Mechanical Loading Induces NRF2 Nuclear Translocation to Epigenetically Remodel Oxidative Stress Defense in Osteocytes
Abstract
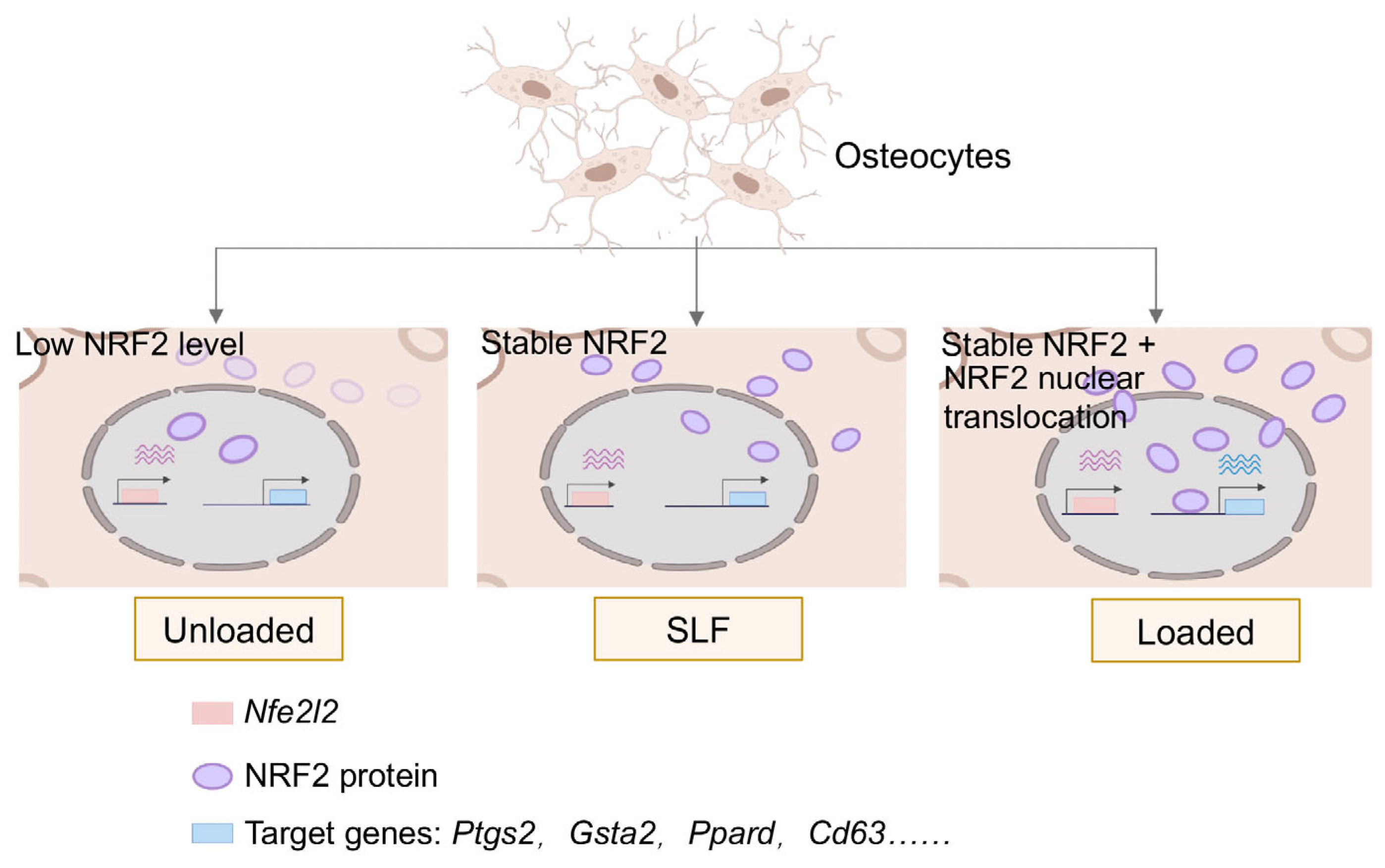
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Mechanical Loading
2.3. RNA-Seq
2.4. ChIP-Seq
2.5. RNA-Seq Analysis
2.6. ChIP-Seq Analysis
2.7. Gene Functional Analysis
2.8. Western Blot
2.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.10. Statistical Analysis:
3. Results
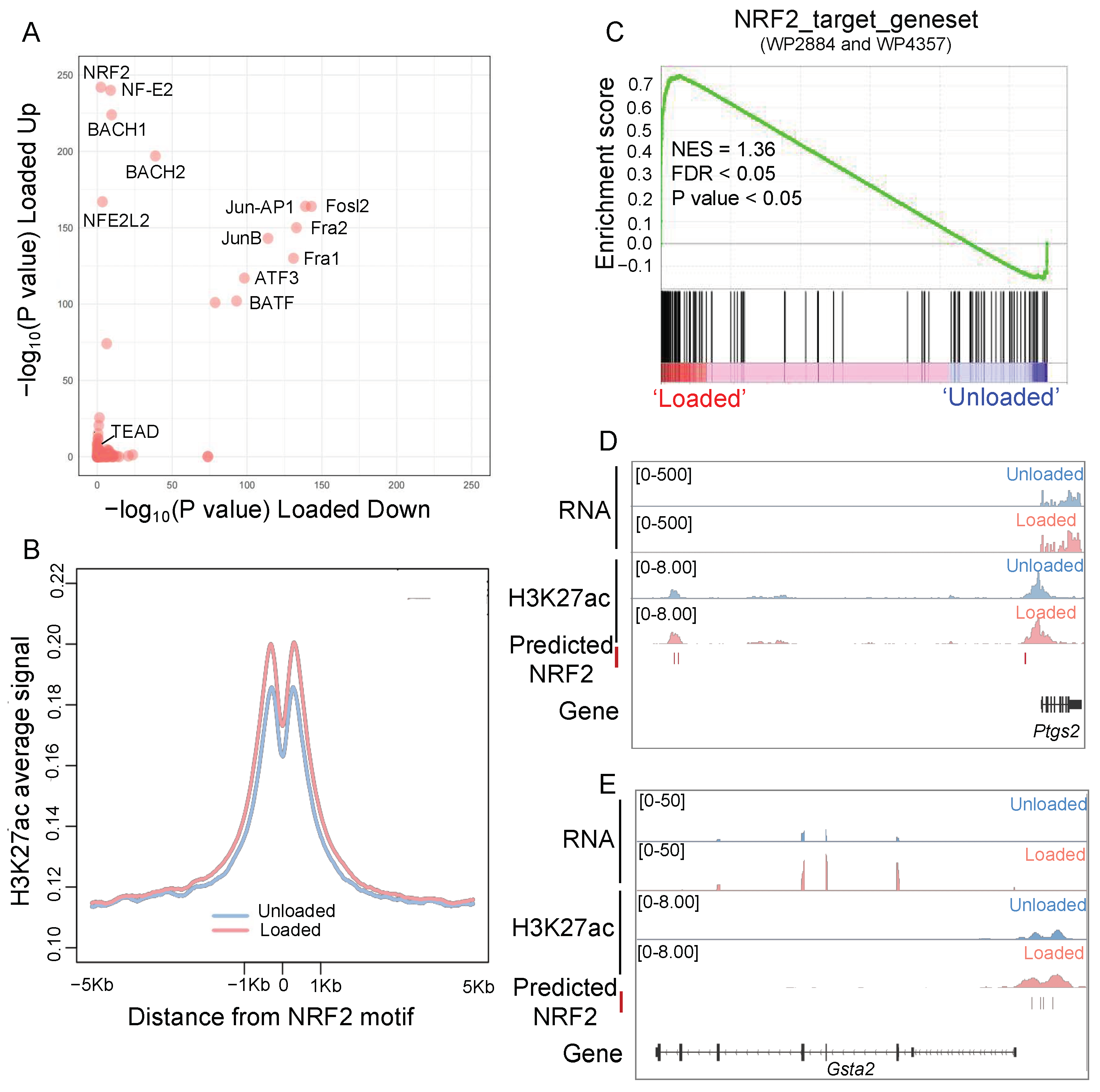
3.1. Mechanical Loading Remodels the Transcriptional and Epigenetic Profile of Oxidative Homeostasis in Osteocytes
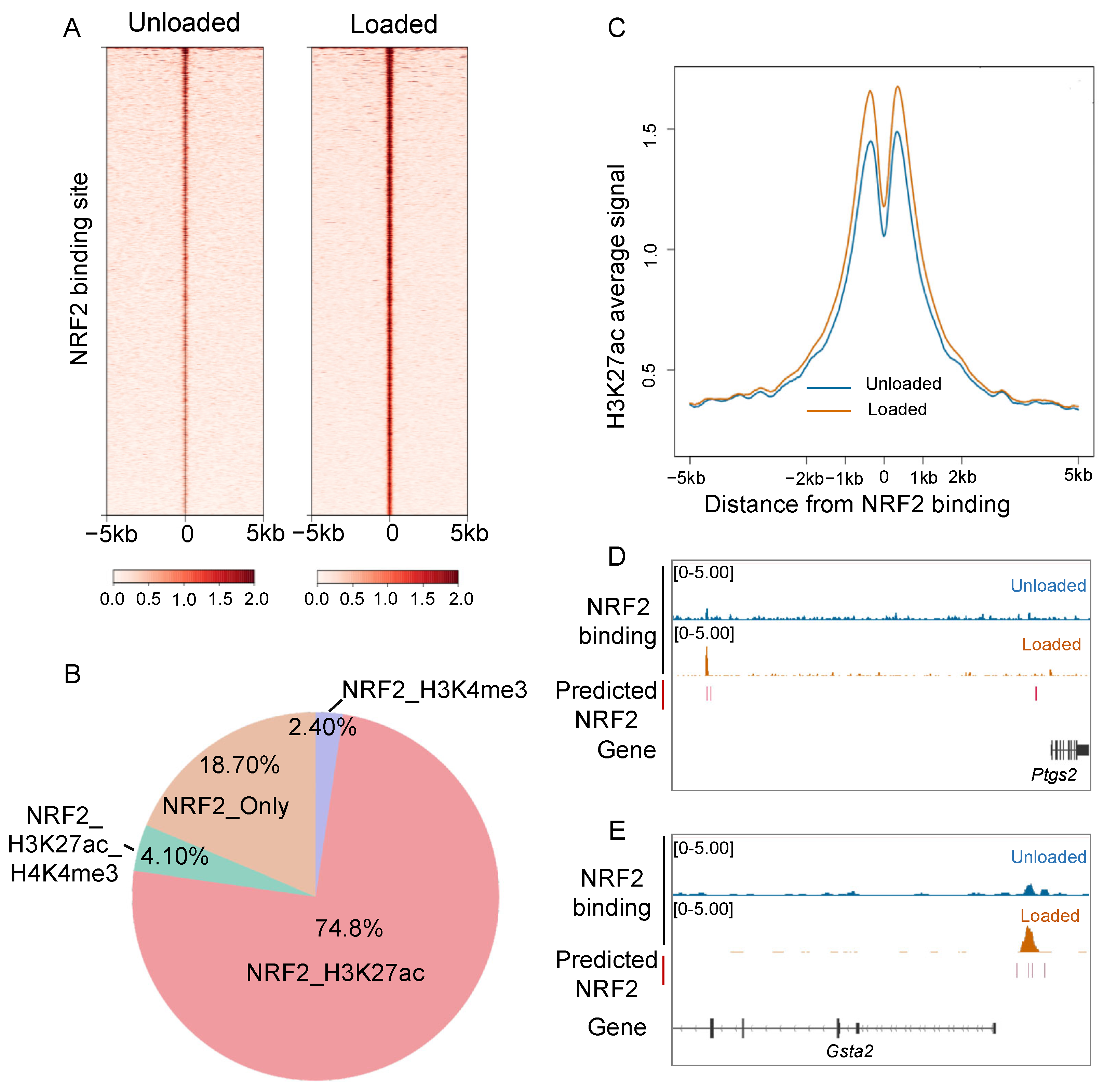
3.2. NRF2 Plays a Vital Role in the Epigenetic Remodeling of Osteocytes
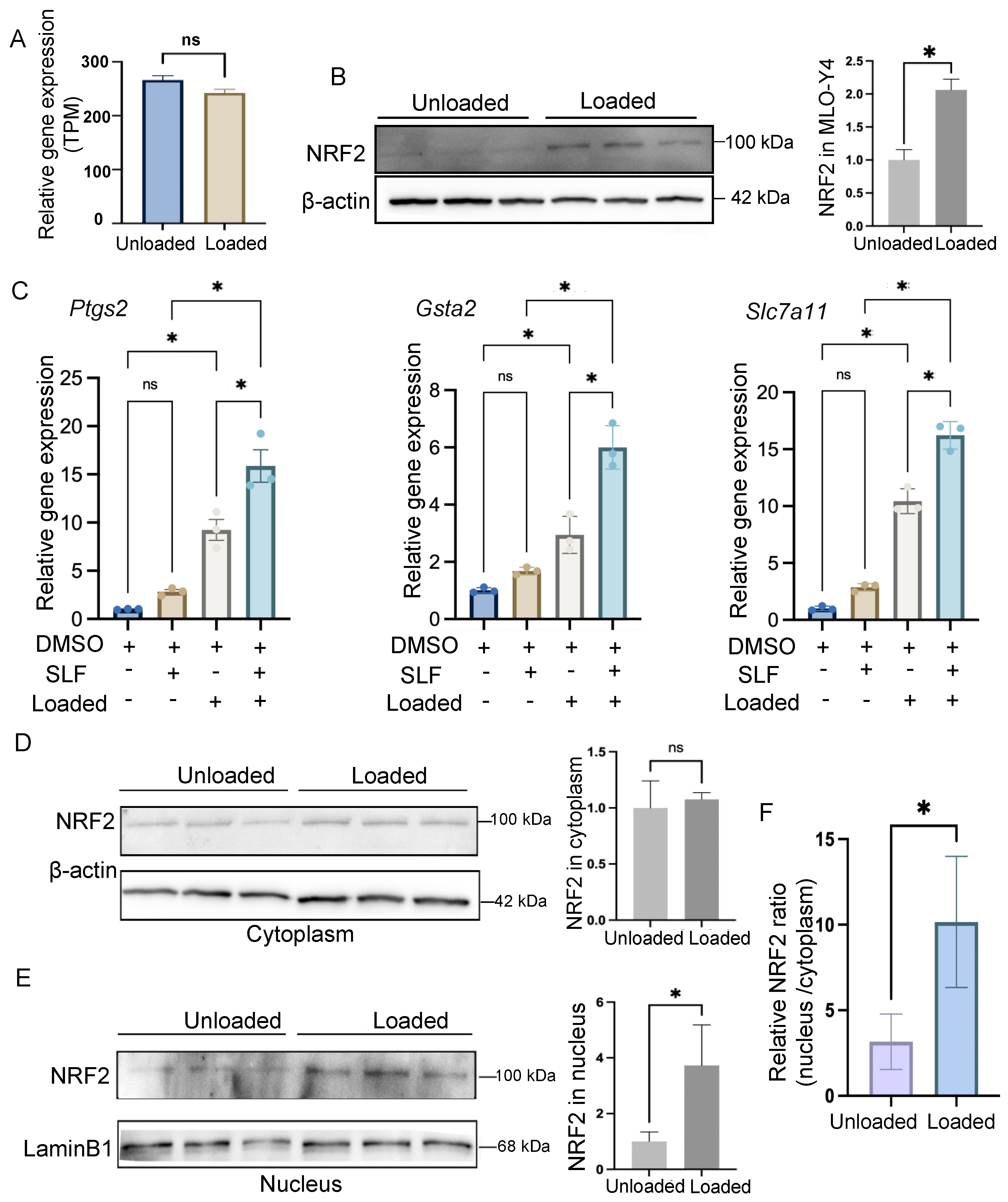
3.3. Mechanical Loading Promotes Nucleus Translocation of NRF2 in Osteocytes
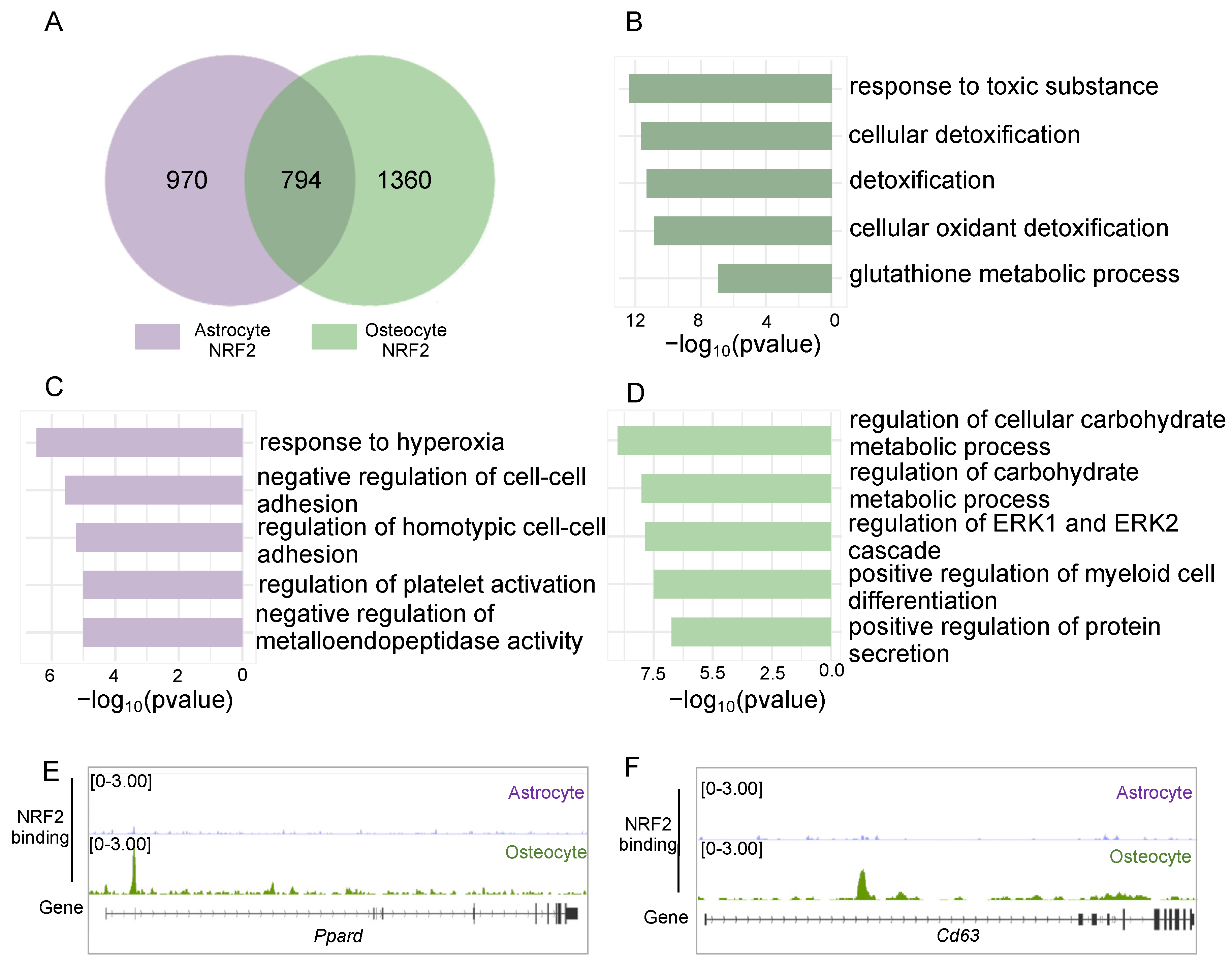
3.4. NRF2 Exerts Both Shared and Cell Type-Specific Function Through Binding to the Genome of Osteocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cauley, J.A.; Giangregorio, L. Physical activity and skeletal health in adults. Lancet Diabetes Endocrinol. 2020, 8, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef]
- Vico, L.; Hargens, A. Skeletal changes during and after spaceflight. Nat. Rev. Rheumatol. 2018, 14, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C. Bone: Oxidative stress and osteoporosis. Nat. Rev. Endocrinol. 2014, 10, 3. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, W.; Liu, T.; Tan, Y.; Chen, C.; Zhao, J.; Geng, H.; Ma, C. The physiological metabolite α-ketoglutarate ameliorates osteoarthritis by regulating mitophagy and oxidative stress. Redox Biol. 2023, 62, 102663. [Google Scholar] [CrossRef]
- Riegger, J.; Schoppa, A.; Ruths, L.; Haffner-Luntzer, M.; Ignatius, A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: A narrative review. Cell. Mol. Biol. Lett. 2023, 28, 76. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, R.; Sun, K.; Yan, C.; Jiang, J.; Kong, F.; Shi, J. The deubiquitinase USP11 ameliorates intervertebral disc degeneration by regulating oxidative stress-induced ferroptosis via deubiquitinating and stabilizing Sirt3. Redox Biol. 2023, 62, 102707. [Google Scholar] [CrossRef]
- Narula, J.; Williams, C.J.; Tiwari, A.; Marks-Bluth, J.; Pimanda, J.E.; Igoshin, O.A. Mathematical model of a gene regulatory network reconciles effects of genetic perturbations on hematopoietic stem cell emergence. Dev. Biol. 2013, 379, 258–269. [Google Scholar] [CrossRef]
- Maurer, M.; Lammerding, J. The Driving Force: Nuclear Mechanotransduction in Cellular Function, Fate, and Disease. Annu. Rev. Biomed. Eng. 2019, 21, 443–468. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, X.; Wei, A.; Chen, F.; Gao, Q.; Lu, K.; Jiang, Q.; Cao, W. Nrf2 epigenetic derepression induced by running exercise protects against osteoporosis. Bone Res. 2021, 9, 15. [Google Scholar] [CrossRef]
- Zhang, K.Q.; Barragan-Adjemian, C.; Ye, L.; Kotha, S.; Dallas, M.; Lu, Y.B.; Zhao, S.J.; Harris, M.; Harris, S.E.; Feng, J.Q.; et al. E11/gp38 selective expression in osteocytes: Regulation by mechanical strain and role in dendrite elongation. Mol. Cell. Biol. 2006, 26, 4539–4552. [Google Scholar] [CrossRef]
- Zarka, M.; Etienne, F.; Bourmaud, M.; Szondi, D.; Schwartz, J.M.; Kampmann, K.; Helary, C.; Rannou, F.; Haÿ, E.; Cohen-Solal, M. Mechanical loading activates the YAP/TAZ pathway and chemokine expression in the MLO-Y4 osteocyte-like cell line. Lab. Investig. 2021, 101, 1597–1604. [Google Scholar] [CrossRef]
- Chang, L.; Azzolin, L.; Di Biagio, D.; Zanconato, F.; Battilana, G.; Xiccato, R.L.; Aragona, M.; Giulitti, S.; Panciera, T.; Gandin, A.; et al. The SWI/SNF complex is a mechanoregulated inhibitor of YAP and TAZ. Nature 2018, 563, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Sifrim, A.; Malfait, M.; Song, Y.; Van Herck, J.; Dekoninck, S.; Gargouri, S.; Lapouge, G.; Swedlund, B.; Dubois, C.; et al. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature 2020, 584, 268–273. [Google Scholar] [CrossRef]
- Santos-Rosa, H.; Caldas, C. Chromatin modifier enzymes, the histone code and cancer. Eur. J. Cancer 2005, 41, 2381–2402. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, J.; Mohagheghian, E.; Wang, N. Force-induced gene up-regulation does not follow the weak power law but depends on H3K9 demethylation. Sci. Adv. 2020, 6, eaay9095. [Google Scholar] [CrossRef]
- Sato, T.; Verma, S.; Andrade, C.D.C.; Omeara, M.; Campbell, N.; Wang, J.S.; Cetinbas, M.; Lang, A.; Ausk, B.J.; Brooks, D.J.; et al. A FAK/HDAC5 signaling axis controls osteocyte mechanotransduction. Nat. Commun. 2020, 11, 3282. [Google Scholar] [CrossRef]
- Yang, J.; Sung, E.; Donlin-Asp, P.G.; Corces, V.G. A subset of Drosophila Myc sites remain associated with mitotic chromosomes colocalized with insulator proteins. Nat. Commun. 2013, 4, 1464. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nusbaum, C.; Myers, R.M.; Brown, M.; Li, W.; et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008, 9, R137. [Google Scholar] [CrossRef] [PubMed]
- Stempor, P.; Ahringer, J. SeqPlots—Interactive software for exploratory data analyses, pattern discovery and visualization in genomics. Wellcome Open Res. 2016, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Heinz, S.; Benner, C.; Spann, N.; Bertolino, E.; Lin, Y.C.; Laslo, P.; Cheng, J.X.; Murre, C.; Singh, H.; Glass, C.K. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 2010, 38, 576–589. [Google Scholar] [CrossRef]
- Gracey, E.; Burssens, A.; Cambré, I.; Schett, G.; Lories, R.; McInnes, I.B.; Asahara, H.; Elewaut, D. Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 193–207. [Google Scholar] [CrossRef]
- Zeng, P.; Klareskog, L.; Alfredsson, L.; Bengtsson, C. Physical workload is associated with increased risk of rheumatoid arthritis: Results from a Swedish population-based case-control study. RMD Open 2017, 3, e000324. [Google Scholar] [CrossRef]
- Nakano-Kobayashi, A.; Canela, A.; Yoshihara, T.; Hagiwara, M. Astrocyte-targeting therapy rescues cognitive impairment caused by neuroinflammation via the Nrf2 pathway. Proc. Natl. Acad. Sci. USA 2023, 120, e2303809120. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Xu, S.; Xu, Y.; Little, P.J.; Jin, Z.G. Targeting Mechanosensitive Transcription Factors in Atherosclerosis. Trends Pharmacol. Sci. 2019, 40, 253–266. [Google Scholar] [CrossRef]
- Müller, D.I.H.; Stoll, C.; Palumbo-Zerr, K.; Böhm, C.; Krishnacoumar, B.; Ipseiz, N.; Taubmann, J.; Zimmermann, M.; Böttcher, M.; Mougiakakos, D.; et al. PPARδ-mediated mitochondrial rewiring of osteoblasts determines bone mass. Sci. Rep. 2020, 10, 8428. [Google Scholar] [CrossRef]
- Chen, M.; Jing, D.; Ye, R.; Yi, J.; Zhao, Z. PPARβ/δ accelerates bone regeneration in diabetic mellitus by enhancing AMPK/mTOR pathway-mediated autophagy. Stem Cell Res. Ther. 2021, 12, 566. [Google Scholar] [CrossRef]
- Ran, N.; Gao, X.; Dong, X.; Li, J.; Lin, C.; Geng, M.; Yin, H. Effects of exosome-mediated delivery of myostatin propeptide on functional recovery of mdx mice. Biomaterials 2020, 236, 119826. [Google Scholar] [CrossRef]
- Tavasolian, F.; Lively, S.; Pastrello, C.; Tang, M.; Lim, M.; Pacheco, A.; Qaiyum, Z.; Yau, E.; Baskurt, Z.; Jurisica, I.; et al. Proteomic and genomic profiling of plasma exosomes from patients with ankylosing spondylitis. Ann. Rheum. Dis. 2023, 82, 1429–1443. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Li, Z.; Liu, H.; Chen, S.; Liu, D. Nrf2 Activation Is Involved in Cyclic Mechanical Stress-Stimulated Osteogenic Differentiation in Periodontal Ligament Stem Cells via PI3K/Akt Signaling and HO1-SOD2 Interaction. Front. Cell Dev. Biol. 2021, 9, 816000. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Tang, Q.; Lu, X.; Chen, G.; Xie, M.; Yang, J.; Yin, Y.; Zheng, W.; Wang, J.; Han, Y.; et al. Time of exercise differentially impacts bone growth in mice. Nat. Metab. 2024, 6, 1036–1052. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.R.; Yen, S.S.; Rubin, J. Vibration therapy: Clinical applications in bone. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 447–453. [Google Scholar] [CrossRef]
- Wysocki, A.; Butler, M.; Shamliyan, T.; Kane, R.L. Whole-body vibration therapy for osteoporosis: State of the science. Ann. Intern. Med. 2011, 155, 680–686. [Google Scholar] [CrossRef]
- Li, L.; Li, W.J.; Zheng, X.R.; Liu, Q.L.; Du, Q.; Lai, Y.J.; Liu, S.Q. Eriodictyol ameliorates cognitive dysfunction in APP/PS1 mice by inhibiting ferroptosis via vitamin D receptor-mediated Nrf2 activation. Mol. Med. 2022, 28, 11. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Chen, F.; Li, Y.; Zhong, Y.; Lu, Y.; Zhang, K.; Yang, F. Vitamin D combined with whole-body vibration training for the treatment of osteo-sarcopenia: Study protocol for a randomized controlled trial. Trials 2024, 25, 638. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410.e14. [Google Scholar] [CrossRef]
- Andreu, I.; Granero-Moya, I.; Chahare, N.R.; Clein, K.; Molina-Jordán, M.; Beedle, A.E.M.; Elosegui-Artola, A.; Abenza, J.F.; Rossetti, L.; Trepat, X.; et al. Mechanical force application to the nucleus regulates nucleocytoplasmic transport. Nat. Cell Biol. 2022, 24, 896–905. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Braga, A.; Yu, Y.; Esteras, N.; Korsak, A.; Theparambil, S.M.; Hadjihambi, A.; Hosford, P.S.; Teschemacher, A.G.; Marina, N.; et al. Mechanosensory Signaling in Astrocytes. J. Neurosci. 2020, 40, 9364–9371. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, N.; Bandyopadhyay, C.; Coon, B.G.; Yun, S.; Schwartz, M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig. 2016, 126, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Zhao, Y.; Liu, H.; Li, Z.; Chen, S.; Liu, D. Nrf2 activation is involved in osteogenic differentiation of periodontal ligament stem cells under cyclic mechanical stretch. Exp. Cell Res. 2021, 403, 112598. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhao, Y.; Zhao, L.; Wang, Z.; Yan, K.; Gao, B.; Zhang, L. The Role of Oxidative Stress in Multiple Exercise-Regulated Bone Homeostasis. Aging Dis. 2023, 14, 1555–1582. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhang, J.; Gong, L.; Liu, N.; Liu, Q.; Liu, Z.; Guo, B.; Yang, J. Mechanical Loading Induces NRF2 Nuclear Translocation to Epigenetically Remodel Oxidative Stress Defense in Osteocytes. Antioxidants 2025, 14, 346. https://doi.org/10.3390/antiox14030346
Guo Y, Zhang J, Gong L, Liu N, Liu Q, Liu Z, Guo B, Yang J. Mechanical Loading Induces NRF2 Nuclear Translocation to Epigenetically Remodel Oxidative Stress Defense in Osteocytes. Antioxidants. 2025; 14(3):346. https://doi.org/10.3390/antiox14030346
Chicago/Turabian StyleGuo, Yue, Jing Zhang, Luyu Gong, Na Liu, Qiaoqiao Liu, Zhaojun Liu, Baosheng Guo, and Jingping Yang. 2025. "Mechanical Loading Induces NRF2 Nuclear Translocation to Epigenetically Remodel Oxidative Stress Defense in Osteocytes" Antioxidants 14, no. 3: 346. https://doi.org/10.3390/antiox14030346
APA StyleGuo, Y., Zhang, J., Gong, L., Liu, N., Liu, Q., Liu, Z., Guo, B., & Yang, J. (2025). Mechanical Loading Induces NRF2 Nuclear Translocation to Epigenetically Remodel Oxidative Stress Defense in Osteocytes. Antioxidants, 14(3), 346. https://doi.org/10.3390/antiox14030346





