Immunomodulatory Effects of Curcumin on CAR T-Cell Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Curcumin
2.3. Quantification of CD69 Expression in Jurkat Cells
2.4. Isolation and Culture of Primary Human T-Cells
2.5. Luciferase-Based Cytotoxicity Assay
2.6. Quantification of Cytokine Production
2.7. Identification of Curcumin Targets in CAR-Related Signaling Pathways
2.8. Protein–Protein Interaction Networks
2.9. Functional Enrichment and Pathway Analysis
2.10. Structural Preparation and Molecular Docking of Curcumin and Its Targets
2.11. Statistical Analysis
3. Results
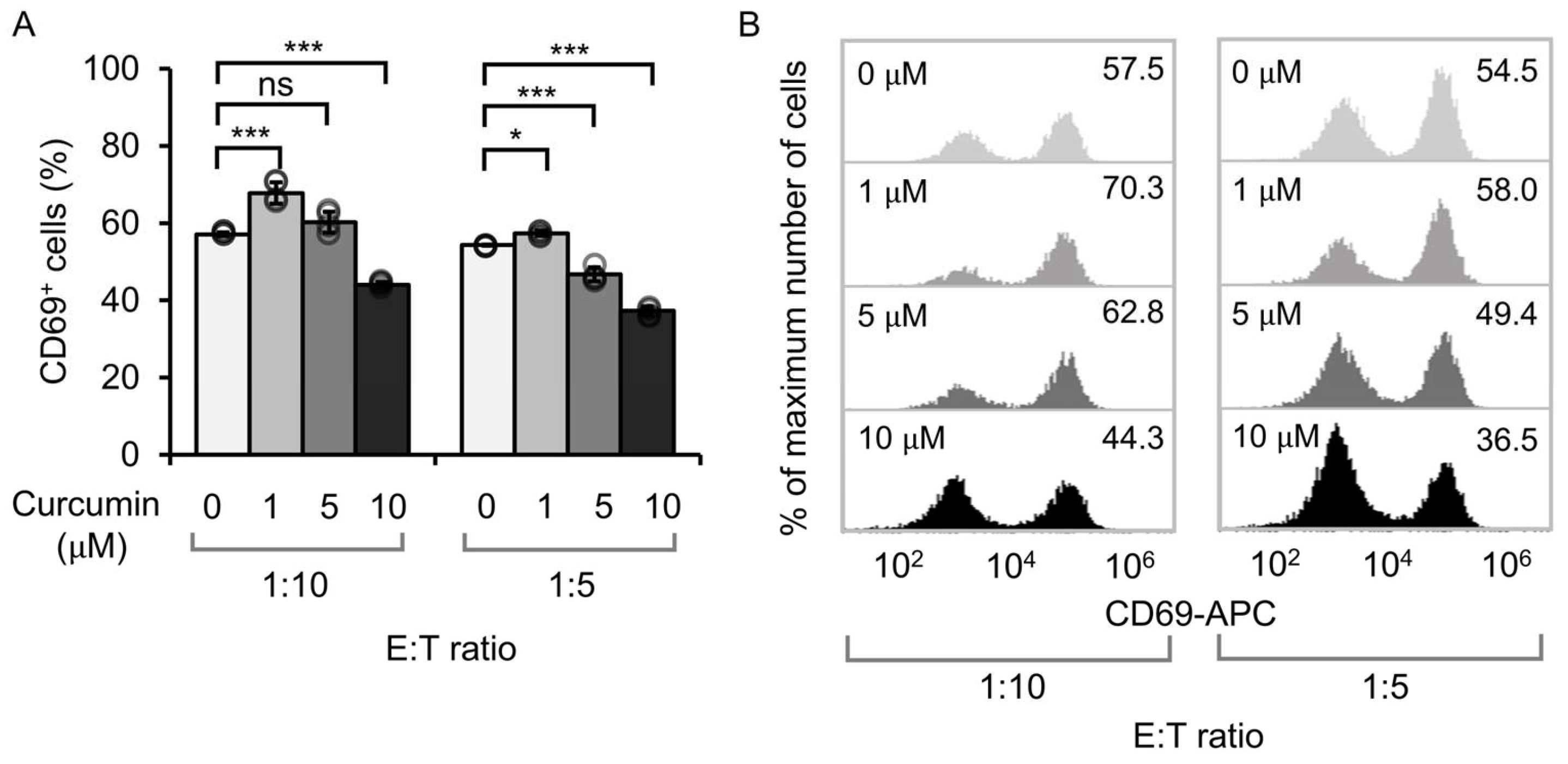
3.1. Effect of Curcumin Dosage on the Activation of Anti-CD19 CAR Jurkat Cells
3.2. Effect of Curcumin on Cytotoxicity and Cytokine Release
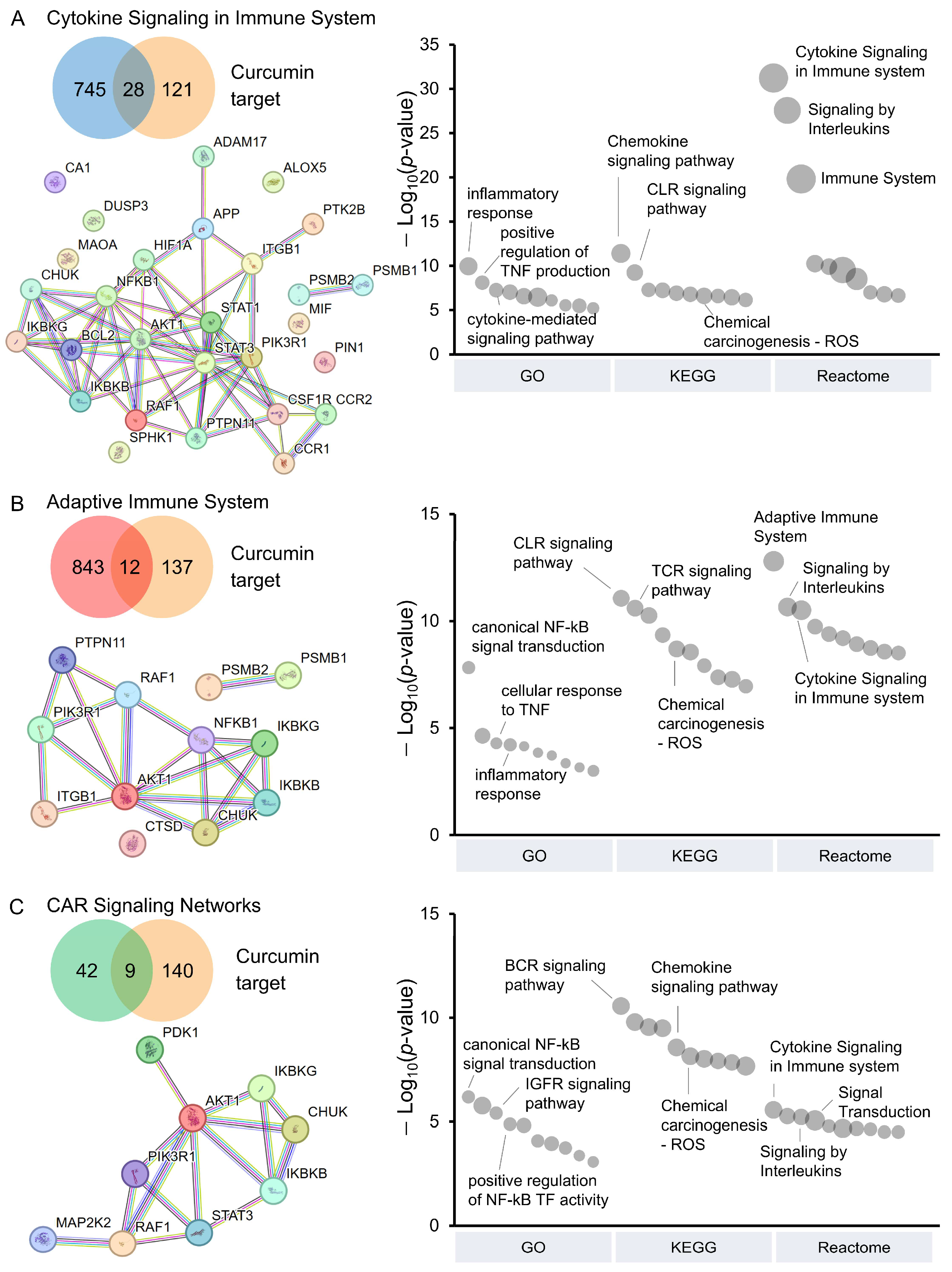
3.3. Pathway Analysis of CAR T-Cells Modulated by Curcumin
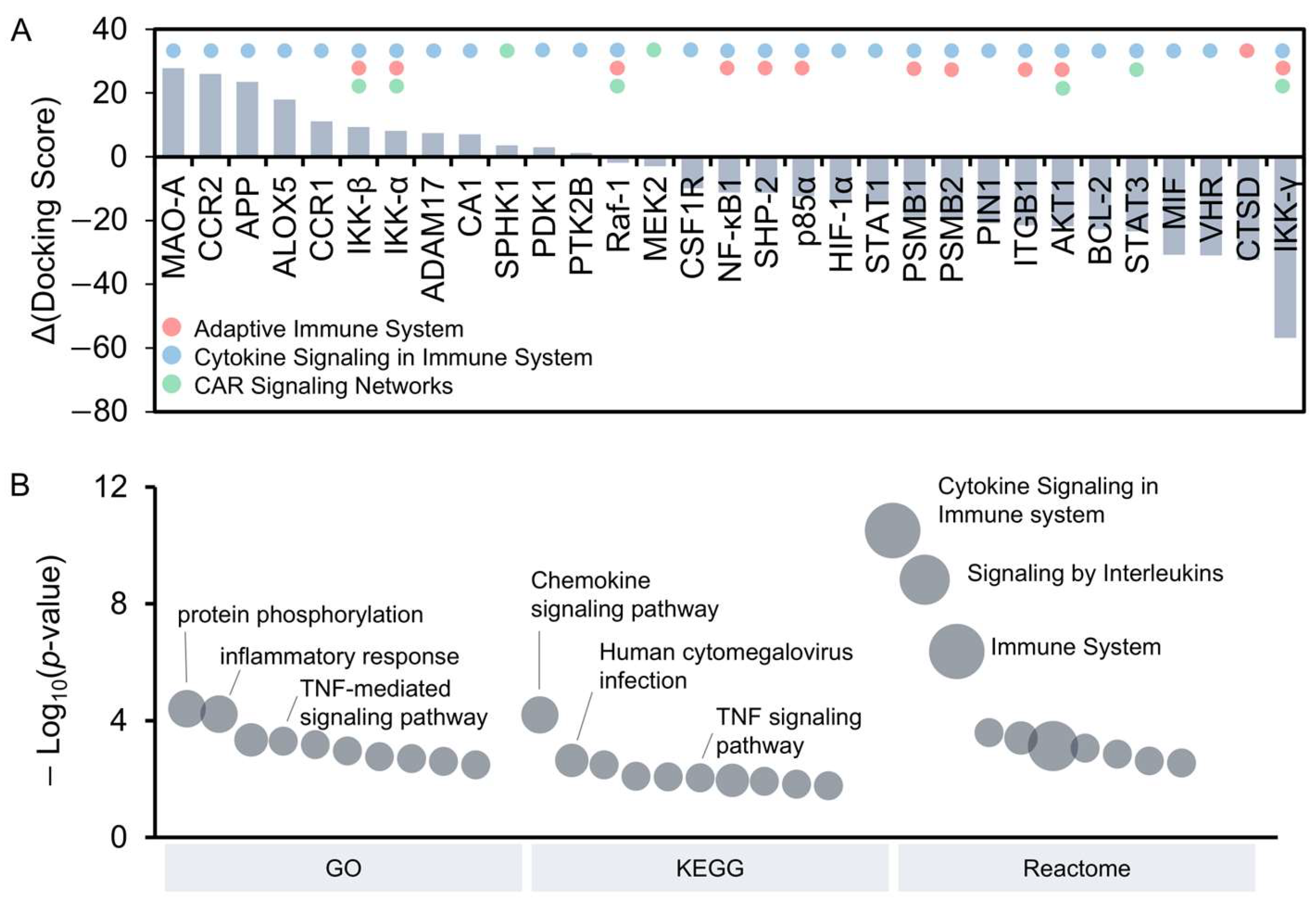
3.4. Identification of Potential Curcumin Targets Through Molecular Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and Anti-Inflammatory Effects of Curcumin/Turmeric Supplementation in Adults: A GRADE-Assessed Systematic Review and Dose–Response Meta-Analysis of Randomized Controlled Trials. Cytokine 2023, 164, 156144. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing up” of the Immune System by Curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by Curcumin as Revealed by Molecular Interaction Studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef]
- Ranjan, D.; Chen, C.; Johnston, T.D.; Jeon, H.; Nagabhushan, M. Curcumin Inhibits Mitogen Stimulated Lymphocyte Proliferation, NFκB Activation, and IL-2 Signaling. J. Surg. Res. 2004, 121, 171–177. [Google Scholar] [CrossRef]
- Yadav, V.S.; Mishra, K.P.; Singh, D.P.; Mehrotra, S.; Singh, V.K. Immunomodulatory Effects of Curcumin. Immunopharmacol. Immunotoxicol. 2005, 27, 485–497. [Google Scholar] [CrossRef]
- Kim, G.; Jang, M.S.; Son, Y.M.; Seo, M.J.; Ji, S.Y.; Han, S.H.; Jung, I.D.; Park, Y.-M.; Jung, H.J.; Yun, C.-H. Curcumin Inhibits CD4+ T Cell Activation, but Augments CD69 Expression and TGF-Β1-Mediated Generation of Regulatory T Cells at Late Phase. PLoS ONE 2013, 8, e62300. [Google Scholar] [CrossRef]
- Ranjan, D.; Johnston, T.D.; Wu, G.; Elliott, L.; Bondada, S.; Nagabhushan, M. Curcumin Blocks Cyclosporine A-Resistant CD28 Costimulatory Pathway of Human T-Cell Proliferation. J. Surg. Res. 1998, 77, 174–178. [Google Scholar] [CrossRef]
- Bose, S.; Panda, A.K.; Mukherjee, S.; Sa, G. Curcumin and Tumor Immune-Editing: Resurrecting the Immune System. Cell Div. 2015, 10, 6. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T Cell Immunotherapy for Human Cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of Chimeric Antigen Receptor T Cells: Recognition and Management. Blood J. Am. Soc. Hematol. 2016, 127, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Rivière, I.; Riddell, S. Therapeutic T Cell Engineering. Nature 2017, 545, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Porter, D.; Frey, N.; Wood, P.A.; Weng, Y.; Grupp, S.A. Grading of Cytokine Release Syndrome Associated with the CAR T Cell Therapy Tisagenlecleucel. J. Hematol. Oncol. 2018, 11, 35. [Google Scholar] [CrossRef]
- Kang, L.; Tang, X.; Zhang, J.; Li, M.; Xu, N.; Qi, W.; Tan, J.; Lou, X.; Yu, Z.; Sun, J. Interleukin-6-Knockdown of Chimeric Antigen Receptor-Modified T Cells Significantly Reduces IL-6 Release from Monocytes. Exp. Hematol. Oncol. 2020, 9, 11. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Current Understanding and Management of CAR T Cell-Associated Toxicities. Nat. Rev. Clin. Oncol. 2024, 21, 501–521. [Google Scholar] [CrossRef]
- Gust, J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A. Endothelial Activation and Blood–Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine Release Syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef]
- Su, M.; Chen, L.; Xie, L.; Fleurie, A.; Jonquieres, R.; Cao, Q.; Li, B.; Liang, J.; Tang, Y. Identification of Early Predictive Biomarkers for Severe Cytokine Release Syndrome in Pediatric Patients with Chimeric Antigen Receptor T-Cell Therapy. Front. Immunol. 2024, 15, 1450173. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Hunter, B.D.; Redd, R.; Rodig, S.J.; Chen, P.-H.; Wright, K.; Lipschitz, M.; Ritz, J.; Kamihara, Y.; Armand, P. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J. Clin. Oncol. 2020, 38, 3095–3106. [Google Scholar] [CrossRef] [PubMed]
- Strati, P.; Ahmed, S.; Furqan, F.; Fayad, L.E.; Lee, H.J.; Iyer, S.P.; Nair, R.; Nastoupil, L.J.; Parmar, S.; Rodriguez, M.A. Prognostic Impact of Corticosteroids on Efficacy of Chimeric Antigen Receptor T-Cell Therapy in Large B-Cell Lymphoma. Blood J. Am. Soc. Hematol. 2021, 137, 3272–3276. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Neelapu, S.S.; Bartlett, N.L.; Lekakis, L.J.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; Timmerman, J.M. Preliminary Results of Prophylactic Tocilizumab after Axicabtageneciloleucel (Axi-Cel; KTE-C19) Treatment for Patients with Refractory, Aggressive Non-Hodgkin Lymphoma (NHL). Blood 2017, 130, 1547. [Google Scholar] [CrossRef]
- Schiff, M.H.; Kremer, J.M.; Jahreis, A.; Vernon, E.; Isaacs, J.D.; van Vollenhoven, R.F. Integrated Safety in Tocilizumab Clinical Trials. Arthritis Res. Ther. 2011, 13, R141. [Google Scholar] [CrossRef]
- Logue, J.M.; Zucchetti, E.; Bachmeier, C.A.; Krivenko, G.S.; Larson, V.; Ninh, D.; Grillo, G.; Cao, B.; Kim, J.; Chavez, J.C. Immune Reconstitution and Associated Infections Following Axicabtagene Ciloleucel in Relapsed or Refractory Large B-Cell Lymphoma. Haematologica 2020, 106, 978. [Google Scholar] [CrossRef]
- Frigault, M.J.; Nikiforow, S.; Mansour, M.K.; Hu, Z.-H.; Horowitz, M.M.; Riches, M.L.; Hematti, P.; Turtle, C.J.; Zhang, M.-J.; Perales, M.-A. Tocilizumab Not Associated with Increased Infection Risk after CAR T-Cell Therapy: Implications for COVID-19? Blood J. Am. Soc. Hematol. 2020, 136, 137–139. [Google Scholar] [CrossRef]
- Martinez, M.; Moon, E.K. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Front. Immunol. 2019, 10, 128. [Google Scholar] [CrossRef]
- Ligtenberg, M.A.; Mougiakakos, D.; Mukhopadhyay, M.; Witt, K.; Lladser, A.; Chmielewski, M.; Riet, T.; Abken, H.; Kiessling, R. Coexpressed Catalase Protects Chimeric Antigen Receptor–Redirected T Cells as Well as Bystander Cells from Oxidative Stress–Induced Loss of Antitumor Activity. J. Immunol. 2016, 196, 759–766. [Google Scholar] [CrossRef]
- Liu, R.; Peng, L.; Zhou, L.; Huang, Z.; Zhou, C.; Huang, C. Oxidative Stress in Cancer Immunotherapy: Molecular Mechanisms and Potential Applications. Antioxidants 2022, 11, 853. [Google Scholar] [CrossRef]
- Beavis, P.A.; Henderson, M.A.; Giuffrida, L.; Mills, J.K.; Sek, K.; Cross, R.S.; Davenport, A.J.; John, L.B.; Mardiana, S.; Slaney, C.Y. Targeting the Adenosine 2A Receptor Enhances Chimeric Antigen Receptor T Cell Efficacy. J. Clin. Investig. 2017, 127, 929–941. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, Y.; Allen, M.E.; Pan, Y.; Kyriakakis, P.; Lu, S.; Chang, Y.-J.; Wang, X.; Chien, S.; Wang, Y. Engineering Light-Controllable CAR T Cells for Cancer Immunotherapy. Sci. Adv. 2020, 6, eaay9209. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Huang, Z.; Wang, X.; Jin, Z.; Li, J.; Limsakul, P.; Zhu, L.; Allen, M.; Pan, Y.; et al. Control of the Activity of CAR-T Cells within Tumours via Focused Ultrasound. Nat. Biomed. Eng. 2021, 5, 1336–1347. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.; Gohlke, B.-O.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on Drug Classification and Target Prediction. Nucleic Acids Res. 2014, 42, W26–W31. [Google Scholar] [CrossRef]
- Milacic, M.; Beavers, D.; Conley, P.; Gong, C.; Gillespie, M.; Griss, J.; Haw, R.; Jassal, B.; Matthews, L.; May, B. The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res. 2024, 52, D672–D678. [Google Scholar] [CrossRef]
- Fabregat, A.; Sidiropoulos, K.; Viteri, G.; Forner, O.; Marin-Garcia, P.; Arnau, V.; D’Eustachio, P.; Stein, L.; Hermjakob, H. Reactome Pathway Analysis: A High-Performance in-Memory Approach. BMC Bioinform. 2017, 18, 142. [Google Scholar] [CrossRef]
- Daniels, K.G.; Wang, S.; Simic, M.S.; Bhargava, H.K.; Capponi, S.; Tonai, Y.; Yu, W.; Bianco, S.; Lim, W.A. Decoding CAR T Cell Phenotype Using Combinatorial Signaling Motif Libraries and Machine Learning. Science 2022, 378, 1194–1200. [Google Scholar] [CrossRef]
- Honikel, M.M.; Olejniczak, S.H. Co-Stimulatory Receptor Signaling in CAR-T Cells. Biomolecules 2022, 12, 1303. [Google Scholar] [CrossRef]
- Alviano, A.M.; Biondi, M.; Grassenis, E.; Biondi, A.; Serafini, M.; Tettamanti, S. Fully Equipped CARs to Address Tumor Heterogeneity, Enhance Safety, and Improve the Functionality of Cellular Immunotherapies. Front. Immunol. 2024, 15, 1407992. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S. The STRING Database in 2023: Protein–Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK Server for Integrated Protein–Protein Docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S.-Y. HDOCK: A Web Server for Protein–Protein and Protein–DNA/RNA Docking Based on a Hybrid Strategy. Nucleic Acids Res. 2017, 45, W365–W373. [Google Scholar] [CrossRef]
- Banerjee, S.; Ji, C.; Mayfield, J.E.; Goel, A.; Xiao, J.; Dixon, J.E.; Guo, X. Ancient Drug Curcumin Impedes 26S Proteasome Activity by Direct Inhibition of Dual-Specificity Tyrosine-Regulated Kinase 2. Proc. Natl. Acad. Sci. USA 2018, 115, 8155–8160. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Kim, Y.J.; Wang, Y.-C.; Li, Z.; Yu, J.; Zeng, S.; Ma, X.; Choi, I.Y.; Di Biase, S. Targeting Monoamine Oxidase A for T Cell–Based Cancer Immunotherapy. Sci. Immunol. 2021, 6, eabh2383. [Google Scholar] [CrossRef]
- Natarajan, C.; Bright, J.J. Curcumin Inhibits Experimental Allergic Encephalomyelitis by Blocking IL-12 Signaling through Janus Kinase-STAT Pathway in T Lymphocytes. J. Immunol. 2002, 168, 6506–6513. [Google Scholar] [CrossRef]
- Abe, Y.; Hashimoto, S.H.U.; Horie, T. Curcumin Inhibition of Inflammatory Cytokine Production by Human Peripheral Blood Monocytes and Alveolar Macrophages. Pharmacol. Res. 1999, 39, 41–47. [Google Scholar] [CrossRef]
- Kliem, C.; Merling, A.; Giaisi, M.; Köhler, R.; Krammer, P.H.; Li-Weber, M. Curcumin Suppresses T Cell Activation by Blocking Ca2+ Mobilization and Nuclear Factor of Activated T Cells (NFAT) Activation. J. Biol. Chem. 2012, 287, 10200–10209. [Google Scholar] [CrossRef]
- Benmebarek, M.-R.; Karches, C.H.; Cadilha, B.L.; Lesch, S.; Endres, S.; Kobold, S. Killing Mechanisms of Chimeric Antigen Receptor (CAR) T Cells. Int. J. Mol. Sci. 2019, 20, 1283. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T. Curcumin Reverses T Cell-Mediated Adaptive Immune Dysfunctions in Tumor-Bearing Hosts. Cell Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Bonacci, T.R.; Shou, P.; Landoni, E.; Woodcock, M.G.; Sun, C.; Savoldo, B.; Herring, L.E.; Emanuele, M.J.; Song, F. 4-1BB-Encoding CAR Causes Cell Death via Sequestration of the Ubiquitin-Modifying Enzyme A20. Cell Mol. Immunol. 2024, 21, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Shou, P.; Du, H.; Hirabayashi, K.; Chen, Y.; Herring, L.E.; Ahn, S.; Xu, Y.; Suzuki, K.; Li, G. THEMIS-SHP1 Recruitment by 4-1BB Tunes LCK-Mediated Priming of Chimeric Antigen Receptor-Redirected T Cells. Cancer Cell 2020, 37, 216–225. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; Ledbetter, J.A.; Gillespie, M.M.; Lindsten, T.; Thompson, C.B. T-Cell Proliferation Involving the CD28 Pathway Is Associated with Cyclosporine-Resistant Interleukin 2 Gene Expression. Mol. Cell Biol. 1987, 7, 4472–4481. [Google Scholar]
- Riley, J.L.; June, C.H. The CD28 Family: A T-Cell Rheostat for Therapeutic Control of T-Cell Activation. Blood 2005, 105, 13–21. [Google Scholar] [CrossRef]
- Philipson, B.I.; O’Connor, R.S.; May, M.J.; June, C.H.; Albelda, S.M.; Milone, M.C. 4-1BB Costimulation Promotes CAR T Cell Survival through Noncanonical NF-ΚB Signaling. Sci. Signal 2020, 13, eaay8248. [Google Scholar] [CrossRef]
- Vafadari, R.; Kraaijeveld, R.; Weimar, W.; Baan, C.C. Tacrolimus Inhibits NF-ΚB Activation in Peripheral Human T Cells. PLoS ONE 2013, 8, e60784. [Google Scholar] [CrossRef]
- Dyer, J.L.; Khan, S.Z.; Bilmen, J.G.; Hawtin, S.R.; Wheatley, M.; Michelangeli, F. Curcumin: A New Cell-Permeant Inhibitor of the Inositol 1, 4, 5-Trisphosphate Receptor. Cell Calcium 2002, 31, 45–52. [Google Scholar] [CrossRef]
- Li, G.; Boucher, J.C.; Kotani, H.; Park, K.; Zhang, Y.; Shrestha, B.; Wang, X.; Guan, L.; Beatty, N.; Abate-Daga, D. 4-1BB Enhancement of CAR T Function Requires NF-ΚB and TRAFs. JCI Insight 2018, 3, e121322. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential Therapeutic Effects of Curcumin, the Anti-Inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Poorebrahim, M.; Melief, J.; de Coaña, Y.P.; Wickström, S.L.; Cid-Arregui, A.; Kiessling, R. Counteracting CAR T Cell Dysfunction. Oncogene 2021, 40, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Renken, S.; Nakajima, T.; Magalhaes, I.; Mattsson, J.; Lundqvist, A.; Arnér, E.S.J.; Kiessling, R.; Wickström, S.L. Targeting of Nrf2 Improves Antitumoral Responses by Human NK Cells, TIL and CAR T Cells during Oxidative Stress. J. Immunother. Cancer 2022, 10, e004458. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Arahori, Y.; Okuyama, M.; Luis, P.B.; Joseph, A.I.; Kitakaze, T.; Goshima, N.; Schneider, C.; Inui, H.; Yamaji, R. Curcumin Activates G Protein-Coupled Receptor 97 (GPR97) in a Manner Different from Glucocorticoid. Biochem. Biophys. Res. Commun. 2022, 595, 41–46. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, T.; Wang, X.; Wei, X.; Chen, Y.; Guo, L.; Zhang, J.; Wang, C. Curcumin Modulates Macrophage Polarization through the Inhibition of the Toll-like Receptor 4 Expression and Its Signaling Pathways. Cell. Physiol. Biochem. 2015, 36, 631–641. [Google Scholar] [CrossRef]
- Mathew, D.; Hsu, W.-L. Antiviral Potential of Curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Hsieh, C. Phase I Clinical Trial of Curcumin, a Chemopreventive Agent, in Patients with High-Risk or Pre-Malignant Lesions. Anticancer. Res. 2001, 21, e2900. [Google Scholar]
- Pan, M.-H.; Huang, T.-M.; Lin, J.-K. Biotransformation of Curcumin through Reduction and Glucuronidation in Mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [CrossRef]
- Sharma, R.A.; Ireson, C.R.; Verschoyle, R.D.; Hill, K.A.; Williams, M.L.; Leuratti, C.; Manson, M.M.; Marnett, L.J.; Steward, W.P.; Gescher, A. Effects of Dietary Curcumin on Glutathione S-Transferase and Malondialdehyde-DNA Adducts in Rat Liver and Colon Mucosa: Relationship with Drug Levels1. Clin. Cancer Res. 2001, 7, 1452–1458. [Google Scholar]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.-M. Enhancing the Bioavailability and Bioactivity of Curcumin for Disease Prevention and Treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limsakul, P.; Srifa, P.; Huang, Z.; Zhu, L.; Wu, Y.; Charupanit, K. Immunomodulatory Effects of Curcumin on CAR T-Cell Therapy. Antioxidants 2025, 14, 454. https://doi.org/10.3390/antiox14040454
Limsakul P, Srifa P, Huang Z, Zhu L, Wu Y, Charupanit K. Immunomodulatory Effects of Curcumin on CAR T-Cell Therapy. Antioxidants. 2025; 14(4):454. https://doi.org/10.3390/antiox14040454
Chicago/Turabian StyleLimsakul, Praopim, Pemikar Srifa, Ziliang Huang, Linshan Zhu, Yiqian Wu, and Krit Charupanit. 2025. "Immunomodulatory Effects of Curcumin on CAR T-Cell Therapy" Antioxidants 14, no. 4: 454. https://doi.org/10.3390/antiox14040454
APA StyleLimsakul, P., Srifa, P., Huang, Z., Zhu, L., Wu, Y., & Charupanit, K. (2025). Immunomodulatory Effects of Curcumin on CAR T-Cell Therapy. Antioxidants, 14(4), 454. https://doi.org/10.3390/antiox14040454







