Dragon Fruit Peel (Hylocereus undatus) Modulates Hepatic Lipid Metabolism and Inflammation in a Rat Model of High-Fat, High-Fructose-Induced Metabolic Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Experimental Protocol
2.3. Blood Pressure Measurement
2.4. Biochemical Analysis
2.5. Hepatic Lipid Accumulation and Oxidative Status Analysis
2.6. Liver Histological Observation
2.7. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR) Analysis
2.8. Data Analysis
3. Results and Discussion
3.1. Effects of DFP on Body Weight Gain, Food Intake, and Organ Weight
3.2. Effects of DFP on Metabolic Parameters
3.3. Effects of DFP on Plasma Oxidative Stress Status
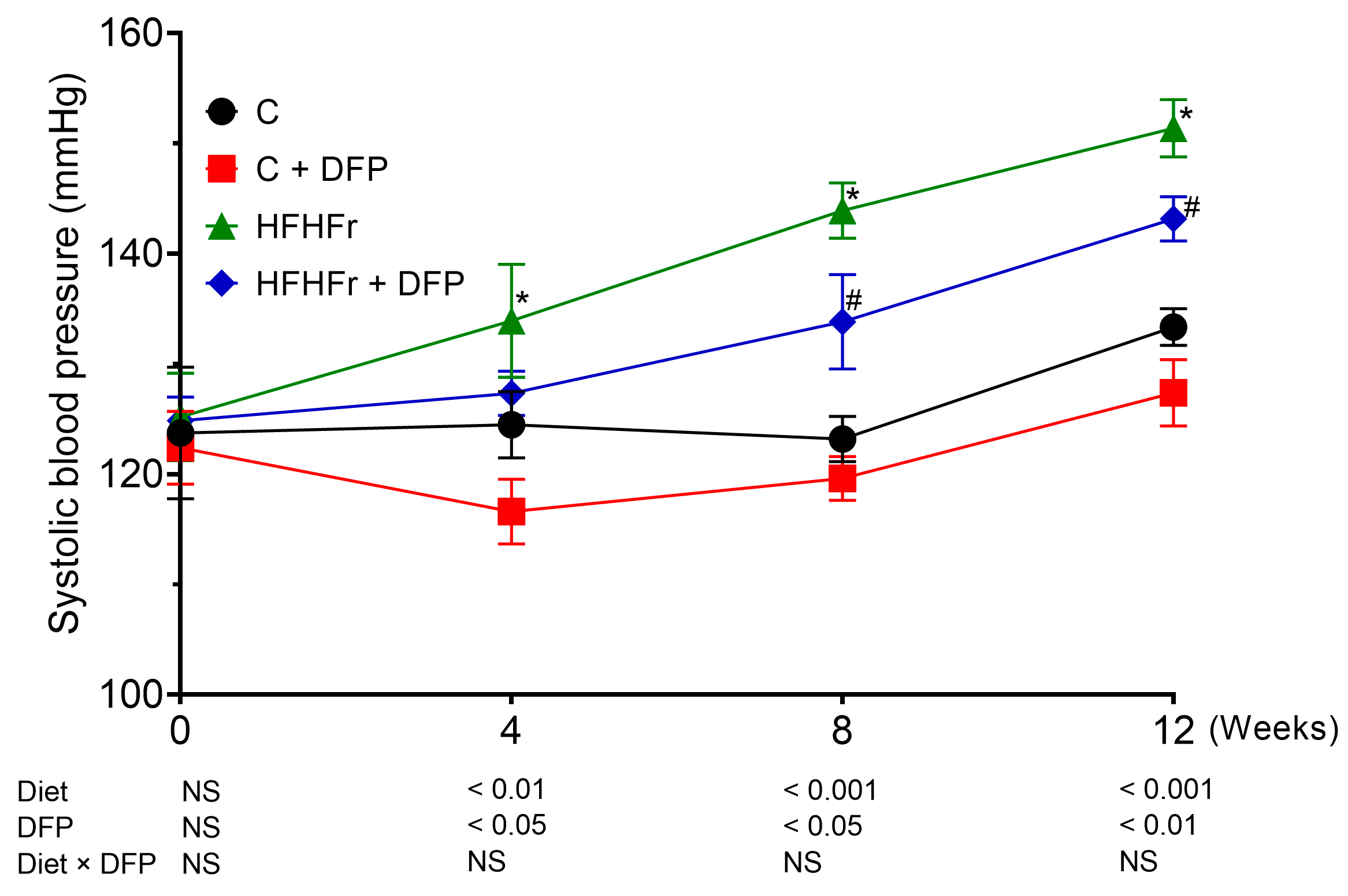
3.4. Effects of DFP on Blood Pressure
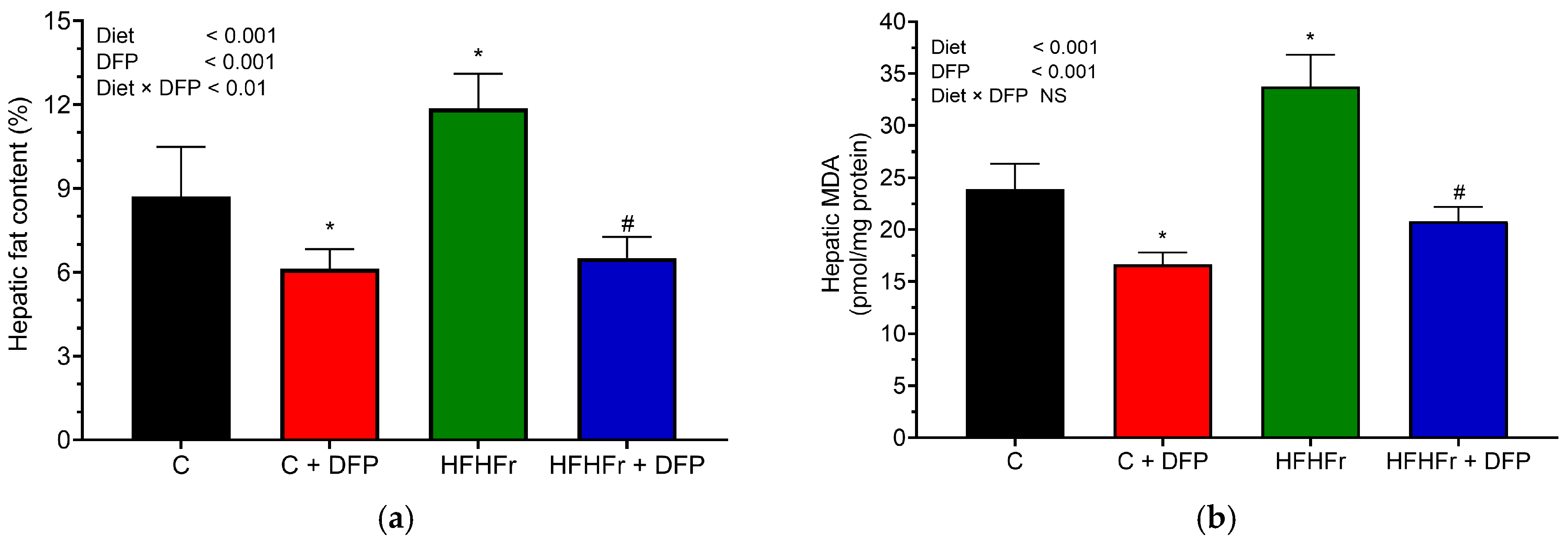
3.5. Effects of DFP on Hepatic Lipid Accumulation, Oxidative Stress, and Histology
3.6. Effects of DFP on Hepatic Gene Expression of Lipid Metabolism
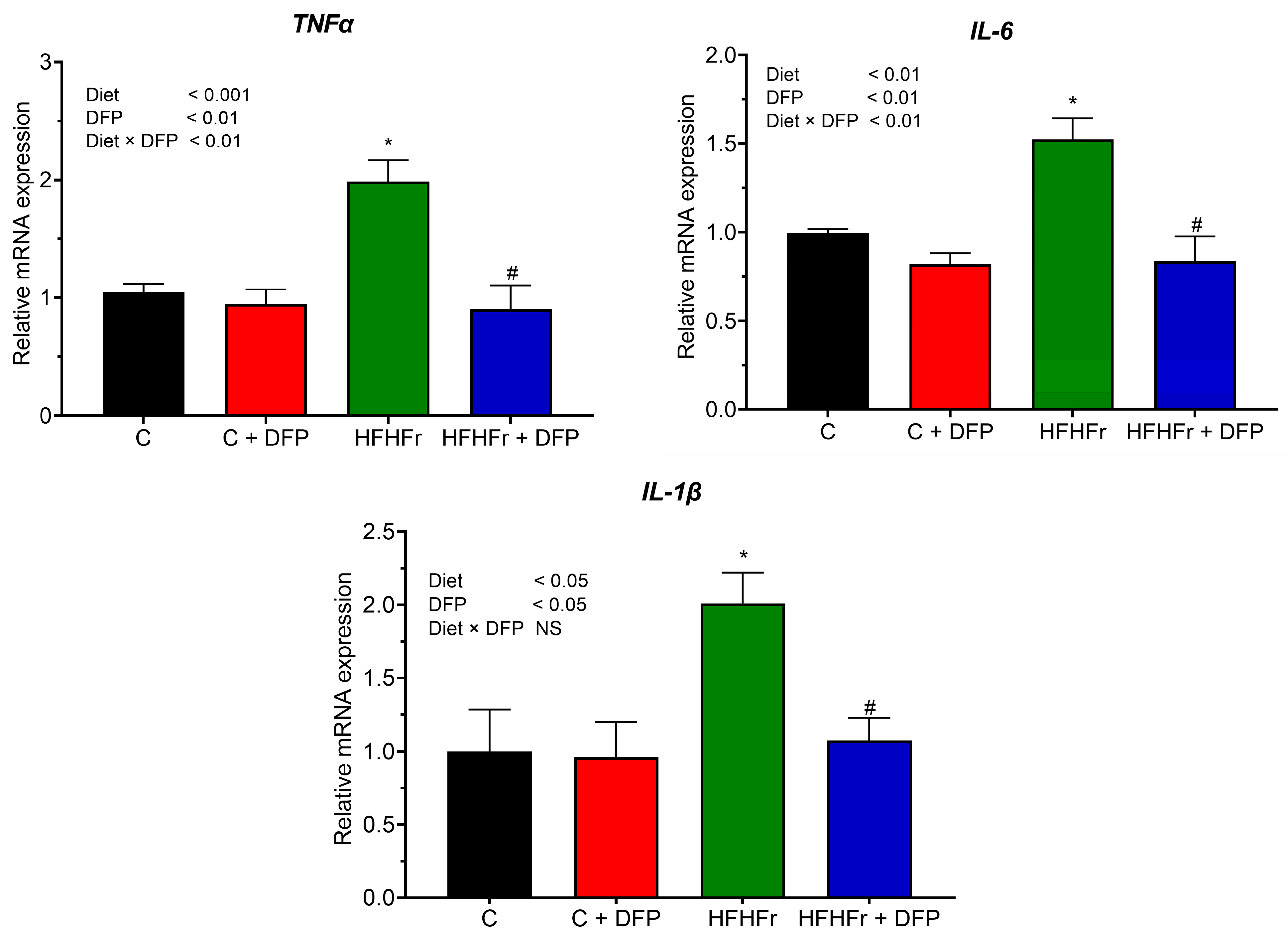
3.7. Effects of DFP on Hepatic Gene Expression of Pro-Inflammatory Cytokines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alamnia, T.T.; Sargent, G.M.; Kelly, M. Dietary patterns and associations with metabolic risk factors for non-communicable disease. Sci. Rep. 2023, 13, 21028. [Google Scholar] [CrossRef] [PubMed]
- Demaria, T.M.; Crepaldi, L.D.; Costa-Bartuli, E.; Branco, J.R.; Zancan, P.; Sola-Penna, M. Once a week consumption of Western diet over twelve weeks promotes sustained insulin resistance and non-alcoholic fat liver disease in C57BL/6 J mice. Sci. Rep. 2023, 13, 3058. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef] [PubMed]
- Badmus, O.O.; Hillhouse, S.A.; Anderson, C.D.; Hinds, T.D.; Stec, D.E. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): Functional analysis of lipid metabolism pathways. Clin. Sci. 2022, 136, 1347–1366. [Google Scholar] [CrossRef]
- Allende, D.S.; Gawrieh, S.; Cummings, O.W.; Belt, P.; Wilson, L.; Van Natta, M.; Behling, C.A.; Carpenter, D.; Gill, R.M.; Kleiner, D.E.; et al. Glycogenosis is common in nonalcoholic fatty liver disease and is independently associated with ballooning, but lower steatosis and lower fibrosis. Liver Int. 2021, 41, 996–1011. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef]
- Arslanow, A.; Teutsch, M.; Walle, H.; Grünhage, F.; Lammert, F.; Stokes, C.S. Short-term hypocaloric high-fiber and high-protein diet improves hepatic steatosis assessed by controlled attenuation parameter. Clin. Transl. Gastroenterol. 2016, 7, e176. [Google Scholar] [CrossRef]
- Salomone, F.; Godos, J.; Zelber-Sagi, S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. 2016, 36, 5–20. [Google Scholar] [CrossRef]
- Wang, R.; Yan, R.; Jiao, J.; Li, F.; Zhang, H.; Chang, Z.; Wei, H.; Yan, S.; Li, J. Fruit and vegetable intake and the risk of non-alcoholic fatty liver disease: A meta-analysis of observational studies. Front. Nutr. 2024, 11, 1398184. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Li, X.; Shu, C.; Jiang, W.; Cao, J. Nutrition, phytochemical profile, bioactivities and applications in food industry of pitaya (Hylocereus spp.) peels: A comprehensive review. Trends Food Sci. Technol. 2021, 116, 199–217. [Google Scholar] [CrossRef]
- Chumroenvidhayakul, S.; Thilavech, T.; Abeywardena, M.; Adisakwattana, S. Investigating the impact of dragon fruit peel waste on starch digestibility, pasting, and thermal properties of flours used in Asia. Foods 2022, 11, 2031. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zheng, Z.; Wu, J.; Lai, J.; Chu, Q.; Zheng, X. White pitaya (Hylocereus undatus) juice attenuates insulin resistance and hepatic steatosis in diet-induced obese mice. PLoS ONE 2016, 11, e0149670. [Google Scholar] [CrossRef]
- Song, H.; Chu, Q.; Xu, D.; Xu, Y.; Zheng, X. Purified betacyanins from Hylocereus undatus peel ameliorate obesity and insulin resistance in high-fat-diet-fed mice. J. Agric. Food Chem. 2016, 64, 236–244. [Google Scholar] [CrossRef]
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Sadie-Van Gijsen, H.; Kotzé-Hörstmann, L. Rat models of diet-induced obesity and metabolic dysregulation: Current trends, shortcomings and considerations for future research. Obes. Res. Clin. Pract. 2023, 17, 449–457. [Google Scholar] [CrossRef]
- Stöppeler, S.; Palmes, D.; Fehr, M.; Hölzen, J.P.; Zibert, A.; Siaj, R.; Schmidt, H.H.-J.; Spiegel, H.-U.; Bahde, R. Gender and strain-specific differences in the development of steatosis in rats. Lab. Anim. 2013, 47, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Patten, G.S.; Abeywardena, M.Y. Effects of antihypertensive agents on intestinal contractility in the spontaneously hypertensive rat: Angiotensin receptor system downregulation by losartan. J. Pharmacol. Exp. Ther. 2017, 360, 260–266. [Google Scholar] [CrossRef]
- Pagadala, P.; Vinutha Shankar, M.S.; Sumathi, M.E. Effect of RFEMR on NSE and MDA levels in Sprague Dawley rats. Bioinformation 2022, 18, 501–505. [Google Scholar] [CrossRef]
- Nakhaee, A.; Bokaeian, M.; Saravani, M.; Farhangi, A.; Akbarzadeh, A. Attenuation of oxidative stress in streptozotocin-induced diabetic rats by Eucalyptus globulus. Indian J. Clin. Biochem. 2009, 24, 419–425. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Mamikutty, N.; Thent, Z.C.; Sapri, S.R.; Sahruddin, N.N.; Mohd Yusof, M.R.; Haji Suhaimi, F. The establishment of metabolic syndrome model by induction of fructose drinking water in male Wistar rats. BioMed Res. Int. 2014, 2014, 263897. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.M.L.; Ng, A.M.H.; Mohd Yunus, M.H.; Idrus, R.B.H.; Law, J.X.; Yazid, M.D.; Chin, K.Y.; Shamsuddin, S.A.; Lokanathan, Y. Recent developments in rodent models of high-fructose diet-induced metabolic syndrome: A systematic review. Nutrients 2021, 13, 2497. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, N.; Liang, S.; Wang, Y.; Liu, S.; Liu, S.; Du, S.; He, H.; Xu, Y.; Cai, H.; et al. The amounts and contributions of total drinking fluids and water from food to total water intake of young adults in Baoding, China. Eur. J. Nutr. 2019, 58, 2669–2677. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.Q.; An, Y.X.; Yu, C.G.; Ke, J.; Zhao, D.; Yu, K. The association between fecal short-chain fatty acids, gut microbiota, and visceral fat in monozygotic twin pairs. Diabetes Metab. Syndr. Obes. 2022, 15, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Izadi, M.S.; Eskandari, F.; Binayi, F.; Salimi, M.; Rashidi, F.S.; Hedayati, M.; Dargahi, L.; Ghanbarian, H.; Zardooz, H. Oxidative and endoplasmic reticulum stress develop adverse metabolic effects due to the high-fat high-fructose diet consumption from birth to young adulthood. Life Sci. 2022, 309, 120924. [Google Scholar] [CrossRef]
- Santos, H.O.; Earnest, C.P.; Tinsley, G.M.; Izidoro, L.F.M.; Macedo, R.C.O. Small dense low-density lipoprotein-cholesterol (sdLDL-C): Analysis, effects on cardiovascular endpoints and dietary strategies. Prog. Cardiovasc. Dis. 2020, 63, 503–509. [Google Scholar] [CrossRef]
- Hernawati; Setiawan, N.A.; Shintawati, R.; Priyandoko, D. The role of red dragon fruit peel (Hylocereus polyrhizus) to improvement blood lipid levels of hyperlipidaemia male mice. J. Phys. Conf. Ser. 2018, 1013, 012167. [Google Scholar] [CrossRef]
- Campos-Perez, W.; Martinez-Lopez, E. Effects of short chain fatty acids on metabolic and inflammatory processes in human health. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158900. [Google Scholar] [CrossRef]
- Cheok, A.; George, T.W.; Rodriguez-Mateos, A.; Caton, P.W. The effects of betalain-rich cacti (dragon fruit and cactus pear) on endothelial and vascular function: A systematic review of animal and human studies. Food Funct. 2020, 11, 6807–6817. [Google Scholar] [CrossRef]
- Vasdev, S.; Gill, V.; Parai, S.; Gadag, V. Fructose-induced hypertension in Wistar-Kyoto rats: Interaction with moderately high dietary salt. Can. J. Physiol. Pharmacol. 2007, 85, 413–421. [Google Scholar] [CrossRef]
- Klein, A.V.; Kiat, H. The mechanisms underlying fructose-induced hypertension: A review. J. Hypertens. 2015, 33, 912–920. [Google Scholar] [CrossRef]
- Mirmiran, P.; Houshialsadat, Z.; Gaeini, Z.; Bahadoran, Z.; Azizi, F. Functional properties of beetroot (Beta vulgaris) in management of cardio-metabolic diseases. Nutr. Metab. 2020, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Topolska, J.; Bączek, N.; Szawara-Nowak, D.; Juśkiewicz, J.; Wiczkowski, W. Characterization of the profile and concentration of betacyanin in the gastric content, blood and urine of rats after an intragastric administration of fermented red beet juice. Food Chem. 2020, 313, 126169. [Google Scholar] [CrossRef] [PubMed]
- Capper, T.E.; Houghton, D.; Stewart, C.J.; Blain, A.P.; McMahon, N.; Siervo, M.; West, D.J.; Stevenson, E.J. Whole beetroot consumption reduces systolic blood pressure and modulates diversity and composition of the gut microbiota in older participants. NFS J. 2020, 21, 28–37. [Google Scholar] [CrossRef]
- Rahimi, P.; Mesbah-Namin, S.A.; Ostadrahimi, A.; Abedimanesh, S.; Separham, A.; Asghary Jafarabadi, M. Effects of betalains on atherogenic risk factors in patients with atherosclerotic cardiovascular disease. Food Funct. 2019, 10, 8286–8297. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Cassard, A.M.; Ciocan, D. Pectin in metabolic liver disease. Nutrients 2022, 15, 157. [Google Scholar] [CrossRef]
- García-Berumen, C.I.; Ortiz-Avila, O.; Vargas-Vargas, M.A.; Del Rosario-Tamayo, B.A.; Guajardo-López, C.; Saavedra-Molina, A.; Rodríguez-Orozco, A.R.; Cortés-Rojo, C. The severity of rat liver injury by fructose and high fat depends on the degree of respiratory dysfunction and oxidative stress induced in mitochondria. Lipids Health Dis. 2019, 18, 78. [Google Scholar] [CrossRef]
- Vulić, J.J.; Ćebović, T.N.; Čanadanović-Brunet, J.M.; Ćetković, G.S.; Čanadanović, V.M.; Djilas, S.M.; Šaponjac, V.T.T. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- DiNunzio, G.; Belew, G.D.; Torres, A.N.; Silva, J.G.; Silva, L.P.; Barosa, C.; Tavares, L.; Jones, J.G. Determining the contribution of a high-fructose corn syrup formulation to hepatic glycogen synthesis during ad-libitum feeding in mice. Sci. Rep. 2020, 10, 12852. [Google Scholar] [CrossRef]
- Lozano, I.; Van der Werf, R.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; et al. High-fructose and high-fat diet-induced disorders in rats: Impact on diabetes risk, hepatic and vascular complications. Nutr. Metab. 2016, 13, 15. [Google Scholar] [CrossRef]
- Erion, D.M.; Popov, V.; Hsiao, J.J.; Vatner, D.; Mitchell, K.; Yonemitsu, S.; Nagai, Y.; Kahn, M.; Gillum, M.P.; Dong, J.; et al. The role of the carbohydrate response element-binding protein in male fructose-fed rats. Endocrinology 2013, 154, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Arguello, G.; Balboa, E.; Arrese, M.; Zanlungo, S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim. Biophys. Acta 2015, 1852, 1765–1778. [Google Scholar] [CrossRef] [PubMed]
- Chyau, C.C.; Wang, H.F.; Zhang, W.J.; Chen, C.C.; Huang, S.H.; Chang, C.C.; Peng, R.Y. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int. J. Mol. Sci. 2020, 21, 360. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, K.; Kopeć, A.; Piątkowska, E.; Leszczyńska, T. High-fructose diet-induced metabolic disorders were counteracted by the intake of fruit and leaves of sweet cherry in Wistar rats. Nutrients 2019, 11, 2638. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, L.; Ruan, Z.; Han, P.; Yu, Y. The regulatory effects of citrus peel powder on liver metabolites and gut flora in mice with non-alcoholic fatty liver disease (NAFLD). Foods 2021, 10, 3022. [Google Scholar] [CrossRef]
- Reyes-García, V.; Botella-Martínez, C.; Juárez-Trujillo, N.; Viuda-Martos, M. Pitahaya (Hylocereus ocamponis) peel flour as new ingredient in the development of beef burgers: Impact on the quality parameters. Eur. Food Res. Technol. 2024, 250, 2375–2385. [Google Scholar] [CrossRef]
- Shiau, S.-Y.; Li, G.-H.; Pan, W.-C.; Xiong, C. Effect of pitaya peel powder addition on the phytochemical and textural properties and sensory acceptability of dried and cooked noodles. J. Food Process. Preserv. 2020, 44, e14491. [Google Scholar] [CrossRef]
- Chumroenvidhayakul, S.; Thilavech, T.; Abeywardena, M.; Adisakwattana, S. Dragon fruit peel waste (Hylocereus undatus) as a potential ingredient for reducing lipid peroxidation, dietary advanced glycation end products, and starch digestibility in cookies. Antioxidants 2023, 12, 1002. [Google Scholar] [CrossRef]


| Parameters | Experimental Groups | Significance of Effects | |||||
|---|---|---|---|---|---|---|---|
| C | C + DFP | HFHFr | HFHFr + DFP | Diet | DFP | Diet × DFP | |
| Initial body weight (g) | 334.5 ± 14.2 | 329.3 ± 14.5 | 339.5 ± 12.3 | 338.6 ± 14.2 | NS | NS | NS |
| Final body weight (g) | 698.9 ± 25.2 | 677.7 ± 29.5 | 679.9 ± 20.1 | 642.6 ± 22.2 | NS | NS | NS |
| Body weight gain (g) | 329.8 ± 24.2 | 279.3 ± 18.9 * | 303.8 ± 11.4 | 248.5 ± 12.2 # | NS | <0.01 | NS |
| Food intake (g/rat/day) | 31.2 ± 0.6 | 28.1 ± 0.3 * | 20.9 ± 0.5 * | 19.9 ± 0.4 | <0.001 | <0.001 | <0.05 |
| Caloric intake from food (kcal/rat/day) | 123.5 ± 2.4 | 109.6 ± 1.3 * | 142.5 ± 2.3 * | 130.1 ± 2.6 # | <0.001 | <0.001 | NS |
| Fluid intake (mL/rat/day) | 37.7 ± 1.1 | 33.5 ± 0.3 | 100.2 ± 3.0 * | 81.1 ± 3.5 # | <0.001 | <0.001 | <0.01 |
| Caloric intake from fluid (kcal/rat/day) | ND | ND | 38.8 ± 1.5 * | 34.0 ± 1.6 # | <0.001 | <0.05 | <0.05 |
| Calorie intake (kcal/rat/day) | 123.5 ± 2.4 | 109.6 ± 1.3 * | 142.5 ± 2.3 * | 130.1 ± 2.6 # | <0.001 | <0.001 | NS |
| Visceral fat tissue (g) | 43.6 ± 2.4 | 31.0 ± 2.0 * | 46.7 ± 2.3 | 33.1 ± 2.9 # | NS | <0.001 | NS |
| Visceral fat tissue to body weight ratio (%) | 7.6 ± 0.1 | 4.4 ± 0.3 * | 6.7 ± 0.5 | 4.7 ± 0.4 # | NS | <0.001 | NS |
| Liver (g) | 22.5 ± 0.6 | 18.1 ± 0.7 | 27.0 ± 0.9 * | 21.0 ± 0.7 # | <0.001 | <0.001 | NS |
| Liver to body weight ratio (%) | 3.2 ± 0.2 | 2.7 ± 0.1 | 4.0 ± 0.2 * | 3.2 ± 0.1 # | <0.001 | <0.01 | NS |
| Glycogen accumulation | 2.4 ± 0.7 | 2.2 ± 0.6 | 3.6 ± 1.02 * | 3.1 ± 0.7 | - | - | - |
| Parameters | Experimental Groups | Significance of Effects | ||||||
|---|---|---|---|---|---|---|---|---|
| C | C + DFP | HFHFr | HFHFr + DFP | Diet | DFP | Diet × DFP | ||
| Plasma glucose (mmol/L) | 9.22 ± 0.16 | 9.08 ± 0.17 | 10.07 ± 0.21 * | 9.02 ± 0.30 # | NS | <0.01 | NS | |
| Serum insulin (μg/L) | 1.13 ± 0.13 | 0.96 ± 0.10 | 1.84 ± 0.25 * | 0.96 ± 0.08 # | <0.05 | <0.01 | <0.05 | |
| Serum TC (mmol/L) | 2.00 ± 0.12 | 1.77 ± 0.07 | 2.66 ± 0.18 * | 2.24 ± 0.12 # | <0.001 | <0.01 | NS | |
| Serum LDL-C (mmol/L) | 0.60 ± 0.04 | 0.42 ± 0.02 * | 0.61 ± 0.03 | 0.51 ± 0.03 # | NS | <0.01 | NS | |
| Serum HDL-C (mmol/L) | 1.26 ± 0.07 | 1.50 ± 0.10 | 0.91 ± 0.02 * | 0.99 ± 0.04 # | <0.001 | <0.01 | NS | |
| Serum TG (mmol/L) | 1.54 ± 0.27 | 1.21 ± 0.17 | 3.83 ± 0.36 * | 1.89 ± 0.17 # | <0.001 | <0.001 | <0.01 | |
| Plasma MDA (μM MDA) | 2.94 ± 0.02 | 2.83 ± 0.03 * | 8.97 ± 0.06 * | 3.65 ± 0.02 # | <0.001 | <0.001 | <0.001 | |
| Plasma FRAP (μM FeSO4) | 163.35 ± 7.36 | 807.50 ± 10.95 * | 130.14 ± 8.29 | 417.63 ± 30.92 # | <0.001 | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chumroenvidhayakul, S.; Thilavech, T.; Abeywardena, M.Y.; Conlon, M.; Dallimore, J.; Adams, M.; Muhlhausler, B.; Adisakwattana, S. Dragon Fruit Peel (Hylocereus undatus) Modulates Hepatic Lipid Metabolism and Inflammation in a Rat Model of High-Fat, High-Fructose-Induced Metabolic Dysfunction. Antioxidants 2025, 14, 319. https://doi.org/10.3390/antiox14030319
Chumroenvidhayakul S, Thilavech T, Abeywardena MY, Conlon M, Dallimore J, Adams M, Muhlhausler B, Adisakwattana S. Dragon Fruit Peel (Hylocereus undatus) Modulates Hepatic Lipid Metabolism and Inflammation in a Rat Model of High-Fat, High-Fructose-Induced Metabolic Dysfunction. Antioxidants. 2025; 14(3):319. https://doi.org/10.3390/antiox14030319
Chicago/Turabian StyleChumroenvidhayakul, Siriwan, Thavaree Thilavech, Mahinda Yapa Abeywardena, Michael Conlon, Julie Dallimore, Michael Adams, Beverly Muhlhausler, and Sirichai Adisakwattana. 2025. "Dragon Fruit Peel (Hylocereus undatus) Modulates Hepatic Lipid Metabolism and Inflammation in a Rat Model of High-Fat, High-Fructose-Induced Metabolic Dysfunction" Antioxidants 14, no. 3: 319. https://doi.org/10.3390/antiox14030319
APA StyleChumroenvidhayakul, S., Thilavech, T., Abeywardena, M. Y., Conlon, M., Dallimore, J., Adams, M., Muhlhausler, B., & Adisakwattana, S. (2025). Dragon Fruit Peel (Hylocereus undatus) Modulates Hepatic Lipid Metabolism and Inflammation in a Rat Model of High-Fat, High-Fructose-Induced Metabolic Dysfunction. Antioxidants, 14(3), 319. https://doi.org/10.3390/antiox14030319









