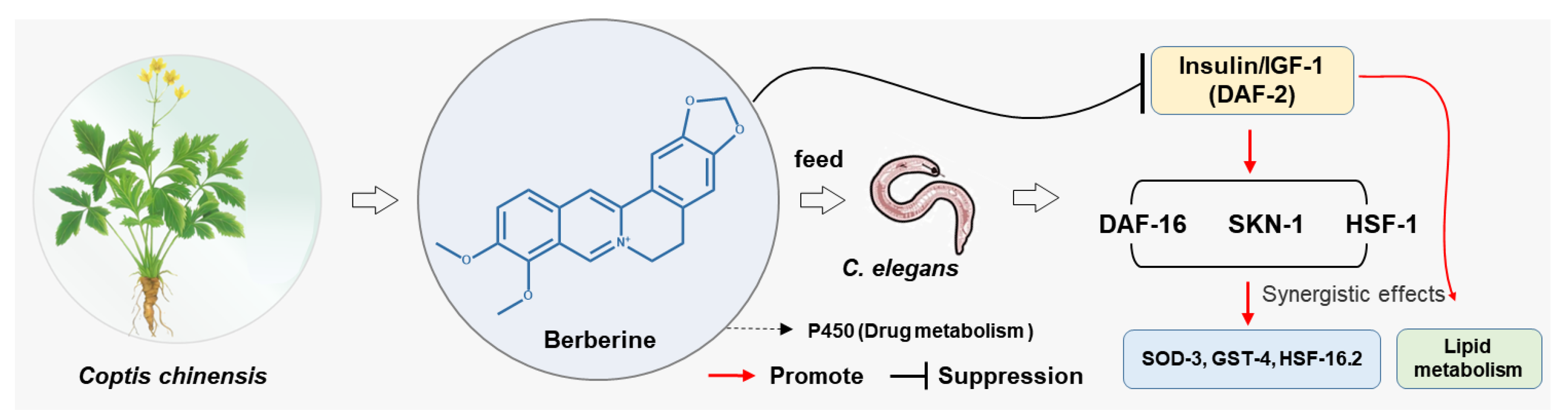
Berberine Extends Lifespan in C. elegans Through Multi-Target Synergistic Antioxidant Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Berberine Solution
2.2. C. elegans Strains
2.3. Synchronization Methods
2.4. Lifespan Assay
2.5. Bacterial Growth Assay
2.6. Growth and Development
2.7. Locomotor Ability
2.8. Pharyngeal Pumping Rate
2.9. Lipofuscin Content
2.10. Fertility Assay
2.11. Heat Stress Assay
2.12. Acute Oxidative Stress Assay
2.13. RNA Extraction, Reverse Transcription, and qRT-PCR
2.14. Whole-Genome Transcriptome
2.15. Statistical Analysis
3. Results
3.1. Effects of Berberine on the Lifespan and Biological Characteristics of C. elegans
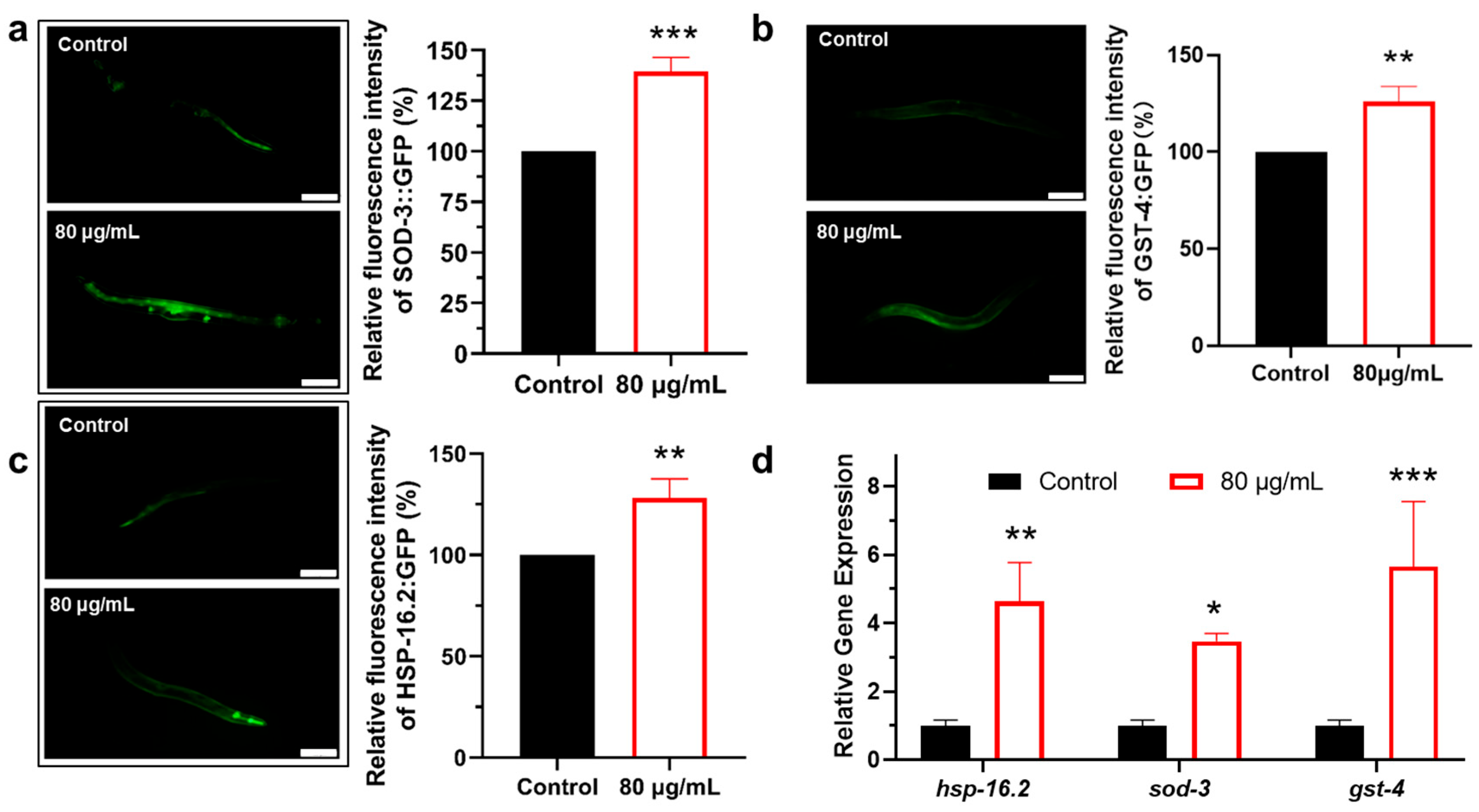
3.2. Berberine Enhances Oxidative Stress Resistance in C. elegans
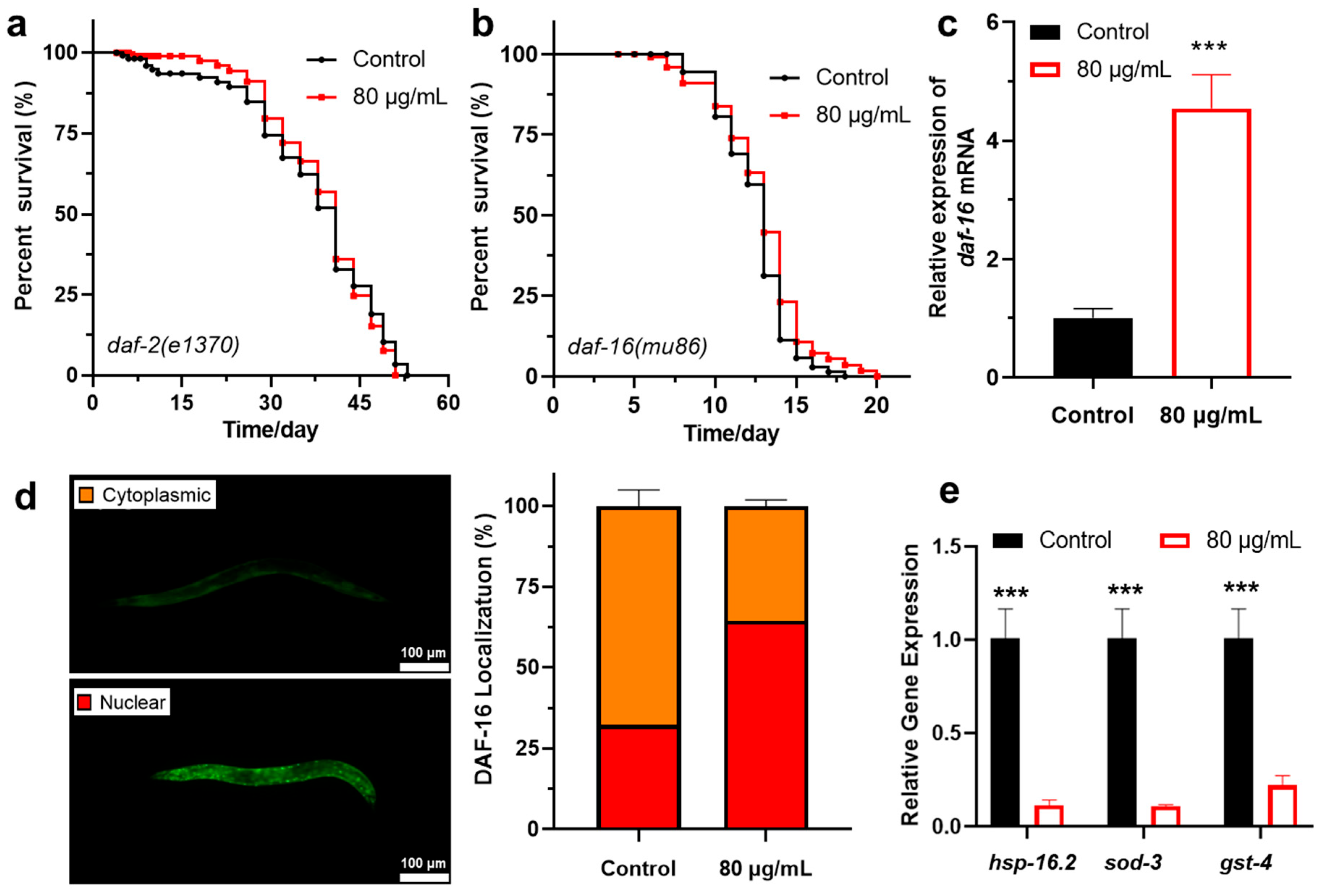
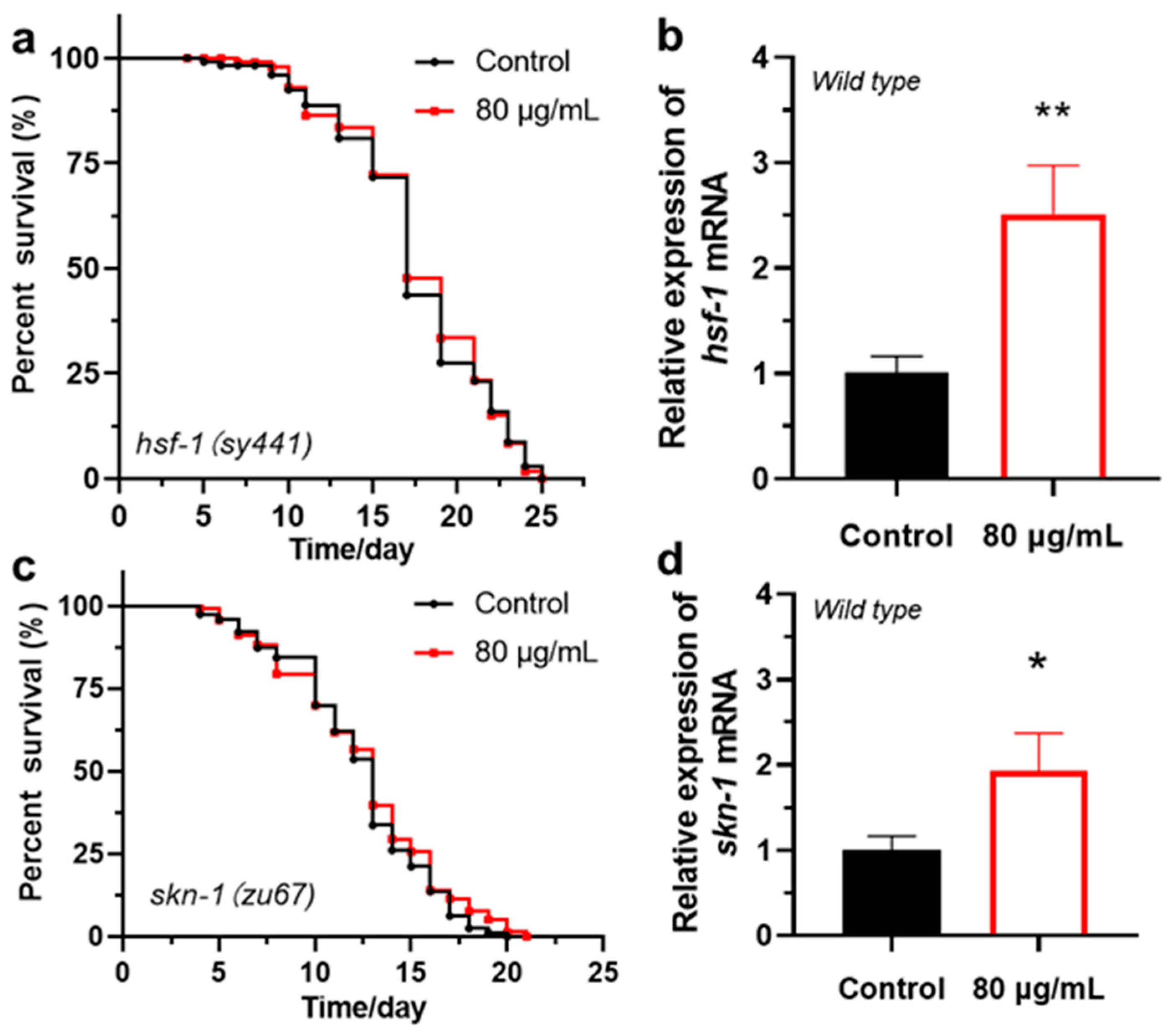
3.3. Berberine Extends the Lifespan of C. elegans via the Insulin/IGF-1 Signaling Pathway
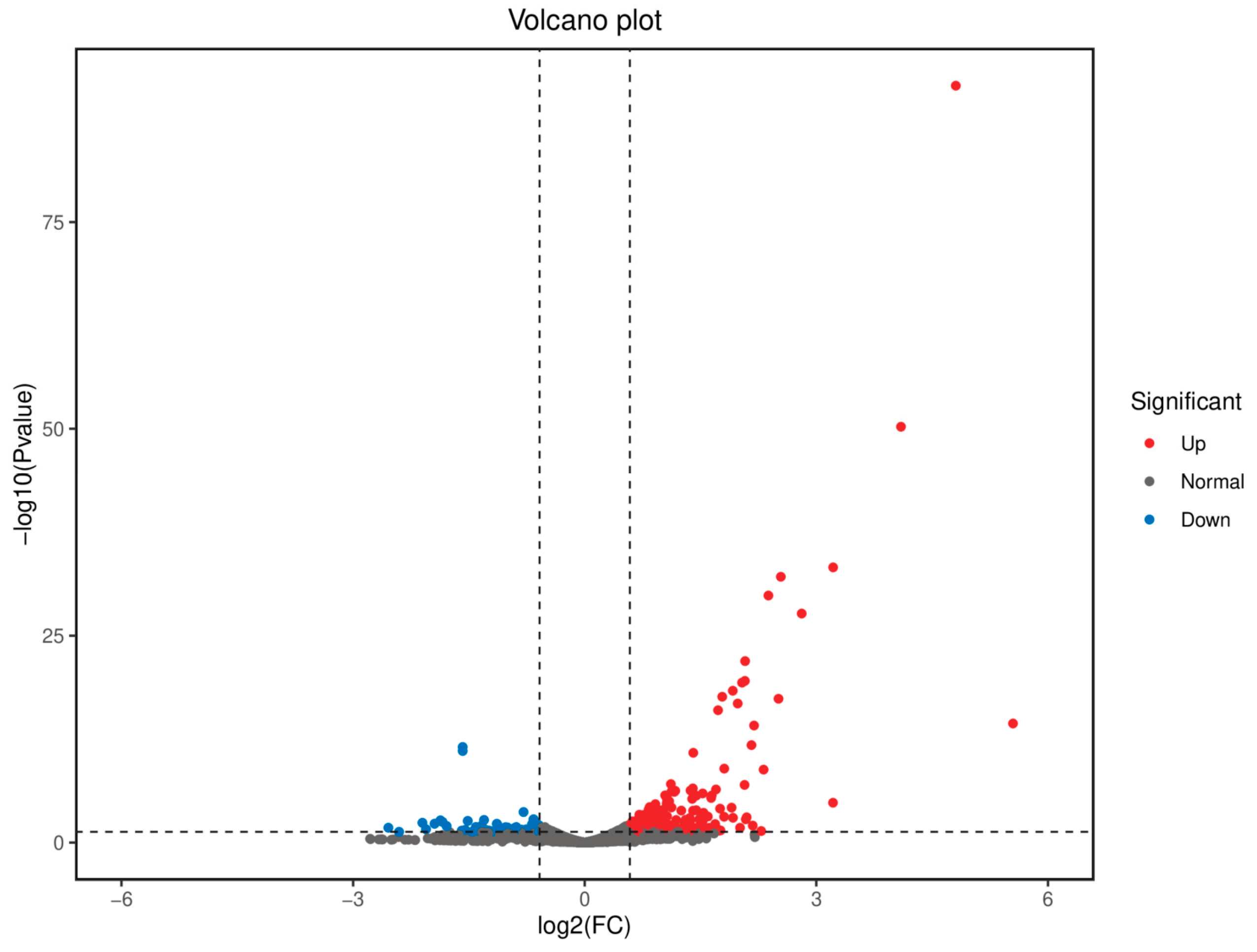
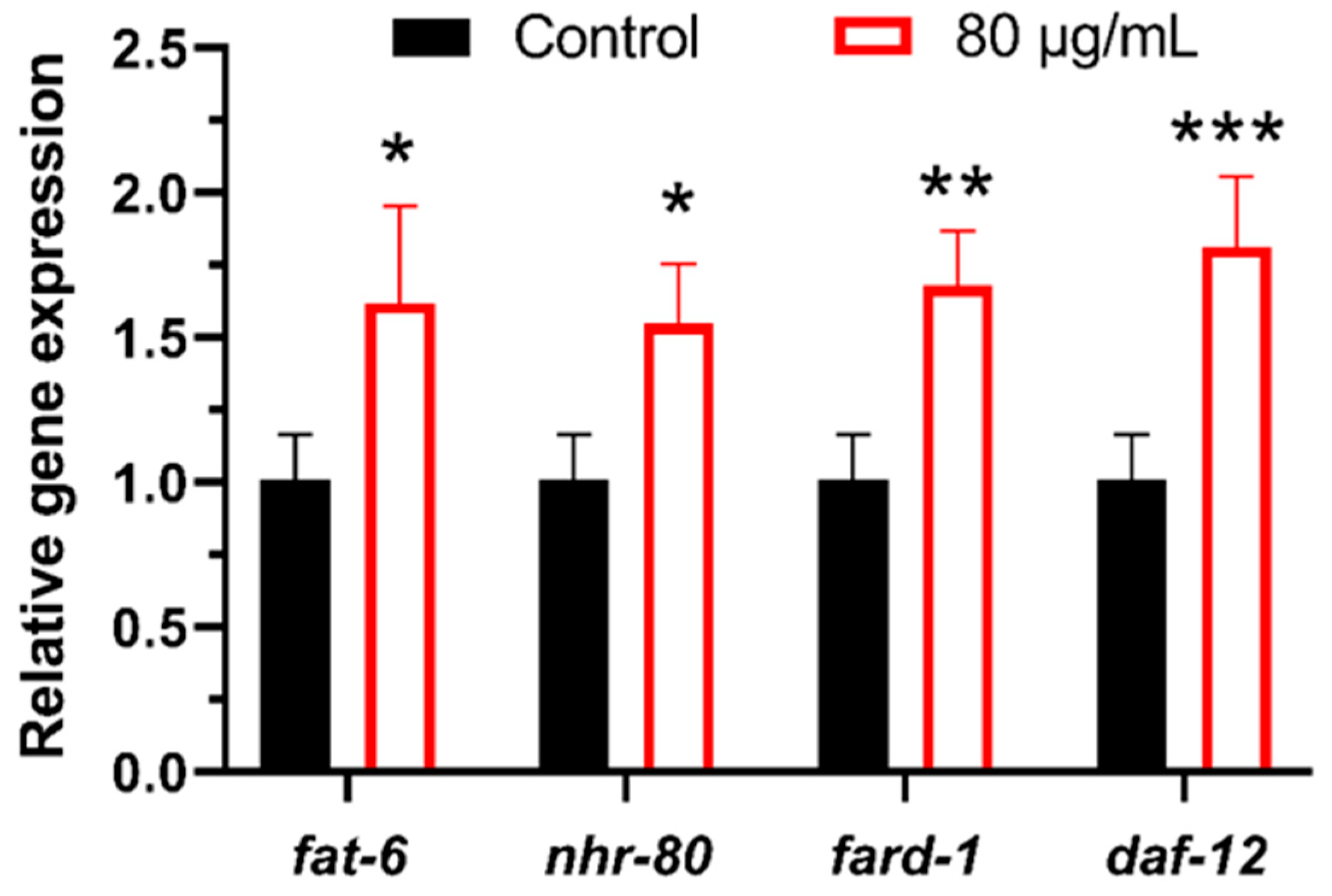
3.4. Whole-Genome Transcriptional Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stroustrup, N.; Anthony, W.E.; Nash, Z.M.; Gowda, V.; Gomez, A.; López-Moyado, I.F.; Apfeld, J.; Fontana, W. The temporal scaling of Caenorhabditis elegans ageing. Nature 2016, 530, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Yao, Z.; van Velthoven, C.T.J.; Kaplan, E.S.; Glattfelder, K.; Barlow, S.T.; Boyer, G.; Carey, D.; Casper, T.; Chakka, A.B.; et al. Brain-wide cell-type-specific transcriptomic signatures of healthy ageing in mice. Nature 2025, 638, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, B. Pro-Aging Metabolic Reprogramming: A Unified Theory of Aging. Engineering 2024, 44, 37–43. [Google Scholar] [CrossRef]
- von Zglinicki, T. Oxidative stress and cell senescence as drivers of ageing: Chicken and egg. Ageing Res. Rev. 2024, 102, 102558. [Google Scholar] [CrossRef]
- Li, D.; Yu, Q.; Wu, R.; Tuo, Z.; Wang, J.; Ye, L.; Shao, F.; Chaipanichkul, P.; Yoo, K.H.; Wei, W.; et al. Interactions between oxidative stress and senescence in cancer: Mechanisms, therapeutic implications, and future perspectives. Redox Biol. 2024, 73, 103208. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, H.-S.; Wang, X.; Dumont, A.S.; Liu, Q. Cellular senescence, DNA damage, and neuroinflammation in the aging brain. Trends Neurosci. 2024, 47, 461–474. [Google Scholar] [CrossRef]
- Cheriki, M.; Habibian, M.; Moosavi, S.J. Curcumin attenuates brain aging by reducing apoptosis and oxidative stress. Metab. Brain Dis. 2024, 39, 833–840. [Google Scholar] [CrossRef]
- Dossena, S.; Marino, A. Oxidative Stress and Antioxidants in Aging. Antioxidants 2024, 13, 1288. [Google Scholar] [CrossRef]
- Meng, J.; Lv, Z.; Qiao, X.; Li, X.; Li, Y.; Zhang, Y.; Chen, C. The decay of Redox-stress Response Capacity is a substantive characteristic of aging: Revising the redox theory of aging. Redox Biol. 2017, 11, 365–374. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Liu, K.; Zhao, J. Immobilization of Superoxide Dismutase in Mesoporous Silica and its Applications in Strengthening the Lifespan and Healthspan of Caenorhabditis elegans. Front. Bioeng. Biotechnol. 2022, 10, 795620. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.-C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The senescence-associated secretory phenotype and its regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, X.; Meng, F.; Zhao, Z.; Guan, S.; Wang, L. Ferulic Acid Supplementation Increases Lifespan and Stress Resistance via Insulin/IGF-1 Signaling Pathway in C. elegans. Int. J. Mol. Sci. 2021, 22, 4279. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.; Luo, Y.; Chen, Q.; Xu, M.; Ji, Y.; Jiang, X.; Qu, C. Poplar Bud (Populus) Extraction and Chinese Propolis Counteract Oxidative Stress in Caenorhabditis elegans via Insulin/IGF-1 Signaling Pathway. Antioxidants 2024, 13, 860. [Google Scholar] [CrossRef]
- Liu, D.; Song, Y.; Zheng, B.; Xie, J.; Chen, Y.; Xie, J.; Chen, X.; Yu, Q. EGCG Alleviates the Aging Toxicity Induced by 3-MCPD via IIS Pathway in Caenorhabditis elegans with Abnormal Reproduction and Heat Shock Protein. J. Agric. Food Chem. 2024, 72, 14315–14325. [Google Scholar] [CrossRef]
- Khan, J.; Pernicova, I.; Nisar, K.; Korbonits, M. Mechanisms of ageing: Growth hormone, dietary restriction, and metformin. Lancet Diabetes Endo. 2023, 11, 261–281. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, X.; Liu, N.; Ma, S.; Zhang, H.; Zhang, Z.; Yang, K.; Jiang, M.; Zheng, Z.; Qiao, Y.; et al. Metformin decelerates aging clock in male monkeys. Cell 2024, 187, 6358–6378.e29. [Google Scholar] [CrossRef]
- Rescher, L.; Singh, S.; Zahn, I.; Paulsen, F.; Schicht, M. Effect of Metformin on Meibomian Gland Epithelial Cells: Implications in Aging and Diabetic Dry Eye Disease. Life 2024, 14, 1682. [Google Scholar] [CrossRef]
- Anisimov, V.N.; Berstein, L.M.; Egormin, P.A.; Piskunova, T.S.; Popovich, I.G.; Zabezhinski, M.A.; Tyndyk, M.L.; Yurova, M.V.; Kovalenko, I.G.; Poroshina, T.E.; et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle 2008, 7, 2769–2773. [Google Scholar] [CrossRef]
- Glossmann, H.H.; Lutz, O.M.D. Metformin and Aging: A Review. Gerontology 2019, 65, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Yonn, M.-S. The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients 2017, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Sun, Y.; Li, J.; Zhang, J.; Hao, D.; Liu, W.; Wu, R.; Kong, F.; Peng, X.; Li, J. Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice. Aging Cell 2017, 16, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.I.; Chen, H.-y.; Cortazar-Chinarro, M.; Maklakov, A.A. Rapamycin additively extends lifespan in short- and long-lived lines of the nematode Caenorhabditis remanei. Exp. Gerontol. 2017, 90, 79–82. [Google Scholar] [CrossRef]
- Milani, G.; Cavalluzzi, M.M.; Solidoro, R.; Salvagno, L.; Quintieri, L.; Di Somma, A.; Rosato, A.; Corbo, F.; Franchini, C.; Duilio, A.; et al. Molecular Simplification of Natural Products: Synthesis, Antibacterial Activity, and Molecular Docking Studies of Berberine Open Models. Biomedicines 2021, 9, 452. [Google Scholar] [CrossRef]
- Jin, B.-R.; An, H.-J. Oral administration of berberine represses macrophage activation-associated benign prostatic hyperplasia: A pivotal involvement of the NF-κB. Aging 2021, 13, 20016–20028. [Google Scholar]
- Mushtaq, Z.; Imran, M.; Saeed, F.; Imran, A.; Ali, S.W.; Shahbaz, M.; Alsagaby, S.A.; Guerrero Sánchez, Y.; Umar, M.; Hussain, M.; et al. Berberine: A comprehensive Approach to combat human maladies. Int. J. Food Prop. 2023, 26, 787–807. [Google Scholar] [CrossRef]
- Purwaningsih, I.; Maksum, I.P.; Sumiarsa, D.; Sriwidodo, S. A Review of Fibraurea tinctoria and Its Component, Berberine, as an Antidiabetic and Antioxidant. Molecules 2023, 28, 1294. [Google Scholar] [CrossRef]
- Qin, S.; Tang, H.; Li, W.; Gong, Y.; Li, S.; Huang, J.; Fang, Y.; Yuan, W.; Liu, Y.; Wang, S.; et al. AMPK and its Activator Berberine in the Treatment of Neurodegenerative Diseases. Curr. Pharm. Des. 2020, 26, 5054–5066. [Google Scholar]
- Gasmi, A.; Asghar, F.; Zafar, S.; Oliinyk, P.; Khavrona, O.; Lysiuk, R.; Peana, M.; Piscopo, S.; Antonyak, H.; Pen, J.J.; et al. Berberine: Pharmacological Features in Health, Disease and Aging. Curr. Med. Chem. 2024, 31, 1214–1234. [Google Scholar]
- Mohammadinejad, R.; Ahmadi, Z.; Tavakol, S.; Ashrafizadeh, M. Berberine as a potential autophagy modulator. J. Cell. Physiol. 2019, 234, 14914–14926. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, L.; Zhou, H.; Cui, Y.; Chen, K.; Zhang, H.; Wu, Q.; Liu, F. Berberine extends healthspan and delays neurodegenerative diseases in Caenorhabditis elegans through ROS-dependent PMK-1/SKN-1 activation. Arch. Gerontol. Geriatr. 2025, 128, 105644. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, E.; Zhang, Y.; Saremi, B.; Yadavali, S.; Hakimi, A.; Dehghani, M.; Goodarzi, M.; Tu, X.; Robertson, S.; Lin, R.; et al. Hydralazine induces stress resistance and extends C. elegans lifespan by activating the NRF2/SKN-1 signalling pathway. Nat. Commun. 2017, 8, 2223. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Lin, D.; Guo, W.; Xu, Z.; Wang, L.; Guan, S. Ginsenoside Prolongs the Lifespan of C. elegans via Lipid Metabolism and Activating the Stress Response Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 9668. [Google Scholar] [CrossRef]
- Delori, F.C. Autofluorescence method to measure macular pigment optical densities fluorometry and autofluorescence imaging. Arch. Biochem. Biophys. 2004, 430, 156–162. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Xu, X.; Song, C.; Zhou, Y.; Xue, D.; Feng, Z.; Zhou, Y.; Li, X. Low methyl-esterified ginseng homogalacturonan pectins promote longevity of Caenorhabditis elegans via impairing insulin/IGF-1 signalling. Carbohyd. Polym. 2024, 346, 122600. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, C.; Cao, Y.; Chen, Y. Caenorhabditis elegans as an in vivo model for the identification of natural antioxidants with anti-aging actions. Biomed. Pharmacother. 2023, 167, 115594. [Google Scholar] [CrossRef]
- Di Somma, A.; Canè, C.; Rotondo, N.P.; Cavalluzzi, M.M.; Lentini, G.; Duilio, A. A Comparative Study of the Inhibitory Action of Berberine Derivatives on the Recombinant Protein FtsZ of E. coli. Int. J. Mol. Sci. 2023, 24, 5674. [Google Scholar] [CrossRef]
- Ansari, M.F.; Tan, Y.-M.; Sun, H.; Li, S.; Zhou, C.-H. Unique iminotetrahydroberberine-corbelled metronidazoles as potential membrane active broad-spectrum antibacterial agents. Bioorg. Med. Chem. Lett. 2022, 76, 129012. [Google Scholar] [CrossRef]
- Petronio Petronio, G.; Cutuli, M.A.; Magnifico, I.; Venditti, N.; Pietrangelo, L.; Vergalito, F.; Pane, A.; Scapagnini, G.; Di Marco, R. In Vitro and In Vivo Biological Activity of Berberine Chloride against Uropathogenic E. coli Strains Using Galleria mellonella as a Host Model. Molecules 2020, 25, 5010. [Google Scholar] [CrossRef]
- Militello, R.; Luti, S.; Gamberi, T.; Pellegrino, A.; Modesti, A.; Modesti, P.A. Physical Activity and Oxidative Stress in Aging. Antioxidants 2024, 13, 557. [Google Scholar] [CrossRef] [PubMed]
- Braendle, C.; Paaby, A. Life history in Caenorhabditis elegans: From molecular genetics to evolutionary ecology. Genetics 2024, 228, iyae151. [Google Scholar] [CrossRef] [PubMed]
- Baldensperger, T.; Jung, T.; Heinze, T.; Schwerdtle, T.; Höhn, A.; Grune, T. The age pigment lipofuscin causes oxidative stress, lysosomal dysfunction, and pyroptotic cell death. Free Radic. Biol. Med. 2024, 225, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; de Toda, I.M.; Cruces, J.; Garrido, A.; Gonzalez-Sanchez, M.; De la Fuente, M. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol. 2017, 12, 423–437. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Wang, Y.; Wang, J.; Wei, H.; Zhang, C.; Zhang, Q. Cyclocodon lancifolius fruit prolongs the lifespan of Caenorhabditis elegans via antioxidation and regulation of purine metabolism. Food Funct. 2024, 15, 3353–3364. [Google Scholar] [CrossRef]
- Almalki, W.H.; Almujri, S.S. Aging, ROS, and cellular senescence: A trilogy in the progression of liver fibrosis. Biogerontology 2024, 26, 10. [Google Scholar] [CrossRef]
- Kuk, M.U.; Kim, D.; Lee, Y.H.; Yoon, J.H.; Park, J.H.; Lee, Y.J.; So, B.H.; Kim, M.; Kwon, H.W.; Byun, Y.; et al. Synergistic ROS Reduction Through the Co-Inhibition of BRAF and p38 MAPK Ameliorates Senescence. Antioxidants 2024, 13, 1465. [Google Scholar] [CrossRef]
- Czachor, J.; Miłek, M.; Galiniak, S.; Stępień, K.; Dżugan, M.; Mołoń, M. Coffee Extends Yeast Chronological Lifespan through Antioxidant Properties. Int. J. Mol. Sci. 2020, 21, 9510. [Google Scholar] [CrossRef]
- Kim, H.-J.; Mun, J.-S.; Oh, S.-H.; Kim, J.-H. Antioxidant and Antiaging Activity of Houttuynia cordata Thunb. Ethyl Acetate Fraction in Caenorhabditis elegans. Nutrients 2024, 16, 4168. [Google Scholar] [CrossRef]
- Xu, Q.; Zheng, B.; Li, T.; Liu, R.H. Black goji berry anthocyanins extend lifespan and enhance the antioxidant defenses in via the JNK-1 and DAF-16/FOXO pathways. J. Sci. Food Agric. 2025, 105, 2282–2293. [Google Scholar] [CrossRef]
- Bahramibanan, F.; Rad, M.V.; Ranjbar, A.; Karbasi, A.; Abbasifard, A. Comparison of oxidative stress status in the kidney tissue of male rats treated with paraquat and nanoparaquat. Sci. Rep. 2025, 15, 389. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Kang, X.; Wang, Q.; Zhou, C.; Mohan, C.; Peng, A. Regulator of G protein signaling-1 modulates paraquat-induced oxidative stress and longevity via the insulin like signaling pathway in Caenorhabditis elegans. Toxicol. Lett. 2017, 273, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Tepper, R.G.; Ashraf, J.; Kaletsky, R.; Kleemann, G.; Murphy, C.T.; Bussemaker, H.J. PQM-1 Complements DAF-16 as a Key Transcriptional Regulator of DAF-2-Mediated Development and Longevity. Cell 2013, 154, 676–690. [Google Scholar] [CrossRef] [PubMed]
- Zečić, A.; Braeckman, B.P. DAF-16/FoxO in Caenorhabditis elegans and Its Role in Metabolic Remodeling. Cells 2020, 9, 109. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, H.; Park, S.-K. Butein Increases Resistance to Oxidative Stress and Lifespan with Positive Effects on the Risk of Age-Related Diseases in Caenorhabditis elegans. Antioxidants 2024, 13, 155. [Google Scholar] [CrossRef]
- Shen, R. Ageing in worms: N-acylethanolamines take control. Protein Cell 2011, 2, 689–690. [Google Scholar] [CrossRef]
- Frankino, P.A.; Siddiqi, T.F.; Bolas, T.; Bar-Ziv, R.; Gildea, H.K.; Zhang, H.; Higuchi-Sanabria, R.; Dillin, A. SKN-1 regulates stress resistance downstream of amino catabolism pathways. iScience 2022, 25, 104571. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, W.; Yue, J.; Xu, S.; Ma, W.; Zhao, C.; Wang, X.; Wang, D. HSF-1 mediated combined ginsenosides ameliorating Alzheimer’s disease like symptoms in Caernorhabditis elegans. Nutr. Neurosci. 2022, 25, 2136–2148. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Si, N.; Han, L.; Ren, W.; Xin, S.; Wang, H.; Zuo, R.; Wei, X.; Yang, J.; et al. Comparative Metabolism Study of Five Protoberberine Alkaloids in Liver Microsomes from Rat, Rhesus Monkey, and Human. Planta Med. 2017, 83, 1281–1288. [Google Scholar] [CrossRef]
- Jiang, B.; Meng, L.; Zhang, F.; Jin, X.; Zhang, G. Enzyme-inducing effects of berberine on cytochrome P450 1A2 in vitro and in vivo. Life Sci. 2017, 189, 1–7. [Google Scholar] [CrossRef]
- Och, A.; Och, M.; Nowak, R.; Podgórska, D.; Podgórski, R. Berberine, a Herbal Metabolite in the Metabolic Syndrome: The Risk Factors, Course, and Consequences of the Disease. Molecules 2022, 27, 1351. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, Z.; Yin, D. Multi- and trans-generational disturbances of perfluorobutane sulfonate and perfluorohexane sulfonate on lipid metabolism in Caenorhabditis elegans. Chemosphere 2021, 280, 130666. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.W.; Smith, R.L.; van Weeghel, M.; Kamble, R.; Janssens, G.E.; Houtkooper, R.H. Identification of key pathways and metabolic fingerprints of longevity in C. elegans. Exp. Gerontol. 2018, 113, 128–140. [Google Scholar] [CrossRef]
- Dumas, K.J.; Guo, C.; Wang, X.; Burkhart, K.B.; Adams, E.J.; Alam, H.; Hu, P.J. Functional divergence of dafachronic acid pathways in the control of C. elegans development and lifespan. Dev. Biol. 2010, 340, 605–612. [Google Scholar] [CrossRef]
- Woodall, B.P.; Gustafsson, Å.B. Autophagy—A key pathway for cardiac health and longevity. Acta Physiol. 2018, 223, e13074. [Google Scholar] [CrossRef]
- Escobar, K.A.; Cole, N.H.; Mermier, C.M.; VanDusseldorp, T.A. Autophagy and aging: Maintaining the proteome through exercise and caloric restriction. Aging Cell 2019, 18, e12876. [Google Scholar] [CrossRef]
- Anjum, J.; Mitra, S.; Das, R.; Alam, R.; Mojumder, A.; Emran, T.B.; Islam, F.; Rauf, A.; Hossain, M.J.; Aljohani, A.S.M.; et al. A renewed concept on the MAPK signaling pathway in cancers: Polyphenols as a choice of therapeutics. Pharmacol. Res. 2022, 184, 106398. [Google Scholar] [CrossRef]
- Yin, S.; Li, J.; Chen, J.; Zhou, Q.; Duan, D.b.p.; Lai, M.; Zhong, J.; He, J.; Chen, D.; Zeng, Z.; et al. LdCyPA attenuates MAPK pathway to assist Leishmania donovani immune escape in host cells. Acta Trop. 2024, 251, 107114. [Google Scholar] [CrossRef]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. BBA-Mol. Cell Res. 2014, 1843, 2150–2163. [Google Scholar] [CrossRef]
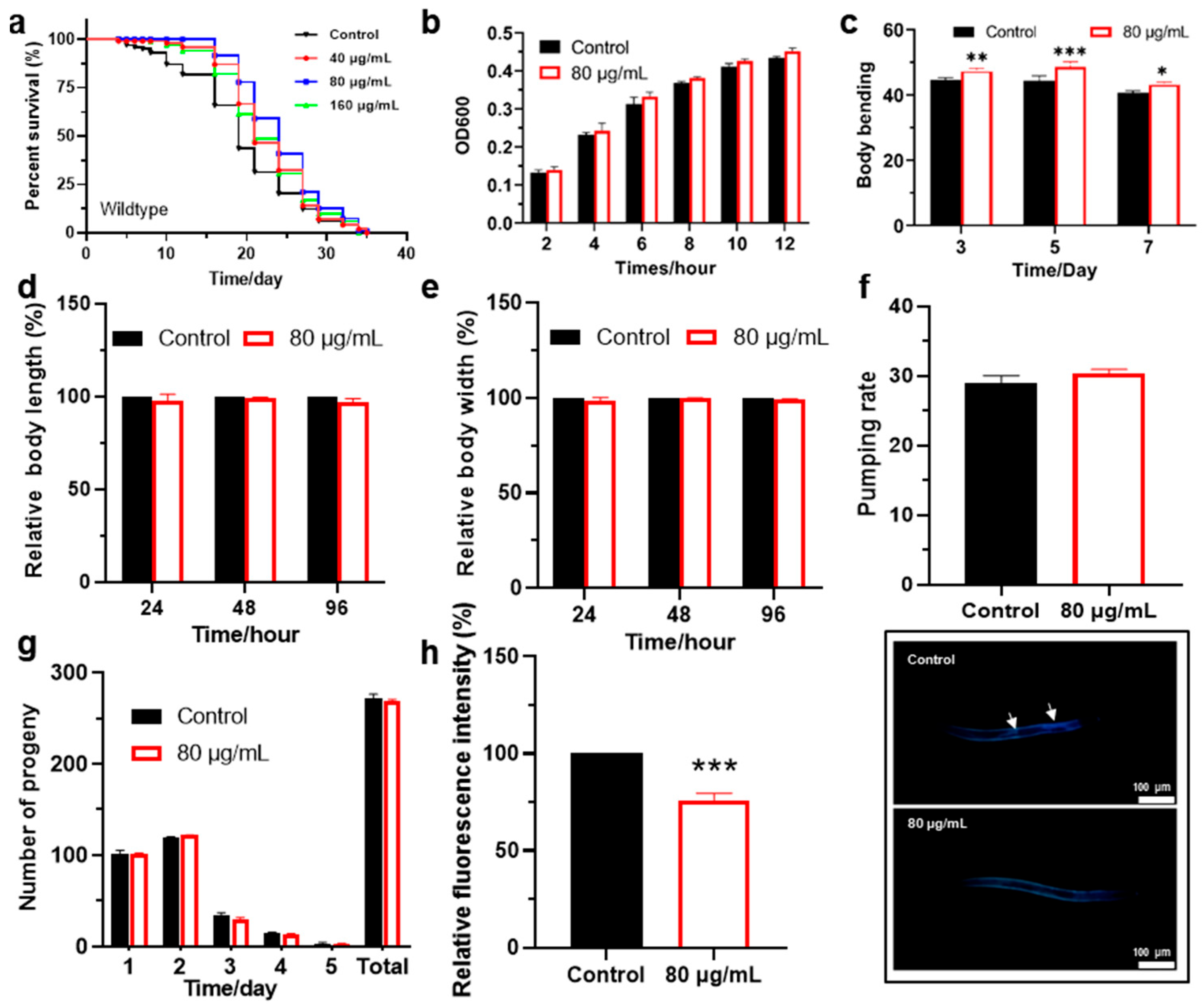


| Genotype | Treatment | Mean Lifespan (Mean ± SD, Day) | % (vs. Control) | p Value (Log-Rank Significance) |
|---|---|---|---|---|
| Wildtype | Control | 19.2 ± 0.7 | - | - |
| Wildtype | 40 μg/mL | 22.9 ± 0.6 | 19.6% | <0.01 ** |
| Wildtype | 80 μg/mL | 24.4 ± 0.6 | 27.3% | <0.001 *** |
| Wildtype | 160 μg/mL | 22.0 ± 0.6 | 14.7% | <0.01 ** |
| Genotype | Treatment | Mean Lifespan (Mean ± SD, Day) | % (vs. Control) | p Value (Log-Rank Significance) |
|---|---|---|---|---|
| daf-2(e1370) | Control | 36.0 ± 12.5 | - | - |
| 80 μg/mL | 38.3 ± 9.4 | 6.3% | 0.97 | |
| daf-16(mu86) | Control | 12.4 ± 2.1 | - | - |
| 80 μg/mL | 12.7 ± 2.8 | 2.3% | 0.11 |
| Genotype | Treatment | Mean Lifespan (Mean ± SD, Day) | % (vs. Control) | p Value (Log-Rank Significance) |
|---|---|---|---|---|
| hsf-1(sy441) | Control | 17.2 ± 4.6 | - | - |
| 80 μg/mL | 17.4 ± 4.4 | 1.0% | 0.87 | |
| skn-1(zu67) | Control | 11.7 ± 3.8 | - | - |
| 80 μg/mL | 12.2 ± 4.1 | 1.8% | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bei, Y.; Wang, T.; Guan, S. Berberine Extends Lifespan in C. elegans Through Multi-Target Synergistic Antioxidant Effects. Antioxidants 2025, 14, 450. https://doi.org/10.3390/antiox14040450
Bei Y, Wang T, Guan S. Berberine Extends Lifespan in C. elegans Through Multi-Target Synergistic Antioxidant Effects. Antioxidants. 2025; 14(4):450. https://doi.org/10.3390/antiox14040450
Chicago/Turabian StyleBei, Yingshuo, Ting Wang, and Shuwen Guan. 2025. "Berberine Extends Lifespan in C. elegans Through Multi-Target Synergistic Antioxidant Effects" Antioxidants 14, no. 4: 450. https://doi.org/10.3390/antiox14040450
APA StyleBei, Y., Wang, T., & Guan, S. (2025). Berberine Extends Lifespan in C. elegans Through Multi-Target Synergistic Antioxidant Effects. Antioxidants, 14(4), 450. https://doi.org/10.3390/antiox14040450






