Lactobacillus plantarum-Derived Inorganic Polyphosphate Regulates Immune Function via Inhibiting M1 Polarization and Resisting Oxidative Stress in Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. DAPI Staining of PolyP in L. plantarum
2.2. Extraction and Purification of PolyP from L. plantarum
2.3. Quantification of Extracted PolyP
2.4. PolyP Chain Length Analysis Using Urea–PAGE
2.5. Cellular Culture and Treatments
2.6. Cellular Proliferation Assays and Morphological Observations
2.7. Nitric Oxide Estimation Using the Griess Reagent
2.8. Reactive Oxygen Species Assay in Macrophages
2.9. ELISAs
2.10. Total RNA Extraction and qRT-PCR
2.11. Statistical Analyses
3. Results
3.1. PolyP Granule Accumulation in L. plantarum
3.2. The Quantification and Chain Length of PolyP Derived from L. plantarum
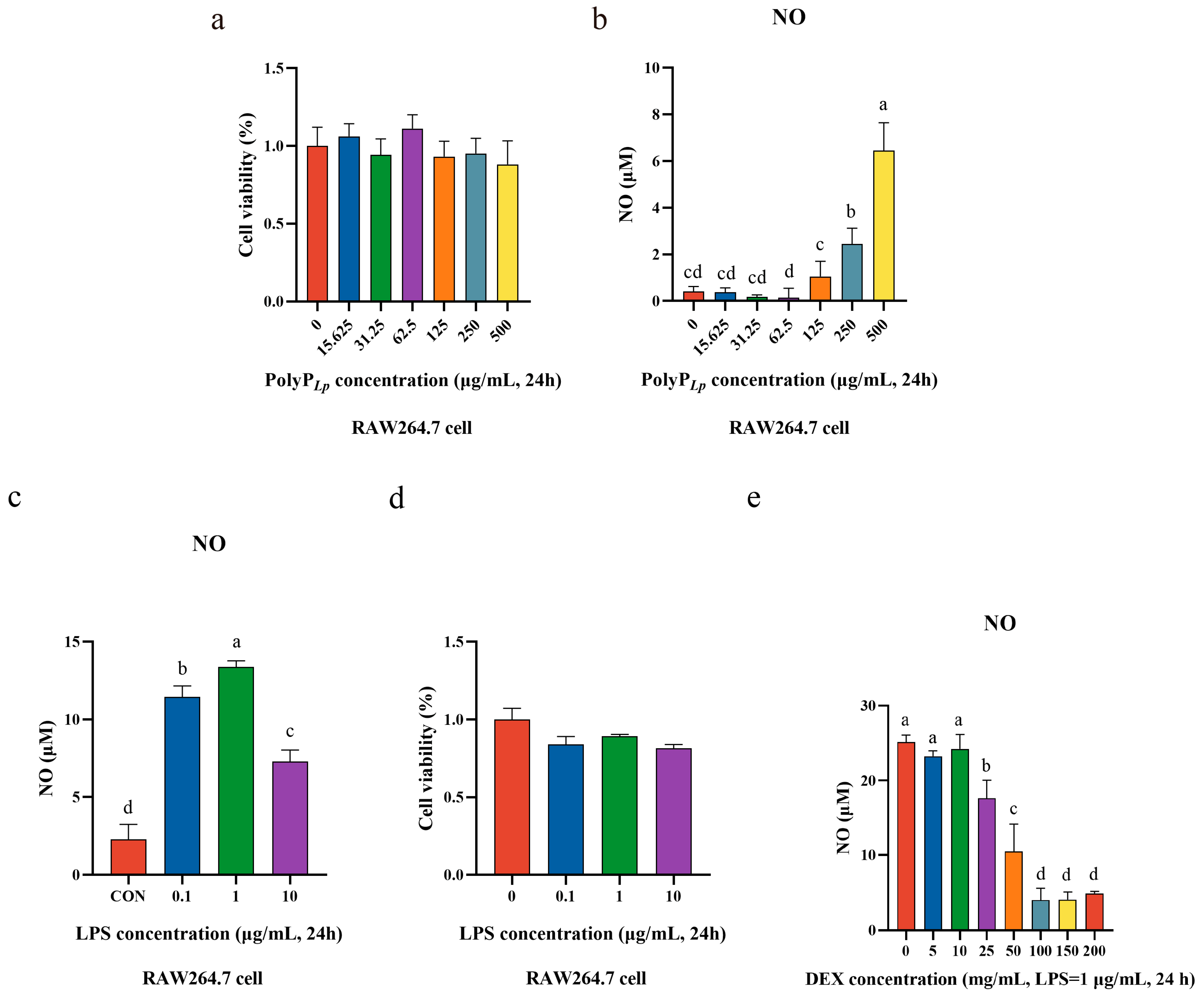
3.3. Oxidative Stress Model Establishment
3.4. PolyP Has No Inhibitory Effect on Cellular Proliferation
3.5. PolyP Repairs the Morphology of LPS-Activated Macrophages
3.6. PolyP Resists LPS-Induced Oxidative Stress in Macrophages
3.7. PolyP Regulates the Immune Response in LPS-Induced Macrophages at the Protein Level
3.8. PolyP Regulates the Immune Response in LPS-Induced Macrophages at the mRNA Level
4. Discussion
4.1. The Chain Length of PolyP Determines Its Immunomodulatory Functions
4.2. PolyP Has No Toxic Effect on Macrophages
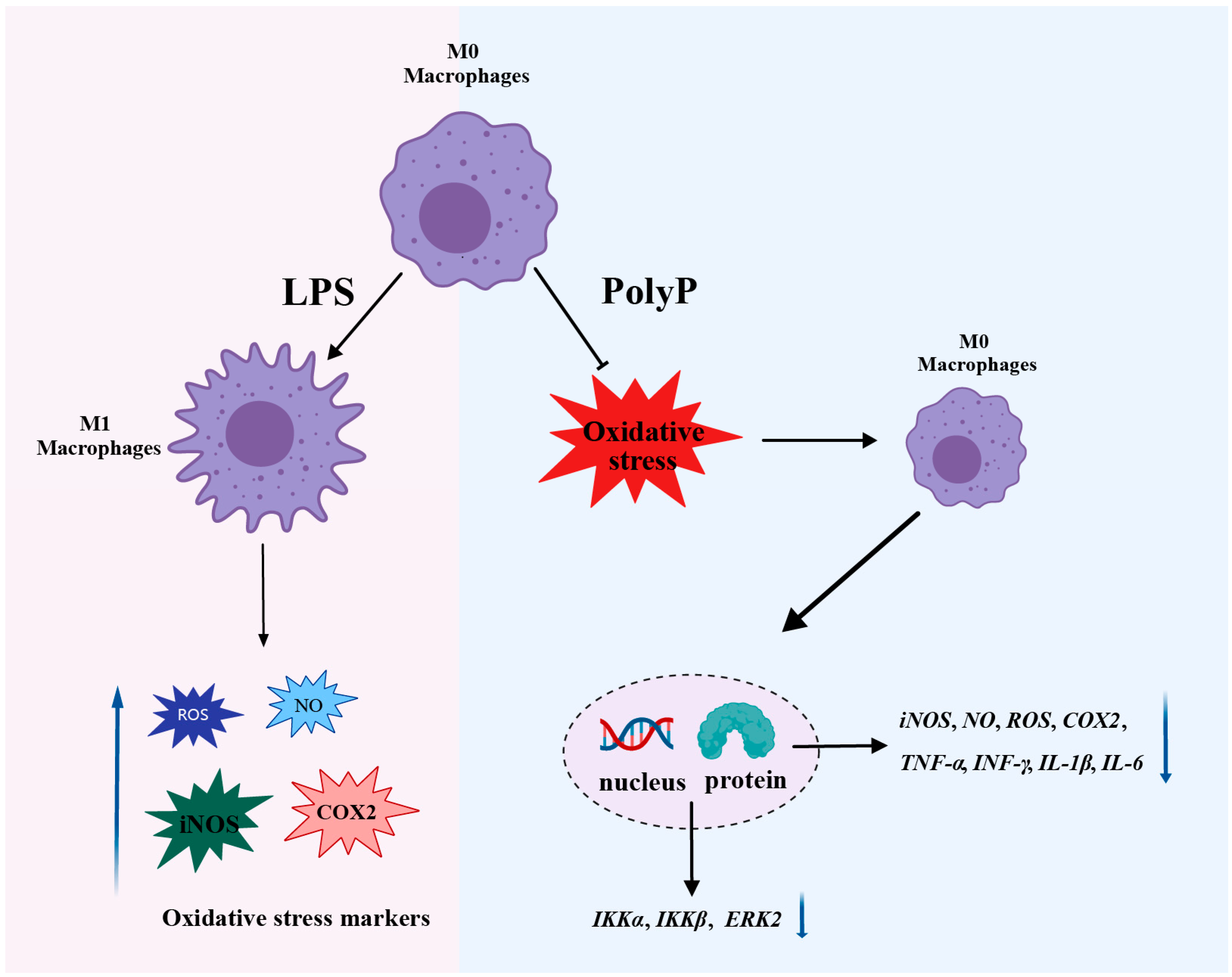
4.3. PolyP Regulates the Immune Response by Targeting the Differentiation of M1 Macrophages
4.4. PolyP Resists LPS-Activated Oxidative Stress in Macrophages
4.5. Superficial Mechanism of the Anti-Inflammatory Properties of PolyP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PolyP | Inorganic polyphosphate |
| LPS | Lipopolysaccharide |
| DEX | Dexamethasone |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| iNOS | Inducible nitric oxide synthase |
| IL-1β | Interleukin-1β |
| INF-γ | Interferon-γ |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| COX-2 | Cyclooxygenase-2 |
| DAPI | 4′,6-Diamidino-2-phenylindole dihydrochloride |
| PAGE | Polyacrylamide gel electrophoresis |
| NF-κB | Nuclear factor kappa-B |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular regulated protein kinase |
| IKK | IκB kinase |
| CXCL15 | C-X-C motif chemokine ligand 15 |
References
- Dahl, J.U.; Gray, M.J.; Bazopoulou, D.; Beaufay, F.; Lempart, J.; Koenigsknecht, M.J.; Wang, Y.; Baker, J.R.; Hasler, W.L.; Young, V.B.; et al. The anti-inflammatory drug mesalamine targets bacterial polyphosphate accumulation. Nat. Microbiol. 2017, 2, 16267. [Google Scholar]
- Hassanian, S.M.; Avan, A.; Ardeshirylajimi, A. Inorganic polyphosphate: A key modulator of inflammation. J. Thromb. Haemost. 2017, 15, 213–218. [Google Scholar] [PubMed]
- Ito, T.; Yamamoto, S.; Yamaguchi, K.; Sato, M.; Kaneko, Y.; Goto, S.; Goto, Y.; Narita, I. Inorganic polyphosphate potentiates lipopolysaccharide-induced macrophage inflammatory response. J. Biol. Chem. 2020, 295, 4014–4023. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, C.; Perez, M.; Huedo, P.; Altadill, T.; Espadaler-Mazo, J.; Arqués, J.L.; Zúñiga, M.; Monedero, V. Study of the biosynthesis and functionality of polyphosphate in Bifidobacterium longum KABP042. Sci. Rep. 2023, 13, 11076. [Google Scholar]
- Bowlin, M.Q.; Gray, M.J. Inorganic polyphosphate in host and microbe biology. Trends Microbiol. 2021, 29, 1013–1023. [Google Scholar] [CrossRef]
- Denoncourt, A.; Downey, M. Model systems for studying polyphosphate biology: A focus on microorganisms. Curr. Genet. 2021, 67, 331–346. [Google Scholar] [PubMed]
- Brown, M.R.W.; Kornberg, A. Inorganic polyphosphate in the origin and survival of species. Proc. Natl. Acad. Sci. USA 2004, 101, 16085–16087. [Google Scholar] [CrossRef] [PubMed]
- Saiki, A.; Ishida, Y.; Segawa, S.; Hirota, R.; Nakamura, T.; Kuroda, A. A Lactobacillus mutant capable of accumulating long-chain polyphosphates that enhance intestinal barrier function. Biosci. Biotechnol. Biochem. 2016, 80, 955–961. [Google Scholar]
- Kashima, S.; Fujiya, M.; Konishi, H.; Ueno, N.; Inaba, Y.; Moriichi, K.; Tanabe, H.; Ikuta, K.; Ohtake, T.; Kohgo, Y. Polyphosphate, an active molecule derived from probiotic Lactobacillus brevis, improves the fibrosis in murine colitis. Transl. Res. 2015, 166, 163–175. [Google Scholar] [CrossRef]
- Correa Deza, M.A.; Rodríguez de Olmos, A.; Suárez, N.E.; Font de Valdez, G.; Salva, S.; Gerez, C.L. Inorganic polyphosphate from the immunobiotic Lactobacillus rhamnosus CRL1505 prevents inflammatory response in the respiratory tract. Saudi J. Biol. Sci. 2021, 28, 5684–5692. [Google Scholar]
- Fujiya, M.; Ueno, N.; Kashima, S.; Tanaka, K.; Sakatani, A.; Ando, K.; Moriichi, K.; Konishi, H.; Kamiyama, N.; Tasaki, Y.; et al. Long-chain polyphosphate is a potential agent for inducing mucosal healing of the colon in ulcerative colitis. Clin. Pharmacol. Ther. 2020, 107, 452–461. [Google Scholar] [CrossRef]
- Segawa, S.; Fujiya, M.; Konishi, H.; Ueno, N.; Kobayashi, N.; Shigyo, T.; Kohgo, Y. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS ONE 2011, 6, e23278. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, S.; Konishi, H.; Fujiya, M.; Tanaka, H.; Murakami, Y.; Kashima, S.; Ando, K.; Ueno, N.; Moriichi, K.; Okumura, T. Probiotic-derived polyphosphate accelerates intestinal epithelia wound healing through inducing platelet-derived mediators. Mediat. Inflamm. 2021, 2021, 5582943. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, C.; Coll-Marqués, J.M.; Jadán-Piedra, C.; Vélez, D.; Devesa, V.; Zúñiga, M.; Monedero, V. Polyphosphate in Lactobacillus and its link to stress tolerance and probiotic properties. Front. Microbiol. 2018, 9, 1944. [Google Scholar]
- Qian, M.; Fang, X.; Wang, X. Autophagy and inflammation. Clin. Transl. Med. 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-inflammatory drugs as anticancer agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [PubMed]
- Biscu, F.; Zouzaf, A.; Cicia, D.; Pridans, C.; Matteoli, G. Innate immunity champions: The diverse functions of macrophages. Eur. J. Immunol. 2024, 54, e2451139. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Han, S.; Hou, Z.; Yang, C.; Zhao, Y. Tumor-associated macrophages within the immunological milieu: An emerging focal point for therapeutic intervention. Heliyon 2024, 10, e36839. [Google Scholar] [CrossRef]
- Hamidzadeh, K.; Christensen, S.M.; Dalby, E.; Chandrasekaran, P.; Mosser, D.M. Macrophages and the recovery from acute and chronic inflammation. Annu. Rev. Physiol. 2017, 79, 567–592. [Google Scholar] [CrossRef]
- Li, C.; Yu, X.; Han, X.; Lian, C.; Wang, Z.; Shao, S.; Shao, F.; Wang, H.; Ma, S.; Liu, J. Innate immune cells in tumor microenvironment: A new frontier in cancer immunotherapy. iScience 2024, 27, 110750. [Google Scholar]
- Yang, P.; Rong, X.; Gao, Z.; Wang, J.; Liu, Z. Metabolic and epigenetic regulation of macrophage polarization in atherosclerosis: Molecular mechanisms and targeted therapies. Pharmacol. Res. 2025, 212, 107588. [Google Scholar]
- Lv, H.; Zhou, Y.; Liu, B.; Guan, J.; Zhang, P.; Deng, X.; Li, D.; Wang, J. Polyphosphate kinase is required for the processes of virulence and persistence in Acinetobacter baumannii. Microbiol. Spectr. 2022, 10, e0123022. [Google Scholar] [CrossRef]
- Kulakova, A.N.; Hobbs, D.; Smithen, M.; Pavlov, E.; Gilbert, J.A.; Quinn, J.P.; McGrath, J.W. Direct quantification of inorganic polyphosphate in microbial cells using 4′-6-diamidino-2-phenylindole (DAPI). Environ. Sci. Technol. 2011, 45, 7799–7803. [Google Scholar]
- Ault-Riché, D.; Fraley, C.D.; Tzeng, C.M.; Kornberg, A. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J. Bacteriol. 1998, 180, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Takauji, S.; Konishi, H.; Fujiya, M.; Ueno, N.; Tanaka, H.; Sato, H.; Isozaki, S.; Kashima, S.; Moriichi, K.; Mizukami, Y.; et al. Polyphosphate, derived from Lactobacillus brevis, modulates the intestinal microbiome and attenuates acute pancreatitis. Dig. Dis. Sci. 2021, 66, 3872–3884. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.U.; Xie, L.; Jakob, U. Extraction and quantification of polyphosphate (polyP) from gram-negative bacteria. Bio Protoc. 2018, 8, e3011. [Google Scholar] [CrossRef] [PubMed]
- Aschar-Sobbi, R.; Abramov, A.Y.; Diao, C.; Kargacin, M.E.; Kargacin, G.J.; French, R.J.; Pavlov, E. High sensitivity, quantitative measurements of polyphosphate using a new DAPI-based approach. J. Fluoresc. 2008, 18, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Lorenzl, B.; Bachinski, N.; Wilhelm, C.; Miillerl, W.E.G.; Schröder, H.C. Osmotic-stress-induced synthesis and degradation of inorganic polyphosphates in the alga Phaeodactylum tricornutum. Mar. Ecol. Prog. Ser. 1995, 121, 279–288. [Google Scholar]
- Ghorbel, S.; Smirnov, A.; Chouayekh, H.; Sperandio, B.; Esnault, C.; Kormanec, J.; Virolle, M.J. Regulation of ppk expression and in vivo function of Ppk in Streptomyces lividans TK24. J. Bacteriol. 2006, 188, 6269–6276. [Google Scholar] [PubMed]
- Rao, N.N.; Gómez-García, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef] [PubMed]
- Sakatani, A.; Fujiya, M.; Ueno, N.; Kashima, S.; Sasajima, J.; Moriichi, K.; Ikuta, K.; Tanabe, H.; Kohgo, Y. Polyphosphate derived from Lactobacillus brevis inhibits colon cancer progression through induction of cell apoptosis. Anticancer Res. 2016, 36, 591–598. [Google Scholar] [PubMed]
- Kus, F.; Smolenski, R.T.; Tomczyk, M. Chain-length dependent effects of inorganic polyphosphate on endothelial function and nucleotide pool. Nucleosides Nucleotides Nucleic Acids 2024, 43, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sanchez, D.; Hernandez-Ruiz, L.; Ruiz, F.A.; Docampo, R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J. Biol. Chem. 2012, 287, 28435–28444. [Google Scholar]
- Diaz, J.M.; Ingall, E.D. Fluorometric quantification of natural inorganic polyphosphate. Environ. Sci. Technol. 2010, 44, 4665–4671. [Google Scholar]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef]
- Terashima-Hasegawa, M.; Ashino, T.; Kawazoe, Y.; Shiba, T.; Manabe, A.; Numazawa, S. Inorganic polyphosphate protects against lipopolysaccharide-induced lethality and tissue injury through regulation of macrophage recruitment. Biochem. Pharmacol. 2019, 159, 96–105. [Google Scholar] [CrossRef]
- Harada, K.; Shiba, T.; Doi, K.; Morita, K.; Kubo, T.; Makihara, Y.; Piattelli, A.; Akagawa, Y. Inorganic polyphosphate suppresses lipopolysaccharide-induced inducible nitric oxide synthase (iNOS) expression in macrophages. PLoS ONE 2013, 8, e74650. [Google Scholar] [CrossRef]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front. Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef] [PubMed]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxid. Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- de Groot, L.E.S.; van der Veen, T.A.; Martinez, F.O.; Hamann, J.; Lutter, R.; Melgert, B.N. Oxidative stress and macrophages: Driving forces behind exacerbations of asthma and chronic obstructive pulmonary disease? Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L369–L384. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, T.; Hu, R.; Zhu, R.; Li, C.; Ruan, Y.; Xie, X.; Li, Y. Next frontier in tumor immunotherapy: Macrophage-mediated immune evasion. Biomark. Res. 2021, 9, 72. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Qu, L.; Yan, S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.R.; Yoon, Y.N.; Son, D.I.; Seok, S.H. Cyclooxygenase-2 inhibition blocks M2 macrophage differentiation and suppresses metastasis in murine breast cancer model. PLoS ONE 2013, 8, e63451. [Google Scholar] [CrossRef]
- Luo, X.; Xiong, H.; Jiang, Y.; Fan, Y.; Zuo, C.; Chen, D.; Chen, L.; Lin, H.; Gao, J. Macrophage reprogramming via targeted ROS scavenging and COX-2 downregulation for alleviating inflammation. Bioconjug. Chem. 2023, 34, 1316–1326. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]





| Gene Name | Primer Sequence |
|---|---|
| IL-1β | Forward: GCAGCAGCACATCAACAAGAGC Reverse: AGGTCCACGGGAAAGACACAGG |
| IL-10 | Forward: TGTCATCGATTTCTCCCCTGTG Reverse: TTCATGGCCTTGTAGACACC |
| iNOS | Forward: TCTGCTGGCTTCCTGCTCTCC Reverse: TCTCCGTGGGCGTGTGATCC |
| TNF-α | Forward: GTGCCAGCCGATGGGTTGTAC Reverse: TGACGGCAGAGAGGAGGTTGAC |
| COX2 | Forward: GACAGATTGCTGGCCGGGTTG Reverse: CAGGGAGAAGCGTTTGCGGTAC |
| CXCL15 | Forward: TGGGTGAAGGCTACTGTTGG Reverse: AGCTTCATTGCCGGTGGAAA |
| IKKα | Forward: GCAGACCGTGAACATCCTCT Reverse: TCCAGGACAGTGAACGAGTG |
| IKKβ | Forward: AGGCGACACGTGAACAGAT Reverse: CTAAGAGCGGATGCGATG |
| ERK | Forward: GCAGATCCAGATCATGATCACAC Reverse: CTGTGACTGAAGATGGTGACTC |
| GADPH | Forward: GTAACCCGTTGAACCCCATT Reverse: CCATCCAATCGGTAGTAGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zheng, A.; Chen, Z.; Wang, X.; Chen, J.; Zou, Z.; Liu, G. Lactobacillus plantarum-Derived Inorganic Polyphosphate Regulates Immune Function via Inhibiting M1 Polarization and Resisting Oxidative Stress in Macrophages. Antioxidants 2025, 14, 428. https://doi.org/10.3390/antiox14040428
Li S, Zheng A, Chen Z, Wang X, Chen J, Zou Z, Liu G. Lactobacillus plantarum-Derived Inorganic Polyphosphate Regulates Immune Function via Inhibiting M1 Polarization and Resisting Oxidative Stress in Macrophages. Antioxidants. 2025; 14(4):428. https://doi.org/10.3390/antiox14040428
Chicago/Turabian StyleLi, Shuzhen, Aijuan Zheng, Zhimin Chen, Xiaoying Wang, Jiang Chen, Zhiheng Zou, and Guohua Liu. 2025. "Lactobacillus plantarum-Derived Inorganic Polyphosphate Regulates Immune Function via Inhibiting M1 Polarization and Resisting Oxidative Stress in Macrophages" Antioxidants 14, no. 4: 428. https://doi.org/10.3390/antiox14040428
APA StyleLi, S., Zheng, A., Chen, Z., Wang, X., Chen, J., Zou, Z., & Liu, G. (2025). Lactobacillus plantarum-Derived Inorganic Polyphosphate Regulates Immune Function via Inhibiting M1 Polarization and Resisting Oxidative Stress in Macrophages. Antioxidants, 14(4), 428. https://doi.org/10.3390/antiox14040428








