | 66.20 ± 1.26 a |
| Aqueous stem | 90.52 ± 1.17 a | 26.27 ± 1.00 a | 55.37 ± 1.05 a | 73.33 ± 1.19 b |
| Aqueous leaves | 349.44 ± 2.53 b | 83.67 ± 0.83 b | 174.87 ± 1.96 b | 137.44 ± 2.16 c |
| Aqueous calyx | 182.54 ± 1.29 c | 53.47 ± 1.02 c | 104.42 ± 1.39 c | 121.63 ± 2.32 d |
| Aqueous fruit | 77.70 ± 0.88 d | 24.83 ± 1.03 a | 55.08 ± 0.96 a | 66.70 ± 0.93 a |
| Ethanolic root | 268.55 ± 2.00 e | 47.73 ± 0.98 d | 86.43 ± 1.29 d | 116.15 ± 1.12 d |
| Ethanolic stem | 177.18 ± 1.31 c | 50.90 ± 0.96 cd | 93.86 ± 1.93 e | 132.43 ± 2.01 c |
| Ethanolic leaves | 517.49 ± 3.60 f | 199.40 ± 1.98 e | 351.24 ± 5.18 f | 476.18 ± 3.72 e |
| Ethanolic calyx | 470.57 ± 4.62 g | 145.79 ± 2.51 f | 153.14 ± 1.94 g | 374.41 ± 4.38 f |
| Ethanolic fruit | 145.36 ± 2.23 h | 44.96 ± 1.00 g | 49.39 ± 1.02 a | 115.36 ± 1.25 d |
| Extract/Assay | AChE IC50 (µg/mL) | BChE IC50 (µg/mL) | α-Glucosidase IC50 (µg/mL) | α-Amylase IC50 (µg/mL) |
|---|---|---|---|---|
| Aqueous root | 46 ± 0.06 a | 205 ± 0.08 a | 1284.249 ± 0.045 a | 116 ± 0.003 a |
| Aqueous stem | 47 ± 0.06 a | 70 ± 0.08 b | ND | 125 ± 0.003 b |
| Aqueous leaves | 68 ± 0.09 b | 86 ± 0.09 c | ND | 197 ± 0.003 c |
| Aqueous calyx | 34 ± 0.08 c | 93 ± 0.09 c | ND | 197 ± 0.007 c |
| Aqueous fruit | 2 ± 0.05 d | 66 ± 0.06 b | ND | 297 ± 0.006 d |
| Ethanolic root | 43 ± 0.05 a | 78 ± 0.09 d | 23.487 ± 0.025 b | 101 ± 0.008 e |
| Ethanolic stem | 27 ± 0.05 e | 51 ± 0.06 e | 73.559 ± 0.043 c | 150 ± 0.005 f |
| Ethanolic leaves | 34 ± 0.06 c | 67 ± 0.10 b | ND | 412 ± 0.003 g |
| Ethanolic calyx | 14 ± 0.06 f | 72 ± 0.05 b | ND | 120 ± 0.005 b |
| Ethanolic fruit | 31 ± 0.04 c | 98 ± 0.06 f | ND | 122 ± 0.005 b |
| Galantamine # | 0.266 ± 0.029 g | 3.824 ± 0.024 g | - | - |
| Acarbose # | - | - | 229.412 ± 0.031 d | 6.477 ± 0.003 h |
| Compounds | Acetylcholinesterase (TcAChE) (Kcal/mol) | Butyrylcholinesterase (hBChE) (Kcal/mol) | α-Amylase (Kcal/mol) | α-Glucosidase (Kcal/mol) |
|---|---|---|---|---|
| 2-Hydroxy-3-methylpentanoic acid | −5.4 | −5.2 | −4.5 | −5.4 |
| 3-O-Caffeoylquinic acid | −9.2 | −9.1 | −7.9 | −6.9 |
| 3-O-p-Coumaroyl quinic acid | −9.4 | −8.7 | −7.9 | −6.9 |
| 5-O-Caffeoyl quinic acid | −9.2 | −8.3 | −7.7 | −7.1 |
| Feruloyltyramine | −9.7 | −8.6 | −7.9 | −7.2 |
| Gluconic acid | −5.3 | −5.4 | −6.1 | −5.1 |
| Malic acid | −4.6 | −5.1 | −4.6 | −4.7 |
| Physagulide P | −9.2 | −10.4 | −8.7 | −9.2 |
| Physagulin A | −13.6 | −10.8 | −11.3 | −8.8 |
| Physagulin F | −8.5 | −11.1 | −10.1 | −8.1 |
| Physalin B | −9.9 | −11.6 | −9.7 | −8.2 |
| Pygenic acid | −9.1 | −11.2 | −9.8 | −8.6 |
| Quinic acid | −6.1 | −6.1 | −6.3 | −5.5 |
| Sclareol | −9.1 | −9.2 | −7.5 | −6.8 |
| Withaminimin | −11.6 | −12.1 | −9.1 | −8.4 |
| Withangulatin A | −1.1 | −1.2 | −1.1 | −1.3 |
| Galanthamine * | −11.30 | −9.20 | -- | -- |
| Acarbose * | -- | -- | −8.10 | −7.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Arana, G.; Torres-Benítez, A.; Ortega-Valencia, J.E.; Merino-Zegarra, C.; Carranza-Rosales, P.; Simirgiotis, M.J. Untargeted Chemical Profile, Antioxidant, and Enzyme Inhibition Activity of Physalis angulata L. from the Peruvian Amazon: A Contribution to the Validation of Its Pharmacological Potential. Antioxidants 2025, 14, 246. https://doi.org/10.3390/antiox14030246
Vargas-Arana G, Torres-Benítez A, Ortega-Valencia JE, Merino-Zegarra C, Carranza-Rosales P, Simirgiotis MJ. Untargeted Chemical Profile, Antioxidant, and Enzyme Inhibition Activity of Physalis angulata L. from the Peruvian Amazon: A Contribution to the Validation of Its Pharmacological Potential. Antioxidants. 2025; 14(3):246. https://doi.org/10.3390/antiox14030246
Chicago/Turabian StyleVargas-Arana, Gabriel, Alfredo Torres-Benítez, José Erick Ortega-Valencia, Claudia Merino-Zegarra, Pilar Carranza-Rosales, and Mario J. Simirgiotis. 2025. "Untargeted Chemical Profile, Antioxidant, and Enzyme Inhibition Activity of Physalis angulata L. from the Peruvian Amazon: A Contribution to the Validation of Its Pharmacological Potential" Antioxidants 14, no. 3: 246. https://doi.org/10.3390/antiox14030246
APA StyleVargas-Arana, G., Torres-Benítez, A., Ortega-Valencia, J. E., Merino-Zegarra, C., Carranza-Rosales, P., & Simirgiotis, M. J. (2025). Untargeted Chemical Profile, Antioxidant, and Enzyme Inhibition Activity of Physalis angulata L. from the Peruvian Amazon: A Contribution to the Validation of Its Pharmacological Potential. Antioxidants, 14(3), 246. https://doi.org/10.3390/antiox14030246









