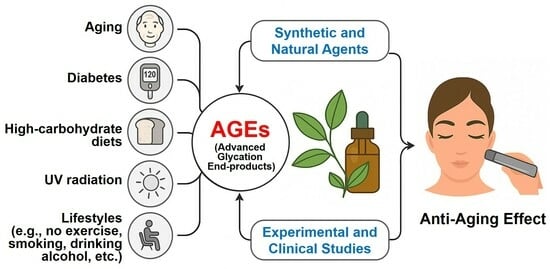
Synthetic and Natural Agents Targeting Advanced Glycation End-Products for Skin Anti-Aging: A Comprehensive Review of Experimental and Clinical Studies
Abstract
1. Introduction
2. Methods
3. Significance of Glycation-Induced Skin Aging
3.1. Experimental Evidence
3.2. Clinical Evidence
4. Interventions for Glycation-Induced Skin Aging
4.1. In Vitro Cell-Free Studies
4.2. In Vitro Studies Using Cultured Cells and Reconstructed Skin Models
4.3. Ex Vivo Studies
4.4. In Vivo Animal Studies
4.5. Clinical Studies
5. Discussion
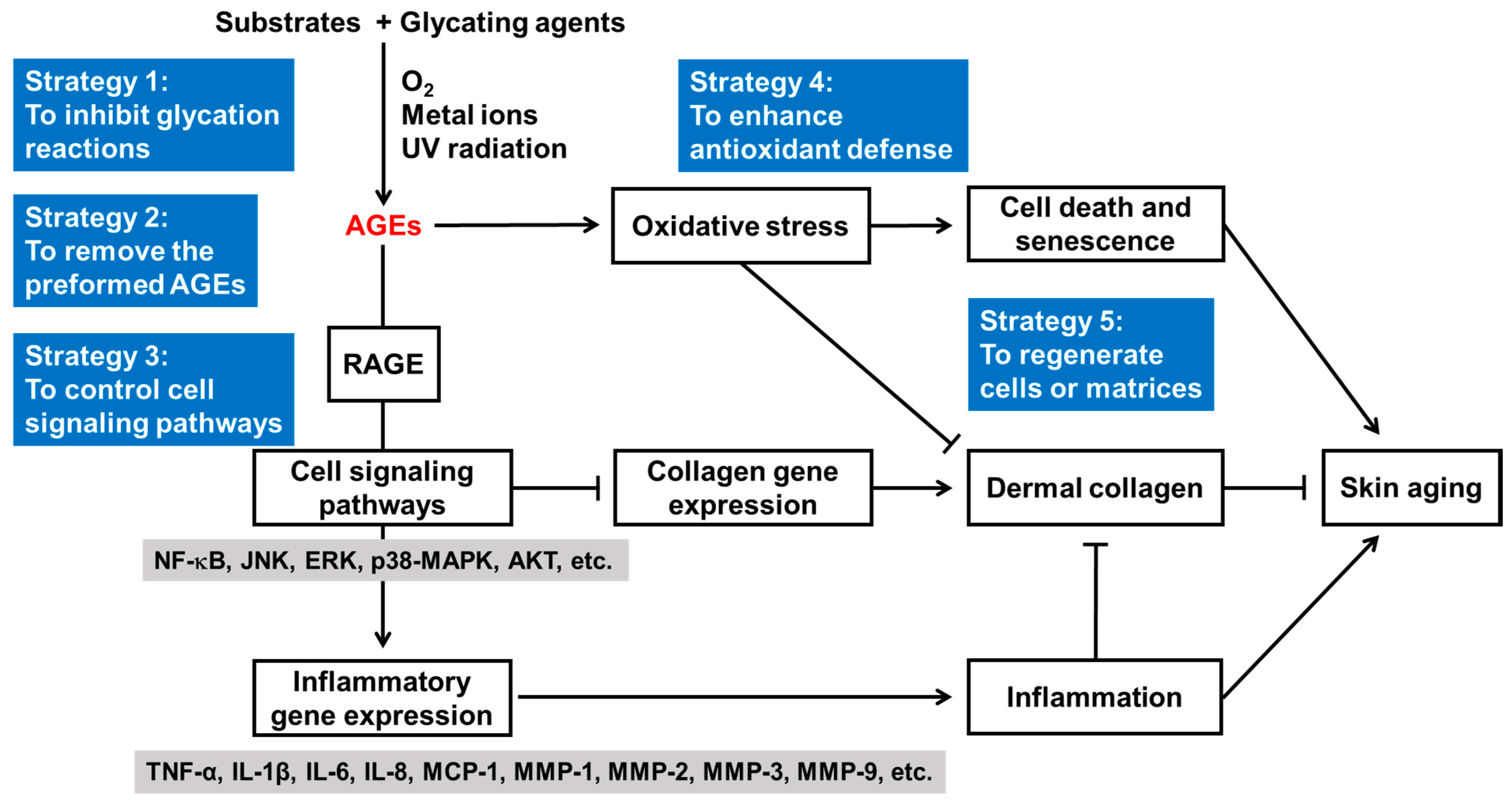
5.1. Skin Anti-Aging Strategies Targeting Glycation
5.2. Synthetic and Natural Agents Targeting Glycation
5.3. Mechanistic Insights and Therapeutic Applications
5.4. Future Tasks and Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGD | aminoguanidine |
| AGE | advanced glycation end-product |
| AKT | protein kinase B; AKT |
| BSA | bovine serum albumin |
| CAT | catalase |
| CCR | C-C chemokine receptor |
| CEL | Nε-(carboxyethyl)lysine |
| CK | cytokeratin |
| CMA | Nω-(carboxymethyl)arginine |
| CML | Nε-(carboxymethyl)lysine |
| COL | collagen |
| COX | cyclooxygenase |
| DTPA | diethylenetriaminepentaacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| ERK | extracellular signal-regulated kinase |
| GPX | glutathione peroxidase |
| Hb | hemoglobin |
| HDF | human dermal fibroblast |
| HEK | human epidermal keratinocyte |
| HUVEC | human umbilical vein endothelial cell |
| Ig | immunoglobulin |
| IκB | inhibitor of NF-κB |
| IL | interleukin |
| ITAo | individual topology angle |
| JNK | c-Jun N-terminal kinase |
| KLF | krüppel-like factor |
| LT | leukotriene |
| MAPK | mitogen-activated protein kinase |
| MCP | monocyte chemoattractant protein |
| MDA | malondialdehyde |
| MG-H1 | methylglyoxal-derived hydroimidazolone 1; Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine |
| MMP | matrix metalloproteinase |
| MPO | myeloperoxidase |
| NBT | nitroblue tetrazolium |
| NF | nuclear factor |
| NRF | nuclear factor erythroid 2-related factor |
| PBS | phosphate-buffered saline |
| PECAM | platelet endothelial cell adhesion molecule |
| PG | prostaglandin |
| PMSF | phenylmethylsulfonyl fluoride |
| RAGE | receptor for advanced glycation end-products |
| ROS | reactive oxygen species |
| RT | room temperature |
| SA-β-gal | senescence-associated β-galactosidase |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SIRT | NAD-dependent deacetylase sirtuin |
| SOD | superoxide dismutase |
| TEWL | transepidermal water loss |
| TNF | tumor necrosis factor |
| UV | ultraviolet |
| VEGF | vascular endothelial growth factor |
References
- Hussein, R.S.; Bin Dayel, S.; Abahussein, O.; El-Sherbiny, A.A. Influences on Skin and Intrinsic Aging: Biological, Environmental, and Therapeutic Insights. J. Cosmet. Dermatol. 2025, 24, e16688. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef]
- Bonté, F.; Girard, D.; Archambault, J.C.; Desmoulière, A. Skin Changes During Ageing. Subcell. Biochem. 2019, 91, 249–280. [Google Scholar] [CrossRef]
- Russell-Goldman, E.; Murphy, G.F. The Pathobiology of Skin Aging: New Insights into an Old Dilemma. Am. J. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef]
- Wong, Q.Y.A.; Chew, F.T. Defining skin aging and its risk factors: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Boo, Y.C. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants 2020, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef]
- He, X.; Gao, X.; Xie, W. Research Progress in Skin Aging, Metabolism, and Related Products. Int. J. Mol. Sci. 2023, 24, 15930. [Google Scholar] [CrossRef]
- Grenier, A.; Morissette, M.C.; Rochette, P.J.; Pouliot, R. The combination of cigarette smoke and solar rays causes effects similar to skin aging in a bilayer skin model. Sci. Rep. 2023, 13, 17969. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Glycolysis: A multifaceted metabolic pathway and signaling hub. J. Biol. Chem. 2024, 300, 107906. [Google Scholar] [CrossRef]
- Yamagishi, S.; Maeda, S.; Matsui, T.; Ueda, S.; Fukami, K.; Okuda, S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim. Biophys. Acta. 2012, 1820, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Uceda, A.B.; Mariño, L.; Casasnovas, R.; Adrover, M. An overview on glycation: Molecular mechanisms, impact on proteins, pathogenesis, and inhibition. Biophys. Rev. 2024, 16, 189–218. [Google Scholar] [CrossRef]
- Crisan, M.; Taulescu, M.; Crisan, D.; Cosgarea, R.; Parvu, A.; Cãtoi, C.; Drugan, T. Expression of advanced glycation end-products on sun-exposed and non-exposed cutaneous sites during the ageing process in humans. PLoS ONE 2013, 8, e75003. [Google Scholar] [CrossRef] [PubMed]
- Isami, F.; West, B.J.; Nakajima, S.; Yamagishi, S.I. Association of advanced glycation end products, evaluated by skin autofluorescence, with lifestyle habits in a general Japanese population. J. Int. Med. Res. 2018, 46, 1043–1051. [Google Scholar] [CrossRef]
- van de Zande, S.C.; de Vries, J.K.; van den Akker-Scheek, I.; Zwerver, J.; Smit, A.J. A physically active lifestyle is related to a lower level of skin autofluorescence in a large population with chronic disease (LifeLines cohort). J. Sport. Health Sci. 2022, 11, 260–265. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.; Zhao, C. The effects of advanced glycation end-products on skin and potential anti-glycation strategies. Exp. Dermatol. 2024, 33, e15065. [Google Scholar] [CrossRef]
- Masaki, H.; Okano, Y.; Sakurai, H. Generation of active oxygen species from advanced glycation end-products (AGE) under ultraviolet light A (UVA) irradiation. Biochem. Biophys. Res. Commun. 1997, 235, 306–310. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T.O. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: Probing the pathogenesis of chronic disease. J. Proteome Res. 2009, 8, 754–769. [Google Scholar] [CrossRef]
- Chellan, P.; Nagaraj, R.H. Early glycation products produce pentosidine cross-links on native proteins. novel mechanism of pentosidine formation and propagation of glycation. J. Biol. Chem. 2001, 276, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, K.; Grune, T. Degradation of oxidized and glycoxidized collagen: Role of collagen cross-linking. Arch. Biochem. Biophys. 2014, 542, 56–64. [Google Scholar] [CrossRef]
- Kinoshita, S.; Mera, K.; Ichikawa, H.; Shimasaki, S.; Nagai, M.; Taga, Y.; Iijima, K.; Hattori, S.; Fujiwara, Y.; Shirakawa, J.I.; et al. N(ω)-(Carboxymethyl)arginine Is One of the Dominant Advanced Glycation End Products in Glycated Collagens and Mouse Tissues. Oxid. Med. Cell Longev. 2019, 2019, 9073451. [Google Scholar] [CrossRef] [PubMed]
- Cefalu, W.T.; Bell-Farrow, A.D.; Wang, Z.Q.; Sonntag, W.E.; Fu, M.X.; Baynes, J.W.; Thorpe, S.R. Caloric restriction decreases age-dependent accumulation of the glycoxidation products, N epsilon-(carboxymethyl)lysine and pentosidine, in rat skin collagen. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, B337–B341. [Google Scholar] [CrossRef] [PubMed]
- Lohwasser, C.; Neureiter, D.; Weigle, B.; Kirchner, T.; Schuppan, D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J. Invest. Dermatol. 2006, 126, 291–299. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef]
- Serban, A.I.; Stanca, L.; Geicu, O.I.; Munteanu, M.C.; Dinischiotu, A. RAGE and TGF-β1 Cross-Talk Regulate Extracellular Matrix Turnover and Cytokine Synthesis in AGEs Exposed Fibroblast Cells. PLoS ONE 2016, 11, e0152376. [Google Scholar] [CrossRef]
- Perrone, A.; Giovino, A.; Benny, J.; Martinelli, F. Advanced Glycation End Products (AGEs): Biochemistry, Signaling, Analytical Methods, and Epigenetic Effects. Oxid. Med. Cell Longev. 2020, 2020, 3818196. [Google Scholar] [CrossRef]
- Wautier, M.P.; Chappey, O.; Corda, S.; Stern, D.M.; Schmidt, A.M.; Wautier, J.L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E685–E694. [Google Scholar] [CrossRef]
- Daffu, G.; del Pozo, C.H.; O’Shea, K.M.; Ananthakrishnan, R.; Ramasamy, R.; Schmidt, A.M. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int. J. Mol. Sci. 2013, 14, 19891–19910. [Google Scholar] [CrossRef] [PubMed]
- Hammes, H.P.; Strödter, D.; Weiss, A.; Bretzel, R.G.; Federlin, K.; Brownlee, M. Secondary intervention with aminoguanidine retards the progression of diabetic retinopathy in the rat model. Diabetologia 1995, 38, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Loske, C.; Neumann, A.; Cunningham, A.M.; Nichol, K.; Schinzel, R.; Riederer, P.; Münch, G. Cytotoxicity of advanced glycation endproducts is mediated by oxidative stress. J. Neural Transm. 1998, 105, 1005–1015. [Google Scholar] [CrossRef]
- Kuzan, A. Toxicity of advanced glycation end products (Review). Biomed. Rep. 2021, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Vasan, S.; Foiles, P.; Founds, H. Therapeutic potential of breakers of advanced glycation end product-protein crosslinks. Arch. Biochem. Biophys. 2003, 419, 89–96. [Google Scholar] [CrossRef]
- Yang, S.Z.; Litchfield, J.E.; Baynes, J.W. AGE-breakers cleave model compounds, but do not break Maillard crosslinks in skin and tail collagen from diabetic rats. Arch. Biochem. Biophys. 2003, 412, 42–46. [Google Scholar] [CrossRef]
- Cheng, G.; Wang, L.L.; Qu, W.S.; Long, L.; Cui, H.; Liu, H.Y.; Cao, Y.L.; Li, S. C16, a novel advanced glycation endproduct breaker, restores cardiovascular dysfunction in experimental diabetic rats. Acta Pharmacol. Sin. 2005, 26, 1460–1466. [Google Scholar] [CrossRef]
- Bakris, G.L.; Bank, A.J.; Kass, D.A.; Neutel, J.M.; Preston, R.A.; Oparil, S. Advanced glycation end-product cross-link breakers. A novel approach to cardiovascular pathologies related to the aging process. Am. J. Hypertens. 2004, 17, 23S–30S. [Google Scholar] [CrossRef]
- Song, Q.; Liu, J.; Dong, L.; Wang, X.; Zhang, X. Novel advances in inhibiting advanced glycation end product formation using natural compounds. Biomed. Pharmacother. 2021, 140, 111750. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zhang, J.Q.; Li, L.; Guo, M.M.; He, Y.F.; Dong, Y.M.; Meng, H.; Yi, F. Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef]
- Gugliucci, A.; Bendayan, M. Histones from diabetic rats contain increased levels of advanced glycation end products. Biochem. Biophys. Res. Commun. 1995, 212, 56–62. [Google Scholar] [CrossRef]
- Levi, B.; Werman, M.J. Long-term fructose consumption accelerates glycation and several age-related variables in male rats. J. Nutr. 1998, 128, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Peppa, M.; Brem, H.; Ehrlich, P.; Zhang, J.G.; Cai, W.; Li, Z.; Croitoru, A.; Thung, S.; Vlassara, H. Adverse effects of dietary glycotoxins on wound healing in genetically diabetic mice. Diabetes 2003, 52, 2805–2813. [Google Scholar] [CrossRef]
- Alikhani, Z.; Alikhani, M.; Boyd, C.M.; Nagao, K.; Trackman, P.C.; Graves, D.T. Advanced Glycation End Products Enhance Expression of Pro-apoptotic Genes and Stimulate Fibroblast Apoptosis through Cytoplasmic and Mitochondrial Pathways. J. Biol. Chem. 2005, 280, 12087–12095. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, J.H.; Jung, M.; Chung, C.H.; Hasham, R.; Park, C.S.; Choi, E.H. A long-standing hyperglycaemic condition impairs skin barrier by accelerating skin ageing process. Exp. Dermatol. 2011, 20, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Dammann, P.; Sell, D.R.; Begall, S.; Strauch, C.; Monnier, V.M. Advanced glycation end-products as markers of aging and longevity in the long-lived Ansell’s mole-rat (Fukomys anselli). J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 573–583. [Google Scholar] [CrossRef]
- Yamanaka, M.; Matsumura, T.; Ohno, R.; Fujiwara, Y.; Shinagawa, M.; Sugawa, H.; Hatano, K.; Shirakawa, J.; Kinoshita, H.; Ito, K.; et al. Non-invasive measurement of skin autofluorescence to evaluate diabetic complications. J. Clin. Biochem. Nutr. 2016, 58, 135–140. [Google Scholar] [CrossRef]
- Hause, F.; Schlote, D.; Simm, A.; Hoffmann, K.; Santos, A.N. Accumulation of glycated proteins suggesting premature ageing in lamin B receptor deficient mice. Biogerontology 2018, 19, 95–100. [Google Scholar] [CrossRef]
- Nowotny, K.; Castro, J.P.; Hugo, M.; Braune, S.; Weber, D.; Pignitter, M.; Somoza, V.; Bornhorst, J.; Schwerdtle, T.; Grune, T. Oxidants produced by methylglyoxal-modified collagen trigger ER stress and apoptosis in skin fibroblasts. Free. Radic. Biol. Med. 2018, 120, 102–113. [Google Scholar] [CrossRef]
- Nicolas, C.; Jaisson, S.; Gorisse, L.; Tessier, F.J.; Niquet-Léridon, C.; Jacolot, P.; Pietrement, C.; Gillery, P. Carbamylation and glycation compete for collagen molecular aging in vivo. Sci. Rep. 2019, 9, 18291. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zheng, C.; Ye, J.; Song, F.; Wang, X.; Liu, Y.; Tian, M.; Dong, J.; Lu, S. Effects of advanced glycation end products on neutrophil migration and aggregation in diabetic wounds. Aging 2021, 13, 12143–12159. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Imai, M.; Tanaka, S.; Ooi, K. Changes in the AGE/Macrophage/TNF-α Pathway Affect Skin Dryness during KK-Ay/Tajcl Mice Aging. Life 2023, 13, 1339. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Z.; Liu, X.-X.; Shi, Y.-J.; Wang, X.-R.; Li, L.; Tai, M.-L.; Yi, F. Unveiling the mechanism of high sugar diet induced advanced glycosylation end products damage skin structure via extracellular matrix–receptor interaction pathway. J. Cosmet. Dermatol. 2024, 23, 2496–2508. [Google Scholar] [CrossRef]
- Odani, H.; Iijima, K.; Nakata, M.; Miyata, S.; Kusunoki, H.; Yasuda, Y.; Hiki, Y.; Irie, S.; Maeda, K.; Fujimoto, D. Identification of Nω-Carboxymethylarginine, a New Advanced Glycation Endproduct in Serum Proteins of Diabetic Patients: Possibility of a New Marker of Aging and Diabetes. Biochem. Biophys. Res. Commun. 2001, 285, 1232–1236. [Google Scholar] [CrossRef]
- Sakai, S.; Kikuchi, K.; Satoh, J.; Tagami, H.; Inoue, S. Functional properties of the stratum corneum in patients with diabetes mellitus: Similarities to senile xerosis. Br. J. Dermatol. 2005, 153, 319–323. [Google Scholar] [CrossRef]
- Ohshima, H.; Oyobikawa, M.; Tada, A.; Maeda, T.; Takiwaki, H.; Itoh, M.; Kanto, H. Melanin and facial skin fluorescence as markers of yellowish discoloration with aging. Skin. Res. Technol. 2009, 15, 496–502. [Google Scholar] [CrossRef]
- Momma, H.; Niu, K.; Kobayashi, Y.; Guan, L.; Sato, M.; Guo, H.; Chujo, M.; Otomo, A.; Yufei, C.; Tadaura, H.; et al. Skin advanced glycation end product accumulation and muscle strength among adult men. Eur. J. Appl. Physiol. 2011, 111, 1545–1552. [Google Scholar] [CrossRef]
- Cleary, P.A.; Braffett, B.H.; Orchard, T.; Lyons, T.J.; Maynard, J.; Cowie, C.; Gubitosi-Klug, R.A.; Way, J.; Anderson, K.; Barnie, A.; et al. Clinical and technical factors associated with skin intrinsic fluorescence in subjects with type 1 diabetes from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes Technol. Ther. 2013, 15, 466–474. [Google Scholar] [CrossRef]
- Mamalis, A.; Fiadorchanka, N.; Adams, L.; Serravallo, M.; Heilman, E.; Siegel, D.; Brody, N.; Jagdeo, J. An immunohistochemical panel to assess ultraviolet radiation-associated oxidative skin injury. J. Drugs Dermatol. 2014, 13, 574–578. [Google Scholar]
- Pepe, D.; Elliott, C.G.; Forbes, T.L.; Hamilton, D.W. Detection of galectin-3 and localization of advanced glycation end products (AGE) in human chronic skin wounds. Histol. Histopathol. 2014, 29, 251–258. [Google Scholar] [CrossRef]
- Spauwen, P.J.; van Eupen, M.G.; Köhler, S.; Stehouwer, C.D.; Verhey, F.R.; van der Kallen, C.J.; Sep, S.J.; Koster, A.; Schaper, N.C.; Dagnelie, P.C.; et al. Associations of advanced glycation end-products with cognitive functions in individuals with and without type 2 diabetes: The maastricht study. J. Clin. Endocrinol. Metab. 2015, 100, 951–960. [Google Scholar] [CrossRef]
- Chen, J.; van der Duin, D.; Campos-Obando, N.; Ikram, M.A.; Nijsten, T.E.C.; Uitterlinden, A.G.; Zillikens, M.C. Serum 25-hydroxyvitamin D(3) is associated with advanced glycation end products (AGEs) measured as skin autofluorescence: The Rotterdam Study. Eur. J. Epidemiol. 2019, 34, 67–77. [Google Scholar] [CrossRef]
- Shirakami, T.; Yamanaka, M.; Fujihara, J.; Matsuoka, Y.; Gohto, Y.; Obana, A.; Tanito, M. Advanced Glycation End Product Accumulation in Subjects with Open-Angle Glaucoma with and without Exfoliation. Antioxidants 2020, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, E.; Kawada, A.; Ono, K.; Fujimoto, E.; Wachi, H.; Harumiya, S.; Nagai, R.; Tajima, S. N(ε)-(carboxymethyl)lysine modification of elastin alters its biological properties: Implications for the accumulation of abnormal elastic fibers in actinic elastosis. J. Invest. Dermatol. 2012, 132, 315–323. [Google Scholar] [CrossRef]
- Mooldijk, S.S.; Lu, T.; Waqas, K.; Chen, J.; Vernooij, M.W.; Ikram, M.K.; Zillikens, M.C.; Ikram, M.A. Skin autofluorescence, reflecting accumulation of advanced glycation end products, and the risk of dementia in a population-based cohort. Sci. Rep. 2024, 14, 1256. [Google Scholar] [CrossRef] [PubMed]
- Hattangadi, S.M.; Lodish, H.F. Regulation of erythrocyte lifespan: Do reactive oxygen species set the clock? J. Clin. Investig. 2007, 117, 2075–2077. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Menini, S.; Oddi, G.; Ricci, C.; Amadio, L.; Pricci, F.; Olivieri, A.; Sorcini, M.; Di Mario, U.; Pesce, C.; et al. Galectin-3/AGE-receptor 3 knockout mice show accelerated AGE-induced glomerular injury: Evidence for a protective role of galectin-3 as an AGE receptor. Faseb J. 2004, 18, 1773–1775. [Google Scholar] [CrossRef]
- Hayashi, C.M.; Nagai, R.; Miyazaki, K.; Hayase, F.; Araki, T.; Ono, T.; Horiuchi, S. Conversion of Amadori products of the Maillard reaction to N(ε)-(carboxymethyl) lysine by short-term heating: Possible detection of artifacts by immunohistochemistry. Lab. Investig. 2002, 82, 795–807. [Google Scholar] [CrossRef]
- Péterszegi, G.; Andrès, E.; Molinari, J.; Ravelojaona, V.; Robert, L. Effect of cellular aging on collagen biosynthesis: I. Methodological considerations and pharmacological applications. Arch. Gerontol. Geriatr. 2008, 47, 356–367. [Google Scholar] [CrossRef]
- Pageon, H.; Técher, M.P.; Asselineau, D. Reconstructed skin modified by glycation of the dermal equivalent as a model for skin aging and its potential use to evaluate anti-glycation molecules. Exp. Gerontol. 2008, 43, 584–588. [Google Scholar] [CrossRef]
- Ravelojaona, V.; Robert, A.M.; Robert, L. Expression of senescence-associated beta-galactosidase (SA-beta-Gal) by human skin fibroblasts, effect of advanced glycation end-products and fucose or rhamnose-rich polysaccharides. Arch. Gerontol. Geriatr. 2009, 48, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Hong, C.O.; Koo, Y.C.; Choi, H.D.; Lee, K.W. Anti-glycation effect of gold nanoparticles on collagen. Biol. Pharm. Bull. 2012, 35, 260–264. [Google Scholar] [CrossRef]
- Pageon, H.; Zucchi, H.; Rousset, F.; Monnier, V.M.; Asselineau, D. Skin aging by glycation: Lessons from the reconstructed skin model. Clin. Chem. Lab. Med. 2014, 52, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Danoux, L.; Mine, S.; Abdul-Malak, N.; Henry, F.; Jeanmaire, C.; Freis, O.; Pauly, G.; Cittadini, L.; André-Frei, V.; Rathjens, A. How to help the skin cope with glycoxidation. Clin. Chem. Lab. Med. 2014, 52, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, J.A.; Kim, M.; Kum, H.; Jung, E.; Park, D. Anti-glycation activities of phenolic constituents from Silybum marianum (Milk Thistle) flower in vitro and on human explants. Molecules 2015, 20, 3549–3564. [Google Scholar] [CrossRef]
- Shin, S.; Son, D.; Kim, M.; Lee, S.; Roh, K.B.; Ryu, D.; Lee, J.; Jung, E.; Park, D. Ameliorating Effect of Akebia quinata Fruit Extracts on Skin Aging Induced by Advanced Glycation End Products. Nutrients 2015, 7, 9337–9352. [Google Scholar] [CrossRef]
- Pennacchi, P.C.; de Almeida, M.E.; Gomes, O.L.; Faião-Flores, F.; de Araújo Crepaldi, M.C.; Dos Santos, M.F.; de Moraes Barros, S.B.; Maria-Engler, S.S. Glycated Reconstructed Human Skin as a Platform to Study the Pathogenesis of Skin Aging. Tissue Eng. Part. A 2015, 21, 2417–2425. [Google Scholar] [CrossRef]
- Han, A.R.; Nam, M.H.; Lee, K.W. Plantamajoside Inhibits UVB and Advanced Glycation End Products-Induced MMP-1 Expression by Suppressing the MAPK and NF-kappaB Pathways in HaCaT Cells. Photochem. Photobiol. 2016, 92, 708–719. [Google Scholar] [CrossRef]
- Ou, J.; Huang, J.; Wang, M.; Ou, S. Effect of rosmarinic acid and carnosic acid on AGEs formation in vitro. Food Chem. 2017, 221, 1057–1061. [Google Scholar] [CrossRef]
- Fernandes, M.F.; Conegundes, J.L.M.; Pinto, N.C.C.; de Oliveira, L.G.; de Aguiar, J.A.K.; Souza-Fagundes, E.M.; Scio, E. Cecropia pachystachya Leaves Present Potential to Be Used as New Ingredient for Antiaging Dermocosmetics. Evid. Based Complement. Alternat Med. 2019, 2019, 8263934. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Gelbrich, T.; Planchenault, P.; Derbré, S.; Schinkovitz, A.; Richomme, P.; Hensel, A.; Ganzera, M. Absolute Configuration of Mycosporine-Like Amino Acids, Their Wound Healing Properties and In Vitro Anti-Aging Effects. Mar. Drugs 2019, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Drouet, S.; Leclerc, E.A.; Garros, L.; Tungmunnithum, D.; Kabra, A.; Abbasi, B.H.; Lainé, É.; Hano, C. A Green Ultrasound-Assisted Extraction Optimization of the Natural Antioxidant and Anti-Aging Flavonolignans from Milk Thistle Silybum marianum (L.) Gaertn. Fruits for Cosmetic Applications. Antioxidants 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; DaSilva, N.A.; Liu, W.; Xu, J.; Dombi, G.W.; Dain, J.A.; Li, D.; Chamcheu, J.C.; Seeram, N.P.; Ma, H. Thymocid(®), a Standardized Black Cumin (Nigella sativa) Seed Extract, Modulates Collagen Cross-Linking, Collagenase and Elastase Activities, and Melanogenesis in Murine B16F10 Melanoma Cells. Nutrients 2020, 12, 2146. [Google Scholar] [CrossRef]
- Freitas, L.d.; Valli, M.; Dametto, A.C.; Pennacchi, P.C.; Andricopulo, A.D.; Maria-Engler, S.S.; Bolzani, V.S. Advanced Glycation End Product Inhibition by Alkaloids from Ocotea paranapiacabensis for the Prevention of Skin Aging. J. Nat. Prod. 2020, 83, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L. Antioxidant and Antiglycation Effects of Polyphenol Compounds Extracted from Hazelnut Skin on Advanced Glycation End-Products (AGEs) Formation. Antioxidants 2021, 10, 424. [Google Scholar] [CrossRef]
- Waditee-Sirisattha, R.; Kageyama, H. Protective effects of mycosporine-like amino acid-containing emulsions on UV-treated mouse ear tissue from the viewpoints of antioxidation and antiglycation. J. Photochem. Photobiol. B. 2021, 223, 112296. [Google Scholar] [CrossRef]
- Lin, H.; Lin, T.Y.; Lin, J.A.; Cheng, K.C.; Santoso, S.P.; Chou, C.H.; Hsieh, C.W. Effect of Pholiota nameko Polysaccharides Inhibiting Methylglyoxal-Induced Glycation Damage In Vitro. Antioxidants 2021, 10, 1589. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.; Yoon, S.H.; Park, D.; Hwang, J.S.; Jung, E. Anti-glycation activities of methyl gallate in vitro and in human explants. J. Cosmet. Dermatol. 2022, 21, 2602–2609. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, M.; Shin, S.; Woo, J.; Son, D.; Ryu, D.; Yoo, J.; Park, D.; Jung, E. Effect of Cirsium japonicum Flower Extract on Skin Aging Induced by Glycation. Molecules 2022, 27, 2093. [Google Scholar] [CrossRef]
- Imai, Y.; Nakashima, Y.; Kanno, T. Inhibitory Effects of Parachlorella Beijerinckii Extracts on the Formation of Advanced Glycation End Products and Glycative Stress-Induced Inflammation in an In Vitro Skin Dermis-Like Model. Evid.-Based Complement. Altern. Med. 2022, 2022, 8789903. [Google Scholar] [CrossRef] [PubMed]
- Alhadid, A.; Bustanji, Y.; Harb, A.; Al-Hiari, Y.; Abdalla, S. Vanillic Acid Inhibited the Induced Glycation Using In Vitro and In Vivo Models. Evid. Based Complement. Alternat Med. 2022, 2022, 7119256. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Johnson, E.; Khoo, C.; Wang, W.; Gu, L. Cranberry Juice Polyphenols Inhibited the Formation of Advanced Glycation End Products in Collagens, Inhibited Advanced Glycation End Product-Induced Collagen Crosslinking, and Cleaved the Formed Crosslinks. J. Agric. Food Chem. 2022, 70, 15560–15569. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, L.; Della Posta, S.; Fanali, C.; Dugo, L.; De Gara, L. Chemical Composition of Hazelnut Skin Food Waste and Protective Role against Advanced Glycation End-Products (AGEs) Damage in THP-1-Derived Macrophages. Molecules 2023, 28, 2680. [Google Scholar] [CrossRef]
- Knoblich, C.; Dunckelmann, K.; Krüger, A.; Küper, T.; Blatt, T.; Weise, J.M. N-acetyl-L-hydroxyproline—A potent skin anti-ageing active preventing advanced glycation end-product formation in vitro and ex vivo. Int. J. Cosmet. Sci. 2024, 46, 297–306. [Google Scholar] [CrossRef]
- Sultana, R.; Parveen, A.; Kang, M.-C.; Hong, S.-M.; Kim, S.Y. Glyoxal-derived advanced glycation end products (GO-AGEs) with UVB critically induce skin inflammaging: In vitro and in silico approaches. Sci. Rep. 2024, 14, 1843. [Google Scholar] [CrossRef]
- Yokota, M.; Tokudome, Y. The Effect of Glycation on Epidermal Lipid Content, Its Metabolism and Change in Barrier Function. Skin. Pharmacol. Physiol. 2016, 29, 231–242. [Google Scholar] [CrossRef]
- Pageon, H.; Asselineau, D. An in vitro approach to the chronological aging of skin by glycation of the collagen: The biological effect of glycation on the reconstructed skin model. Ann. N. Y. Acad. Sci. 2005, 1043, 529–532. [Google Scholar] [CrossRef]
- Pageon, H.; Zucchi, H.; Dai, Z.; Sell, D.R.; Strauch, C.M.; Monnier, V.M.; Asselineau, D. Biological Effects Induced by Specific Advanced Glycation End Products in the Reconstructed Skin Model of Aging. BioResearch Open Access 2015, 4, 54–64. [Google Scholar] [CrossRef]
- Robert, L.; Molinari, J.; Ravelojaona, V.; Andrès, E.; Robert, A.M. Age- and passage-dependent upregulation of fibroblast elastase-type endopeptidase activity. Role of advanced glycation endproducts, inhibition by fucose- and rhamnose-rich oligosaccharides. Arch. Gerontol. Geriatr. 2010, 50, 327–331. [Google Scholar] [CrossRef]
- Cadau, S.; Leoty-okombi, S.; Pain, S.; Béchetoille, N.; Andre-frei, V.; Berthod, F. In vitro glycation of an endothelialized and innervated tissue-engineered skin to screen anti-AGE molecules. Biomaterials 2015, 51, 216–225. [Google Scholar] [CrossRef]
- Balansin Rigon, R.; Kaessmeyer, S.; Wolff, C.; Hausmann, C.; Zhang, N.; Sochorová, M.; Kováčik, A.; Haag, R.; Vávrová, K.; Ulrich, M.; et al. Ultrastructural and Molecular Analysis of Ribose-Induced Glycated Reconstructed Human Skin. Int. J. Mol. Sci. 2018, 19, 3521. [Google Scholar] [CrossRef]
- Li, X.; Yang, K.; Gao, S.; Zhao, J.; Liu, G.; Chen, Y.; Lin, H.; Zhao, W.; Hu, Z.; Xu, N. Carnosine Stimulates Macrophage-Mediated Clearance of Senescent Skin Cells Through Activation of the AKT2 Signaling Pathway by CD36 and RAGE. Front. Pharmacol. 2020, 11, 593832. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Lee, S.G.; Jung, I.; Suk, J.; Lee, M.H.; Kim, D.U.; Lee, J.H. Effect of a Topical Collagen Tripeptide on Antiaging and Inhibition of Glycation of the Skin: A Pilot Study. Int. J. Mol. Sci. 2022, 23, 1101. [Google Scholar] [CrossRef]
- Lyu, J.L.; Liu, Y.J.; Wen, K.C.; Chiu, C.Y.; Lin, Y.H.; Chiang, H.M. Protective Effect of Djulis (Chenopodium formosanum) Extract against UV- and AGEs-Induced Skin Aging via Alleviating Oxidative Stress and Collagen Degradation. Molecules 2022, 27, 2332. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Jiang, L.I.; Kononov, T.; Zahr, A.S. A case study investigating the short-term efficacy and tolerability of a daily serum composed from a unique sunflower sprout extract. J. Cosmet. Dermatol. 2022, 21, 4410–4421. [Google Scholar] [CrossRef]
- Markiewicz, E.; Jerome, J.; Mammone, T.; Idowu, O.C. Anti-Glycation and Anti-Aging Properties of Resveratrol Derivatives in the in-vitro 3D Models of Human Skin. Clin. Cosmet. Investig. Dermatol. 2022, 15, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Ceccacci, S.; Lucia, A.; Tortora, A.; Colantuono, A.; Carotenuto, G.; Tito, A.; Monti, M.C. Jasminum sambac Cell Extract as Antioxidant Booster against Skin Aging. Antioxidants 2022, 11, 2409. [Google Scholar] [CrossRef]
- Wattanapitayakul, S.K.; Jarisarapurin, W.; Kunchana, K.; Setthawong, V.; Chularojmontri, L. Unripe Carica papaya Fresh Fruit Extract Protects against Methylglyoxal-Mediated Aging in Human Dermal Skin Fibroblasts. Prev. Nutr. Food Sci. 2023, 28, 235–245. [Google Scholar] [CrossRef]
- Bai, D.; Wang, Z.; Xie, L.; Lu, B.; Wang, M.; Zhang, J. Topical Transdermal Administration of Supramolecular Self-Assembled Carnosine for Anti-Melanin and Anti-Aging. Adv. Healthc. Mater. 2024, 13, e2401960. [Google Scholar] [CrossRef]
- Augello, F.R.; Lombardi, F.; Ciafarone, A.; Ciummo, V.; Altamura, S.; Giuliani, M.; Cinque, B.; Palumbo, P. Efficacy of an Innovative Poly-Component Formulation in Counteracting Human Dermal Fibroblast Aging by Influencing Oxidative and Inflammatory Pathways. Biomedicines 2024, 12, 2030. [Google Scholar] [CrossRef] [PubMed]
- Pageon, H. Reaction of glycation and human skin: The effects on the skin and its components, reconstructed skin as a model. Pathologie Biologie 2010, 58, 226–231. [Google Scholar] [CrossRef]
- Narda, M.; Peno-Mazzarino, L.; Krutmann, J.; Trullas, C.; Granger, C. Novel Facial Cream Containing Carnosine Inhibits Formation of Advanced Glycation End-Products in Human Skin. Ski. Pharmacol. Physiol. 2018, 31, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Havas, F.; Krispin, S.; Cohen, M.; Loing, E.; Farge, M.; Suere, T.; Attia-Vigneau, J. A Dunaliella salina Extract Counteracts Skin Aging under Intense Solar Irradiation Thanks to Its Antiglycation and Anti-Inflammatory Properties. Mar. Drugs 2022, 20, 104. [Google Scholar] [CrossRef]
- De Decker, I.; Notebaert, M.; Speeckaert, M.M.; Claes, K.E.Y.; Blondeel, P.; Van Aken, E.; Van Dorpe, J.; De Somer, F.; Heintz, M.; Monstrey, S.; et al. Enzymatic Deglycation of Damaged Skin by Means of Combined Treatment of Fructosamine-3-Kinase and Fructosyl-Amino Acid Oxidase. Int. J. Mol. Sci. 2023, 24, 8981. [Google Scholar] [CrossRef] [PubMed]
- Tohgasaki, T.; Nishizawa, S.; Kondo, S.; Ishiwatari, S.; Sakurai, T. Long Hanging Structure of Collagen VII Connects the Elastic Fibers and the Basement Membrane in Young Skin Tissue. J. Histochem. Cytochem. 2022, 70, 751–757. [Google Scholar] [CrossRef]
- Odetti, P.R.; Borgoglio, A.; De Pascale, A.; Rolandi, R.; Adezati, L. Prevention of diabetes-increased aging effect on rat collagen-linked fluorescence by aminoguanidine and rutin. Diabetes 1990, 39, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Lipman, R.D.; Jahngen-Hodge, J.; Palmer, V.; Smith, D.; Padhye, N.; Dallal, G.E.; Cyr, D.E.; Laxman, E.; Shepard, D.; et al. Dietary calorie restriction in the Emory mouse: Effects on lifespan, eye lens cataract prevalence and progression, levels of ascorbate, glutathione, glucose, and glycohemoglobin, tail collagen breaktime, DNA and RNA oxidation, skin integrity, fecundity, and cancer. Mech. Ageing Dev. 1995, 79, 33–57. [Google Scholar] [CrossRef]
- Sell, D.R. Ageing promotes the increase of early glycation Amadori product as assessed by epsilon-N-(2-furoylmethyl)-L-lysine (furosine) levels in rodent skin collagen. The relationship to dietary restriction and glycoxidation. Mech. Ageing Dev. 1997, 95, 81–99. [Google Scholar] [CrossRef]
- Novelli, M.; Masiello, P.; Bombara, M.; Bergamini, E. Protein glycation in the aging male Sprague-Dawley rat: Effects of antiaging diet restrictions. J. Gerontol. A Biol. Sci. Med. Sci. 1998, 53, B94–B101. [Google Scholar] [CrossRef][Green Version]
- Yamada, S.; Ohkubo, C. The influence of frequent and excessive intake of glucose on microvascular aging in healthy mice. Microcirculation 1999, 6, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Probert, L.L.; Alhumadi, N.H.; Klandorf, H. Protein glycosylation and advanced glycosylated endproducts (AGEs) accumulation: An avian solution? J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, B171–B176; discussion B177–B178. [Google Scholar] [CrossRef]
- Song, X.; Bao, M.; Li, D.; Li, Y.M. Advanced glycation in D-galactose induced mouse aging model. Mech. Ageing Dev. 1999, 108, 239–251. [Google Scholar] [CrossRef]
- Lingelbach, L.B.; Mitchell, A.E.; Rucker, R.B.; McDonald, R.B. Accumulation of Advanced Glycation Endproducts in Aging Male Fischer 344 Rats during Long-Term Feeding of Various Dietary Carbohydrates. J. Nutr. 2000, 130, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Klandorf, H.; Rathore, D.S.; Iqbal, M.; Shi, X.; Van Dyke, K. Accelerated tissue aging and increased oxidative stress in broiler chickens fed allopurinol. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 129, 93–104. [Google Scholar] [CrossRef]
- Sell, D.R.; Nelson, J.F.; Monnier, V.M. Effect of chronic aminoguanidine treatment on age-related glycation, glycoxidation, and collagen cross-linking in the Fischer 344 rat. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B405–B411. [Google Scholar] [CrossRef] [PubMed]
- Rutter, K.; Sell, D.R.; Fraser, N.; Obrenovich, M.; Zito, M.; Starke-Reed, P.; Monnier, V.M. Green Tea Extract Suppresses the Age-Related Increase in Collagen Crosslinking and Fluorescent Products in C57BL/6 Mice. IJVNR 2003, 73, 453–460. [Google Scholar] [CrossRef]
- Sell, D.R.; Lane, M.A.; Obrenovich, M.E.; Mattison, J.A.; Handy, A.; Ingram, D.K.; Cutler, R.G.; Roth, G.S.; Monnier, V.M. The Effect of Caloric Restriction on Glycation and Glycoxidation in Skin Collagen of Nonhuman Primates. J. Gerontol. Ser. A 2003, 58, B508–B516. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Z.; Peng, Z.; Lu, F. Anti-aging effect of adipose-derived stem cells in a mouse model of skin aging induced by D-galactose. PLoS ONE 2014, 9, e97573. [Google Scholar] [CrossRef]
- Wang, H.; Wei, S.; Xue, X.; You, Y.; Ma, Q. Adipose stem cells’ antagonism in glycosylation of D-galactose-induced skin aging of nude mice and its skin recovery function. Int. J. Immunopathol. Pharmacol. 2016, 29, 376–385. [Google Scholar] [CrossRef]
- Dorr, B.S.; Stahl, R.S.; Hanson-Dorr, K.C.; Furcolow, C.A. Using pentosidine and hydroxyproline to predict age and sex in an avian species. Ecol. Evol. 2017, 7, 8999–9005. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Du, H.-h.; Jiang, M.; Zhou, C.; Deng, Y.; Long, X.; Zhao, X. Antioxidant Effect of Lactobacillus fermentum CQPC04-Fermented Soy Milk on D-Galactose-Induced Oxidative Aging Mice. Front. Nutr. 2021, 8, 727467. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.J.; Lee, H.W.; Yoo, G.; Kim, D.; Choi, I.W.; Kim, Y.; Ha, S.K. Protective effect of Schizonepeta tenuifolia Briq. ethanolic extract against UVB-induced skin aging and photodamage in hairless mice. Front. Pharmacol. 2023, 14, 1176073. [Google Scholar] [CrossRef]
- Xie, Y.; Ye, J.; Ouyang, Y.; Gong, J.; Li, C.; Deng, Y.; Mai, Y.; Liu, Y.; Deng, W. Microneedle-Assisted Topical Delivery of Idebenone-Loaded Bioadhesive Nanoparticles Protect against UV-Induced Skin Damage. Biomedicines 2023, 11, 1649. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Lee, J.S.; Jang, J.H.; Jung, J.I.; Kim, E.J.; Choi, S.Y. AGEs Blocker™ (Goji Berry, Fig, and Korean Mint Mixed Extract) Inhibits Skin Aging Caused by Streptozotocin-Induced Glycation in Hairless Mice. Prev. Nutr. Food Sci. 2023, 28, 134–140. [Google Scholar] [CrossRef]
- Shihab, E.M.; Kadhim, H.M.; Shahooth, S.S. Dapagliflozin mitigates oxidative stress, inflammatory, and histopathological markers of aging in mice. J. Med. Life. 2024, 17, 157–163. [Google Scholar] [CrossRef]
- Konen, J.C.; Summerson, J.H.; Kirk, J.K. Measurement feasability of advanced glycated end-products from skin samples after antioxidant vitamin supplementation in patients with type 2 diabetes. J. Nutr. Health Aging. 2000, 4, 81–84. [Google Scholar]
- Chiu, A.E.; Chan, J.L.; Kern, D.G.; Kohler, S.; Rehmus, W.E.; Kimball, A.B. Double-blinded, placebo-controlled trial of green tea extracts in the clinical and histologic appearance of photoaging skin. Dermatol. Surg. 2005, 31, 855–860; discussion 860. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Yatskayer, M.; Raab, S.; Oresajo, C. An evaluation of the effect of a topical product containing C-xyloside and blueberry extract on the appearance of type II diabetic skin. J. Cosmet. Dermatol. 2009, 8, 147–151. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Deyev, A.I.; Savel’yeva, E.L.; Lankin, V.Z.; Yegorov, Y.E. Skin beautification with oral non-hydrolized versions of carnosine and carcinine: Effective therapeutic management and cosmetic skincare solutions against oxidative glycation and free-radical production as a causal mechanism of diabetic complications and skin aging. J. Dermatolog Treat. 2012, 23, 345–384. [Google Scholar] [CrossRef]
- Yoshikata, R.; Myint, K.Z.Y.; Ohta, H.; Ishigaki, Y. Effects of an equol-containing supplement on advanced glycation end products, visceral fat and climacteric symptoms in postmenopausal women: A randomized controlled trial. PLoS ONE 2021, 16, e0257332. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Okada, Y.; Miura, S.; Imai, Y.; Igase, K.; Ohyagi, Y.; Igase, M. Ingestion of a collagen peptide containing high concentrations of prolyl-hydroxyproline and hydroxyprolyl-glycine reduces advanced glycation end products levels in the skin and subcutaneous blood vessel walls: A randomized, double-blind, placebo-controlled study. Biosci. Biotechnol. Biochem. 2023, 87, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, D.; Wang, F. Facial assessment methods for inhibiting glycation and aging effects and the correlation between glycation and aging parameters. Sci. Rep. 2025, 15, 1935. [Google Scholar] [CrossRef] [PubMed]
- Guiotto, A.; Pecorelli, A.; Draelos, Z.D.; Gueniche, A.; Yatskayer, M.; Nelson, D.B. Reversing Oxinflammation Associated with Glycative Stress and Formation of Advanced Glycation End Products with a Dietary Supplement Containing Rosemary Extract. J. Clin. Aesthet. Dermatol. 2025, 18, 34–38. [Google Scholar]
- Papaccio, F.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Bavkar, L.N.; Patil, R.S.; Rooge, S.B.; Nalawade, M.L.; Arvindekar, A.U. Acceleration of protein glycation by oxidative stress and comparative role of antioxidant and protein glycation inhibitor. Mol. Cell Biochem. 2019, 459, 61–71. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Hongsprabhas, P. Genistein as antioxidant and antibrowning agents in vivo and in vitro: A review. Biomed. Pharmacother. 2016, 82, 379–392. [Google Scholar] [CrossRef]
- Geng, R.; Kang, S.G.; Huang, K.; Tong, T. Boosting the Photoaged Skin: The Potential Role of Dietary Components. Nutrients 2021, 13, 1691. [Google Scholar] [CrossRef]
- Umbayev, B.; Askarova, S.; Almabayeva, A.; Saliev, T.; Masoud, A.R.; Bulanin, D. Galactose-Induced Skin Aging: The Role of Oxidative Stress. Oxid. Med. Cell Longev. 2020, 2020, 7145656. [Google Scholar] [CrossRef]
- Chmielewski, R.; Lesiak, A. Mitigating Glycation and Oxidative Stress in Aesthetic Medicine: Hyaluronic Acid and Trehalose Synergy for Anti-AGEs Action in Skin Aging Treatment. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2701–2712. [Google Scholar] [CrossRef]
- Draelos, Z.D. INDIVIDUAL ARTICLE: Sugar Sag: What Is Skin Glycation and How Do You Combat It? J. Drugs Dermatol. 2024, 23, S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.K.A.; Brech, G.C.; Luna, N.M.S.; Iborra, R.T.; Soares-Junior, J.M.; Baracat, E.C.; Greve, J.M.D.; Alonso, A.C.; Machado-Lima, A. Advanced glycation end products consumption and the decline of functional capacity in patients with Parkinson’s disease: Cross-sectional study. Clinics 2024, 79, 100320. [Google Scholar] [CrossRef] [PubMed]
- Drenth, H.; Zuidema, S.U.; Krijnen, W.P.; Bautmans, I.; Smit, A.J.; van der Schans, C.; Hobbelen, H. Advanced Glycation End Products Are Associated with Physical Activity and Physical Functioning in the Older Population. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1545–1551. [Google Scholar] [CrossRef]
- Thornalley, P.J. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch. Biochem. Biophys. 2003, 419, 31–40. [Google Scholar] [CrossRef]
- Harper, E.I.; Siroky, M.D.; Hilliard, T.S.; Dominique, G.M.; Hammond, C.; Liu, Y.; Yang, J.; Hubble, V.B.; Walsh, D.J.; Melander, R.J.; et al. Advanced Glycation End Products as a Potential Target for Restructuring the Ovarian Cancer Microenvironment: A Pilot Study. Int. J. Mol. Sci. 2023, 24, 9804. [Google Scholar] [CrossRef]
- Martinovic, D.; Tokic, D.; Usljebrka, M.; Lupi-Ferandin, S.; Cigic, L.; Vanjaka Rogosic, L.; Ercegovic, S.; Kontic, M.; Kumrić, M.; Rusic, D.; et al. The Association between the Level of Advanced Glycation End Products and Objective Skin Quality Parameters. Life 2023, 13, 256. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Sell, D.R.; Zhang, J.; Nemet, I.; Theves, M.; Lu, J.; Strauch, C.; Halushka, M.K.; Monnier, V.M. Anaerobic vs aerobic pathways of carbonyl and oxidant stress in human lens and skin during aging and in diabetes: A comparative analysis. Free. Radic. Biol. Med. 2010, 49, 847–856. [Google Scholar] [CrossRef]
- Fu, M.X.; Wells-Knecht, K.J.; Blackledge, J.A.; Lyons, T.J.; Thorpe, S.R.; Baynes, J.W. Glycation, glycoxidation, and cross-linking of collagen by glucose. Kinetics, mechanisms, and inhibition of late stages of the Maillard reaction. Diabetes 1994, 43, 676–683. [Google Scholar] [CrossRef]
- Stamatas, G.N.; Estanislao, R.B.; Suero, M.; Rivera, Z.S.; Li, J.; Khaiat, A.; Kollias, N. Facial skin fluorescence as a marker of the skin’s response to chronic environmental insults and its dependence on age. Br. J. Dermatol. 2006, 154, 125–132. [Google Scholar] [CrossRef]
- Qu, D.; Venzon, D.; Murray, M.; Depauw, M. Noninvasive measurement of advanced glycation end-products in the facial skin: New data for skin aging studies. J. Cosmet. Sci. 2017, 68, 195–204. [Google Scholar]
- Xin, C.; Wang, Y.; Liu, M.; Zhang, B.; Yang, S. Correlation analysis between advanced glycation end products detected noninvasively and skin aging factors. J. Cosmet. Dermatol. 2021, 20, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.S.; Razavi, Z.; Ehsani, A.H.; Firooz, A.; Afazeli, S. Clinical significance of non-invasive skin autofluorescence measurement in patients with diabetes: A systematic review and meta-analysis. Eclinicalmedicine 2021, 42, 101194. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, P.J.; Howell, S.; Mackenzie, T.; Corstjens, H.; Muizzuddin, N.; Matsui, M.S. Two fluorescent wavelengths, 440(ex)/520(em) nm and 370(ex)/440(em) nm, reflect advanced glycation and oxidation end products in human skin without diabetes. Diabetes Technol. Ther. 2012, 14, 285–292. [Google Scholar] [CrossRef]
- Delanghe, J.R.; Beeckman, J.; Beerens, K.; Himpe, J.; Bostan, N.; Speeckaert, M.M.; Notebaert, M.; Huizing, M.; Van Aken, E. Topical Application of Deglycating Enzymes as an Alternative Non-Invasive Treatment for Presbyopia. Int. J. Mol. Sci. 2023, 24, 7343. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Watanabe, K.; Beppu, M.; Kikugawa, K.; Yasuda, H. Identification of oxidized protein hydrolase of human erythrocytes as acylpeptide hydrolase. Biochim. Et. Biophys. Acta BBA-Protein Struct. Mol. Enzymology 2000, 1478, 102–112. [Google Scholar] [CrossRef]
- Shimizu, K.; Ikegami-Kawai, M.; Takahashi, T. Increased oxidized protein hydrolase activity in serum and urine of diabetic rat models. Biol. Pharm. Bull. 2009, 32, 1632–1635. [Google Scholar] [CrossRef][Green Version]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermatoendocrinol 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Couturaud, V.; Le Fur, M.; Pelletier, M.; Granotier, F. Reverse skin aging signs by red light photobiomodulation. Skin. Res. Technol. 2023, 29, e13391. [Google Scholar] [CrossRef]
- Oh, S.; Rho, N.K.; Byun, K.A.; Yang, J.Y.; Sun, H.J.; Jang, M.; Kang, D.; Son, K.H.; Byun, K. Combined Treatment of Monopolar and Bipolar Radiofrequency Increases Skin Elasticity by Decreasing the Accumulation of Advanced Glycated End Products in Aged Animal Skin. Int. J. Mol. Sci. 2022, 23, 2993. [Google Scholar] [CrossRef]
- Ding, B.; Lin, C.; Liu, Q.; He, Y.; Ruganzu, J.B.; Jin, H.; Peng, X.; Ji, S.; Ma, Y.; Yang, W. Tanshinone IIA attenuates neuroinflammation via inhibiting RAGE/NF-κB signaling pathway in vivo and in vitro. J. Neuroinflammation 2020, 17, 302. [Google Scholar] [CrossRef]
- Hudson, B.I.; Lippman, M.E. Targeting RAGE Signaling in Inflammatory Disease. Annu. Rev. Med. 2018, 69, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Boo, Y.C. Natural Nrf2 Modulators for Skin Protection. Antioxidants 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.W.; Wan, J.; Yoon, S.E.; Wong, S.; Yi, K.H. Conditions to Consider When Choosing Fillers. J. Cosmet. Dermatol. 2025, 24, e70075. [Google Scholar] [CrossRef]
- Rho, N.K.; Kim, H.S.; Kim, S.Y.; Lee, W. Injectable Skin Boosters in Aging Skin Rejuvenation: A Current Overview. Arch. Plast. Surg. 2024, 51, 528–541. [Google Scholar] [CrossRef]
- Han, J.; Sun, Y.; Wu, T.; Hou, X.; Zheng, S.; Zhang, H.; Lin, T.; Liu, H.; Sun, T. Echinacoside-Zinc Nanomaterial Inhibits Skin Glycation by Suppressing the Transcriptional Activation of the Receptor for Advanced Glycation End-Products. ACS Nano. 2023, 17, 14123–14135. [Google Scholar] [CrossRef]

| Model | Glycation Inducer | Findings | References |
|---|---|---|---|
| Sprague–Dawley rats | Diabetes induced by streptozotocin (70 mg kg−1, i.p.) | 1. Increased glycated plasma proteins, glycated hemoglobin (Hb), and fructosamine. 2. The AGE levels of liver histones and skin collagen increased with the duration of diabetes and animal age. | Gugliucci and Bendayan, 1995 [42] |
| Sprague–Dawley rats | Glucose, sucrose, or fructose in drinking water (250 g L−1) | 1. Blood glycated Hb levels and urine lipid peroxidation products were higher in fructose-fed rats. 2. Insoluble collagen and collagen-bound fluorescence were higher in fructose-fed rats. | Levi and Werman, 1998 [43] |
| db/db(+/+) mice | High-AGE diets | 1. Increased the levels of AGEs, such as Nε-(carboxymethyl)lysine (CML) and methylglyoxal derivatives, of skin proteins and collagen in diabetic mice. 2. Delayed skin wound closure in diabetic mice. | Peppa et al., 2003 [44] |
| CD1 mice | CML-collagen injection | 1. Induced fibroblast apoptosis mediated by the receptor for advanced glycation end-products (RAGE) and caspases 3, 8, and 9. 2. Enhanced mRNA levels of pro-apoptotic genes. | Alikhani et al., 2005 [45] |
| Fatty OLETF rats and control LETO rats | Long-standing hyperglycemia | 1. Fatty OLETF rats with diabetes or impaired glucose tolerance had higher serum AGE levels and epidermal RAGE levels. 2. Impaired skin barrier functions, including transdermal permeability and antimicrobial barriers. | Park et al., 2011 [46] |
| Ansell’s mole-rats (Fukomys anselli) | Age (1–19 years) and breeding status | 1. Glucosepane, pentosidine, CML, and Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1) in insoluble skin collagen increased with age. 2. Glucosepane and CML were higher in breeders versus nonbreeders. 3. The pentosidine formation rate was lower in mole-rats than in other short-lived rodents. | Dammann et al., 2012 [47] |
| C57BL6/J mice | Age | 1. Argpyrimidine and pentosidine, but not protein carbonyls, increased in the skin of old mice. | Nowotny and Grune, 2014 [24] |
| ddY mice | Diabetes induced by streptozotocin (150 mg kg−1, abdominal injection) | 1. Increased blood glucose and fluorescent AGE levels in the auricle and decreased body weight. | Yamanaka et al., 2016 [48] |
| Lamin B receptor-deficient icJ/icJ mice (NMRI background) | Progeria (premature aging symptoms) | 1. Pentosidine and argpyrimidine, but not CML, increased in the heart and liver (not skin) of icJ/icJ mice. | Hause et al., 2018 [49] |
| C57BL/6J mice | Age | 1. Increased the levels of methylglyoxal-modified AGEs (argpyrimidine and MG-H1) in brain tissue and collagen. | Nowotny et al., 2018 [50] |
| ddY mice | 20 weeks | 1. The skin level of Nω-(carboxymethyl)arginine (CMA) was higher than CML, Nε-(carboxyethyl)lysine (CEL), and MG-H1. | Kinoshita et al., 2019 [25] |
| db/db [C57Bl/KsJ-db/db] mice and the corresponding controls (db/+) | Chronic kidney disease by nephrectomy or cyanate-supplemented water | 1. Diabetes increased glycation products (furosine and CML), whereas chronic kidney disease increased carbamylation products (homocitrulline) in the skin and aorta and in skin type I collagen. 2. Carbamylation of proteins precedes their glycation, with the former competitively inhibiting the latter protein modification. | Nicolas et al., 2019 [51] |
| Sprague–Dawley rats | Diabetes induced by streptozotocin (50 mg kg−1, i.p.) | 1. Full-thickness excisional wounds increased AGE levels, interleukin (IL) 8 receptor A, leukotriene (LT) B4, and myeloperoxidase (MPO) in the skin wound edges more highly in the diabetic group. | Kang et al., 2021 [52] |
| KK-Ay/TaJcl mice (C57BL/6N background) and control C57BL/6j mice | Ages (10, 27, 40, and 50 weeks) | 1. The body weight, blood glucose, skin thickness, transepidermal water loss (TEWL), AGE levels, and expression of RAGE, prostaglandin (PG) E2, tumor necrosis factor (TNF)-α, monocyte chemoattractant protein (MCP)-1, and C-C chemokine receptor (CCR) 2 were higher and the skin conductance and collagen expression were lower in the test groups compared to the age-matched control groups. 2. The age-dependent changes in these parameters were greater in the test groups than in the age-matched control groups. | Hiramoto et al., 2023 [53] |
| C57BL/6J mice | High-sugar feed | 1. Changed toward a redder, yellower, and darker skin color. 2. Increased sebum secretion and lowered TEWL. 3. Increased skin AGE levels and decreased the expression of collagen type I, fibronectin 1, laminin-5, and tenascin C. | Li et al., 2024 [54] |
| Model | Factors | Findings | References |
|---|---|---|---|
| Plasma samples from 30 living human subjects | Diabetes | CMA level of serum proteins was elevated in the diabetic group compared to the age-matched control group. | Odani et al., 2001 [55] |
| 49 Japanese patients with diabetes | Diabetes | 1. The high-frequency conductance (skin hydration) and skin surface lipid levels were lower in the group with high fasting plasma glucose (>110 mg dL−1). 2. The TEWL was slightly reduced in the group with high HbA1c levels (>5.8%). | Sakai et al., 2005 [56] |
| 40 healthy Japanese women | Age | 1. Cheek skin yellowness (b* value) and the AGE index, but not the melanin index, increased with age. 2. The b* value was correlated with the AGE index or the melanin index. | Ohshima et al., 2009 [57] |
| Group I (232 men) and group II (138 men) among 1263 participants enrolled in annual examinations | Age | 1. Skin autofluorescence increased with age in group I and group II. 2. Participants with higher skin autofluorescence had lower grip strength (group I) and leg extension power (group II). | Momma et al., 2011 [58] |
| 1441 human subjects with type I diabetes (726 with no retinopathy and 715 with nonproliferative retinopathy) | Diabetes | 1. Skin autofluorescence in the log scale was correlated with mean HbA1c over time, age, smoking, skin tone, renal damage, and locational latitude. | Cleary et al., 2013 [59] |
| Five patient-matched skin biopsy specimens from chronic solar ultraviolet (UV) radiation-exposed and protected skin | UV exposure | 1. Protein damage (e.g., AGEs) was higher in the UV-exposed skin. 2. DNA damage (e.g., 8-hydroxy deoxyguanosine) was higher in the UV-exposed skin. 3. Lipid peroxidation (e.g., 4-hydroxynonenal) was higher in the UV-exposed skin. | Mamalis et al., 2014 [60] |
| 16 human subjects with diabetes (13 with type II diabetes, one with type I diabetes, and 2 without diabetes) | Wound | 1. Expression of galectin-3, a potential receptor for AGEs, was reduced at the wound edge and in the wound bed where the AGE level was increased. | Pepe et al., 2014 [61] |
| A population-based cohort study involving 215 participants with type II diabetes | Diabetes | 1. Higher skin autofluorescence levels were associated with delayed word recall and response inhibition. 2. Higher plasma levels of pentosidine, not CML or CEL, were associated with worse global cognitive functioning. 3. Associations did not differ between individuals with and without diabetes. | Spauwen et al., 2015 [62] |
| A total of 168 human subjects, including 82 subjects with type 2 diabetes | Diabetes | 1. The autofluorescence intensity of fingertip skin and serum MG-H1, but not HbA1c, increased with the number of varied diabetic complications. | Yamanaka et al., 2016 [48] |
| A population-based prospective cohort study involving 2388 human subjects | Diabetes | 1. Skin autofluorescence was higher in the diabetic group than in the non-diabetic group. 2. Serum 25-hydroxyvitamin D3 concentration was inversely associated with skin autofluorescence. | Chen et al., 2019 [63] |
| 576 Japanese patients with primary open-angle glaucoma, exfoliation glaucoma, and non-glaucomatous controls | Glaucoma | 1. AGE level (measured by fingertip skin autofluorescence) was higher in the exfoliation glaucoma group than in the primary open-angle glaucoma or control group. 2. Male sex, exfoliation glaucoma, and diabetes, but not age, visual acuity, intraocular pressure, glaucoma medications, lens status, and systemic hypertension, were associated with higher AGE levels. | Shirakami et al., 2020 [64] |
| 2 healthy volunteers | UV exposure | 1. Skin CML was increased by UV irradiation. 2. The CML level was higher in the paraffin-embedded skin specimens from sun-exposed areas than sun-protected areas of human subjects, and the increase was age-dependent. | Yoshinaga et al., 2012 [65] |
| Protein Substrates | Glycating Agents | Media and Additives | Reaction Conditions | Measurements | Test Materials | Positive Controls | References |
|---|---|---|---|---|---|---|---|
| Human serum albumin | Glucose (1.6 M) | Na-P buffer (50 mM, pH 7.2), 2 mM diethylenetriaminepentaacetic acid (DTPA) | 37 °C, 7 days | Colorimetry with nitroblue tetrazolium (NBT) reaction. | NaBH4, DTPA | Aminoguanidine (AGD) | Hayashi et al., 2002 [69] |
| Bovine serum albumin (BSA) (0.149 mM) | Glucose (11 mM) | Physiological saline, 10 mM FeCl2 | 37 °C, 4 weeks | Spectrophotometry | Fucose- or rhamnose-rich oligo- and polysaccharides | Péterszegi et al., 2008 [70] | |
| Bovine collagen type I | Ribose (10 mM) | Room temperature (RT), 1 month | Fluorimetry | Blueberry (Vaccinium angustifolium) extract | AGD | Pageon et al., 2008 [71] | |
| BSA (1%) | Glucose 11 mM | 10 mM FeCl2 | 37 °C, 4 weeks | Ravelojaona et al., 2009 [72] | |||
| α-Elastin (10 μg mL−1) | Ribose (0.2 M) | Phosphate-buffered saline (PBS) | 37 °C, 1 week | Colorimetry (Elastase digestion) | AGD | Yoshinaga et al., 2012 [65] | |
| Collagen (3 mg mL−1) | Glycolaldehyde (10 mM) | 1 mM HCl, 0.02% NaN3, 1 mM DTPA | 37 °C, 7 days | Fluorimetry | Gold nanoparticles | AGD | Kim et al., 2012 [73] |
| Bovine skin collagen type I (4–5 mg mL−1) | Ribose (10 mM) | RT, 3 weeks | Fluorimetry | AGD | Pageon et al., 2014 [74] | ||
| Collagen type I (1 mg mL−1) | Glucose (55 mM) | Dulbecco’s phosphate-buffered saline | 45 °C, 3 weeks | Fluorimetry | Manilkara extract | AGD AGD AGD | Danoux et al., 2014 [75] |
| Bovine elastin (6 mg mL−1) | Glycolaldehyde (10 mM) | 37 °C, 2 days | Manilkara and Argania extracts | ||||
| Albumin (6 mg mL−1) | Argania extract | ||||||
| BSA (10 mg mL−1) | Glucose (0.5 M) | Phosphate buffer (0.1 M, pH 7.4), 0.02% NaN3 | 37 °C, 3 weeks | Fluorimetry | Silybum marianum flower extract, silibinin | AGD | Shin et al., 2015 [76] |
| Akebia quinata fruit extract | AGD | Shin et al., 2015 [77] | |||||
| Rat tail collagen type I (at (3–4 mg mL−1) | Sodium glyoxylate | 0.5 N acetic acid, sodium cyanoborohydride | 37 °C, 24 h | Fluorimetry | AGD | Pennacchi et al., 2015 [78] | |
| BSA (20 mg mL−1) | Glyceraldehyde (20 mM) | K-phosphate buffer (0.1 M, pH 7.4), 1 mM DTPA | 37 °C, 7 days | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) | Han et al., 2016 [79] | ||
| BSA (20 mg mL−1) | Glucose (20 mM), glyoxal or methylglyoxal (29 μM) | PBS (0.2 M, pH 7.4), 1 mM NaN3 | 37 °C, 7 days | Fluorimetry | Rosmarinic acid, carnosine | Ou et al., 2017 [80] | |
| BSA (10 mg mL−1) | Fructose (1.6 M) | Na-phosphate buffer (0.1 M, pH 7.4), 0.8% NaN3 | 37 °C, 7 days | Fluorimetry | Cecropia pachystachya leaf extract, quercetin | AGD | Fernandes et al., 2019 [81] |
| BSA (10 mg mL−1) | Ribose (0.5 M) | Na-phosphate buffer (50 mM, pH 7.4) | 37 °C for 24 h | Fluorimetry | Mycosporine-like amino acids | AGD | Orfanoudaki et al., 2019 [82] |
| BSA (20 mg mL−1) | Glucose (0.5 M) | Phosphate buffer (0.1 M, pH 7.4), 0.02% NaN3 | 37 °C, 5 days | Fluorimetry | Silybum marianum flavonolignans | Drouet et al., 2019 [83] | |
| BSA (10 mg mL−1) | Fructose (100 mM) | PBS (0.2 M, pH 7.2) | 37 °C, 14 days | Fluorimetry | Black cumin (Nigella sativa) seed extract (rich in thymoquinone) | AGD | Li et al., 2020 [84] |
| Bovine collagen type I (1.5 mg mL−1) | Methylglyoxal (5 mM) | PBS, 10 mM NaN3 | 37 °C, 30 days | ||||
| BSA (1 mg mL−1) | Methylglyoxal (5 mM) | Phosphate buffer (pH 7.4), 150 mM NaCl | 37 °C, 3 days | Fluorimetry | Alkaloids from Ocotea paranapiacabensis | AGD | Freitas et al., 2020 [85] |
| Collagen type I (2.5 mg mL−1) | Ribose (10 mM), glucose (200 mM) | RT, 7 days | |||||
| BSA (4 mg mL−1) | Methylglyoxal (20 mM) | PBS, 0.02% NaN3 | 37 °C, 7 days | Fluorimetry | Hazelnut (Corylus avellana) skin extracts, gallic acid | AGD | Spagnuolo et al., 2021 [86] |
| Collagen, elastin, BSA | Glyceraldehyde | Enzyme-linked immunosorbent assay (ELISA) for AGEs | Mycosporine-like amino acids | AGD | Waditee-Sirisattha and Kageyama, 2021 [87] | ||
| BSA (10 mg mL−1) | Glucose (0.5 M) | PBS, 1.5 mM phenylmethylsulfonyl fluoride (PMSF) | 37 °C, 2 months | Fluorimetry | Kang et al., 2021 [52] | ||
| BSA (5 mg mL−1) | Methylglyoxal (25 mM) | Phosphate buffer (0.1 M, pH 8.0) | 37 °C, 24 h | Colorimetry with NBT reaction | Pholiota nameko polysaccharides | AGD | Lin et al., 2021 [88] |
| BSA (10 mg mL−1) | Glucose (0.5 M) | Phosphate buffer (0.1 M, pH 7.4) | 37 °C, 3 weeks | Fluorimetry for AGEs, colorimetry for protein carbonyls, and ELISA for CML | Methyl gallate | AGD | Shin et al., 2022 [89] |
| BSA (10 mg mL−1) | Glucose (0.5 M) | Phosphate buffer (0.1 M, pH 7.4) | 37 °C, 28 days | Fluorimetry for AGEs, colorimetry for protein carbonyls, and ELISA for CML | Cirsium japonicum flower extract, apigenin, and chlorogenic acid | AGD | Yoon et al., 2022 [90] |
| BSA (10 mg mL−1) | Glycolaldehyde (10 mM) | Phosphate buffer (0.1 M, pH 7.4), 0.02% NaN3 | 37 °C, 5 days | ||||
| BSA (10 mg mL−1) | Glucose (0.5 M) | Phosphate buffer (0.1 M, pH 7.4) | 37 °C, 4 weeks | Fluorimetry, ELISA for CML | Chlorella (Parachlorella beijerinckii) extract | AGD | Imai et al., 2022 [91] |
| BSA (2 mg mL−1) | Glyoxal (20 mM) | 37 °C, 1 week | |||||
| Collagen (2 mg mL−1) | Glucose (100 mM) | 37 °C, 4 weeks | |||||
| Collagen (1 mg mL−1) | Glyoxal (1 mM) | 37 °C, 1 week | |||||
| BSA (50 mg mL−1) | Glucose (50 mM) | Na-phosphate buffer (0.2 M, pH 7.4), 0.02% NaN3 | 37 °C, 1 week | Fluorimetry | Vanillic acid | AGD | Alhadid et al., 2022 [92] |
| Collagen | Methylglyoxal, dehydroascorbic acid | PBS (0.1 M, pH 7.4) | 37 °C, 7 days | Fluorimetry | Cranberry juice (rich in polyphenols) | AGD, alagebrium (ALT-711) | Chang et al., 2022 [93] |
| BSA (4 mg mL−1) | Methylglyoxal (20 mM) | PBS (pH 7.4) | 37 °C, 168 h | Fluorimetry, dot blot for CML | Spagnuolo et al., 2023 [94] | ||
| BSA (10.35 mg mL−1) | Glucose (1 M) | PBS | 50 °C, 6–7 days | Fluorimetry | N-Acetylhydroxyproline | Knoblich et al., 2024 [95] | |
| BSA (10 mg mL−1) | Glyoxal (10 mM) | PBS (pH 7.4), 0.2% NaN3 | 37 °C, 7 days | Sultana et al., 2024 [96] |
| Model | Glycation Inducers | Induced Changes | Intervention | Outcomes | References |
|---|---|---|---|---|---|
| Reconstructed skin model | Ribose (10 mM) | 1. CML, pentosidine (↑) 2. Integrin, collagen, procollagen, matrix metalloproteinases (MMPs) (↑) | AGD | 1. CML, pentosidine (↓) 2. Integrin, collagen, procollagen (↓) | Pageon and Asselineau, 2005 [98] |
| A reconstructed skin model | CEL, CML, MG-H1, or pentosidine | 1. Epidermal integrin α6 (↑) 2. Laminin-5 (↓) 3. Procollagen type I (↑) 4. MCP-1 (↑) 5. IL-6, MMP-1, MMP-3, vascular endothelial growth factor (VEGF) (↓) | Pageon et al., 2015 [99] | ||
| Reconstructed skin model | Ribose (10 mM) | 1. Diameter of collagen lattice (↑) 2. Epidermal thickness (↑) 3. Dermal thickness (↓) 4. Dermal collagen aggregation (↑) 5. CML (↑) 6. Suprabasal integrin β1 (↑) | Blueberry extract, AGD | 1. Diameter of collagen lattice (↓) 2. Epidermal thickness (↓) 3. Dermal thickness (↑) 4. Dermal collagen aggregation (↓) 5. CML (↓) 6. Suprabasal integrin β1 (↓) | Pageon et al., 2008 [71] |
| Human dermal fibroblasts (HDFs) | AGEs of BSA | 1. Senescence-associated β-galactosidase (SA-β-gal)-positive cells (↑) | Fucose- or rhamnose-rich oligo- and polysaccharides | 1. SA-β-gal-positive cells (↓) | Ravelojaona et al., 2009 [72] |
| HDFs | AGEs of BSA | 1. MMP-2, MMP-9 (↑) | Fucose- or rhamnose-rich oligosaccharides | 1. MMP-2, MMP-9 (↓) | Robert et al., 2010 [100] |
| Reconstructed skin model | Ribose (10 mM) | 1. Dermal thickness (↓) 2. CML (↑) 3. Collagen type VII, procollagen type III, glycosaminoglycans (↑) 4. MMP-2 (↑) | AGD | 1. Collagen type VII, procollagen type III, glycosaminoglycans (↓) 2. MMP-2 (↓) | Pageon et al., 2014 [74] |
| Reconstructed skin model (MimeskinTM) | Ribose (0.5 M) | Diameter of collagen fibers (↓) | Davilla extract, AGD | Diameter of collagen fibers (↑) | Danoux et al., 2014 [75] |
| Dead human dermis | UV irradiation | 1. Soluble fluorescent AGE levels (↑) 2. Soluble pentosidine-like AGE levels (↑) 3. Reactive oxygen species (ROS) production (↑) | Argania extract, AGD | 1. Soluble fluorescent AGE levels (↓) 2. Soluble pentosidine-like AGE levels (↓) 3. ROS production (↓) | |
| HDFs | AGEs of BSA (100 μg mL−1) | 1. ROS production (↑) 2. SA-β-gal-positive cells (↑) | Akebia quinata fruit extract | 1. ROS production (↓) 2. SA-β-gal-positive cells (↓) | Shin et al., 2015 [77] |
| Endothelialized and innervated reconstructed skin model | Glyoxal (200–500 μM) | 1. Increased CML (↑) 2. Number of capillaries (↓) 3. Platelet endothelial cell adhesion molecule (PECAM) 1, loricrin, filaggrin, and Krüppel-like factor (KLF) 4 (↓) | AGD, alagebrium (ALT-711) | 1. Increased CML (↓) 2. Number of capillaries (↑) 3. PECAM-1, loricrin, filaggrin, and KLF-4 (↑) | Cadau et al., 2015 [101] |
| Reconstructed skin model | Sodium glyoxylate | 1. Dermal thickness (↓) 2. CML expression (↑) 3. Cytokeratin (CK) 10 and CK-14 (↑) 4. E-cadherin and desmoglein (↑) 5. Gaps in the dermis (↑) 6. Collagen flattening/compression (↑) | AGD | 1. Dermal thickness (↑) 2. CML expression (↓) 3. CK-10 and CK-14 proteins and mRNAs (↓) | Pennacchi et al., 2015 [78] |
| HDFs | UVB radiation with glyceraldehyde-induced AGEs of BSA (100 μg mL−1) | 1. ROS production (↑) 2. RAGE protein (↑) 3. MMP-1 protein (↑) 4. TNF-α, IL-1β, and IL-6 (↑) 5. Phosphorylation of extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK) (↑) 6. Nuclear factor (NF)-κB/p65 subunit nuclear translocation and inhibitor of NF-κB (IκBα) phosphorylation (↑) | Plantamajoside | 1. ROS production (↓) 2. RAGE protein (↓) 3. MMP-1 protein (↓) 4. TNF-α, IL-1β, and IL-6 (↓) 5. Phosphorylation of ERK, p38-MAPK, and JNK (↓) 6. IκBα phosphorylation and p65 nuclear translocation (↓) | Han et al., 2016 [79] |
| HaCaT keratinocytes | |||||
| HaCaT keratinocytes | Glyoxal (5 mM) | 1. Cell viability (↓) 2. Fluorescent AGE levels (↑) 3. Free fatty acids (↑) | Yokota and Tokudome, 2016 [97] | ||
| Reconstructed skin model | Glyoxal (2.5–10 mM) | 1. Free fatty acids (↑) 2. TEWL (↑) | |||
| Dead mouse skin | Glyoxal 50 mM | 1. Skin color yellowness (↑) 2. TEWL (↑) | |||
| HDFs | Methylglyoxal-modified collagen | 1. Cell viability (↓) 2. Apoptosis (↑) 3. Endoplasmic reticulum stress (↑) 4. Oxidative stress (↑) | N-Acetylcysteine | 1. Apoptosis (↓) 2. Oxidative stress (↓) | Nowotny et al., 2018 [50] |
| Reconstructed skin model | Ribose (10 mM) | 1. Epidermal thickness (↑) 2. Collagen aggregates (↑) 3. Fibril and filament (↑) 4. Integrin β1, laminin-5, loricrin (↑) 5. Filaggrin (↓) | Balansin Rigon et al., 2018 [102] | ||
| Reconstructed skin model | Ribose (10 mM), glucose (200 mM) | 1. Dermis thickness (↓) | Alkaloids from Ocoteaparanapiacabensis, AGD | 1. Dermis thickness (↑) | Freitas et al., 2020 [85] |
| HaCaT keratinocytes, human foreskin fibroblasts, THP-1 cells | t-Butylhydroperoxide | 1. SA-β-gal-positive cells (↑) 2. IL-6, IL-8, MMP-1, MMP-3 (↑) | Carnosine | 1. CD36, RAGE in macrophages derived from THP-1 cells (↑) 2. Protein kinase B (AKT) 2 phosphorylation (↑) 3. Macrophage-mediated elimination of senescent skin cells (↑). | Li et al., 2020 [103] |
| Hs68 cells | Methylglyoxal (400 μM) | 1. Cell viability (↓) 2. ROS generation (↑) | Pholiota nameko polysaccharides | 1. Cell viability (↑) 2. ROS generation (↓) | Lin et al., 2021 [88] |
| HL-60 cells | AGEs of BSA (0.5 μg mL−1) | 1. Neutrophil migration and cluster formation (↓) | Kang et al., 2021 [52] | ||
| HDFs | UVB radiation | 1. Pentosidine, AGEs, methylglyoxal (↑) 2. Denatured collagen (↑) 3. MMPs 1, 3, and 9 (↑) | Hydrolyzed fish collagen (25% tripeptide) | 1. Pentosidine, AGEs, methylglyoxal (↓) 2. Denatured collagen (↓) 3. Collagen expression (↑) 4. MMPs 1, 3, and 9 (↓) | Lee et al., 2022 [104] |
| RAW264.7 cells | Methylglyoxal (100–400 μM) | 1. Cell viability (↓) | Vanillic acid | 1. Cell viability (↑) | Alhadid et al., 2022 [92] |
| HDFs | Glucose-induced AGEs (0.5 mM) | 1. MMP-1 mRNA (↑) | Cirsium japonicum flower extract | 1. MMP-1 mRNA (↓) | Yoon et al., 2022 [90] |
| Hs68 Cells | CML (100 μg mL−1) | 1. ROS production (↑) 2. RAGE protein (↑) 3. Collagen contents (↓) | Djulis (Chenopodium formosanum) extract | 1. ROS generation (↓) 2. RAGE protein (↓) 3. Collagen contents (↑) | Lyu et al., 2022 [105] |
| HaCaT keratinocytes | UVA radiation | 1. AGE levels (↑) | Sunflower sprout extract | 1. AGE levels (↓) | Barua et al., 2022 [106] |
| Fibroblasts in collagen gels | Glyoxal (400, 1000 μM) | 1. Contraction of collagen gel (↓) 2. Fluorescent AGE levels (↑) 3. CML-protein (↑) 4. CMA-protein (↑) 5. RAGE protein (↑) 6. IL-8 mRNA (↑) | Chlorella (Parachlorella beijerinckii) extract, AGD | 1. Contraction of collagen gel (↑) 2. Fluorescent AGE levels (↓) 3. CML-protein (↓) 4. CMA-protein (↑) 5. RAGE protein (↓) 6. IL-8 mRNA (↓) | Imai et al., 2022 [91] |
| HDFs | Methylglyoxal (500 μM) | 1. Autofluorescence (AGEs) (↑) 2. Cell density and proliferation (↓) 3. ROS production (↑) | Carnosine, resveratrol, oxyresveratrol, piceatannol | 1. Autofluorescence (AGEs) (↑) 2. Cell density and proliferation (↓) 3. ROS production (↑) | Markiewicz et al., 2022 [107] |
| Full-thickness skin model (EpiDermFT) | 1. Skin model diameter (↓) 2. Autofluorescence (AGEs) (↑) 3. CML (↑) 4. Eosin fluorescence (↓) 5. Epidermal thickness (↓) 6. Cell density and proliferation (↓) 7. Collagen density (↓) | 1. Autofluorescence (AGEs) (↓) 2. CML (↓) 3. Eosin fluorescence (↑) 4. Epidermal thickness (↑) 5. Cell density and proliferation (↑) 6. Collagen density (↑) | |||
| HDFs (formaldehyde-fixed) | Glyoxal (0.5%) | 1. AGE levels (↑) | Jasminum sambac cell extract | 1. AGE levels (↓) | Ceccacci et al., 2022 [108] |
| Macrophages derived from THP-1 cells | Methylglyoxal-modified BSA (300 μg mL−1) | 1. Cell viability (↓) 2. ROS production (↑) 3. TNF-α and IL-1β (↑) | Hazelnut (Corylus avellana) skin extract (rich in polyphenols) | 1. Cell viability (↑) 2. ROS production (↓) 3. TNF-α and IL-1β (↓) | Spagnuolo et al., 2023 [94] |
| HDFs | Methylglyoxal (400 μM) | 1. Cell viability (↓) 2. SA-β-gal-positive cells (↑) 3. Collagen type I alpha 1 chain (COL1A1) (↓) 4. AKT, JNK, p38-MAPK, c-Jun, and NF-κB phosphorylation (↑) 5. MMP-1 (↑) | Carica papaya fruit extract, AGD | 1. Cell viability (↑) 2. SA-β-gal-positive cells (↓) 3. COL1A1 (↑) 4. AKT, JNK, p38-MAPK, c-Jun, and NF-κB phosphorylation (↓) 5. MMP-1 (↓) | Wattanapitayakul et al., 2023 [109] |
| Fibroblasts in collagen gels | Glyoxal (600 μM) | 1. Contraction of collagen gel (↓) | N-Acetylhydroxyproline | 1. Contraction of collagen gel (↑) | Knoblich et al., 2024 [95] |
| HDFs | Methylglyoxal (500 μM) | 1. AGE levels (↑) 2. RAGE protein (↑) | Supramolecular carnosine | 1. AGE levels (↓) 2. RAGE protein (↓) | Bai et al., 2024 [110] |
| HDFs | H2O2 (25 μM) | 1. SA-β-gal-positive cells (↑) 2. Superoxide dismutase (SOD), catalase (CAT) activity (↓) 3. p-NF-κB/NF-κB ratio (↑) 4. IL-1β, IL-6 (↑) 5. MMP-1, MMP-9 (↑) 6. AGE levels (↑) | K formulation (containing hyaluronan, collagen type I peptide) | 1. SOD, CAT activity (↑) 2. Nuclear factor erythroid 2-related factor (NRF) 2 expression (↑) 3. p-NF-κB/NF-κB ratio (↓) 4. IL-1β, IL-6 (↓) 5. MMP-1, MMP-9 (↓) 6. AGE levels (↓) | Augello et al., 2024 [111] |
| HDFs, human epidermal keratinocytes (HEKs), HaCaT keratinocytes | Glyoxal-modified BSA (100 μg mL−1), UVB radiation | 1. Cell viability (↓) 2. Nitric oxide and ROS production (↑) 3. TNF-α, IL-1β, IL-6, IL-8 (↑) 4. NF-κB/p65 phosphorylation 5. RAGE and cyclooxygenase (COX) 2 (↑) 6. COL1A and NAD-dependent deacetylase sirtuin-1 (SIRT1) (↓) | Sultana et al., 2024 [96] |
| Model | Glycation Inducers | Induced Changes | Interventions | Outcomes | References |
|---|---|---|---|---|---|
| Human skin explants | Methylglyoxal (500 μM) | 1. Fibrillin-1 protein (↓) 2. CML expression (↑) | Silybum marianum flower extract, AGD | 1. Fibrillin-1 protein (↑) 2. CML expression (↓) | Shin et al., 2015 [76] |
| Human skin explants | Methylglyoxal (500 μM) | 1. Fibrillin-1 protein (↓) 2. CML expression (↑) | Akebia quinata fruit extract, AGD | 1. Fibrillin-1 protein (↑) 2. CML expression (↓) | Shin et al., 2015 [77] |
| Human skin explants | Methylglyoxal (500 μM) | 1. Pentosidine expression (↑) 2. CML expression (↑) | Carnosine | 1. Pentosidine expression (↓) 2. CML expression (↓) | Narda et al., 2018 [113] |
| Human skin explants | Methylglyoxal (500 μM) | 1. Fibrillin-1 protein (↓) 2. CML expression (↑) | Methyl gallate | 1. Fibrillin-1 protein (↑) 2. CML expression (↓) | Shin et al., 2022 [89] |
| Human skin explants | Methylglyoxal (500 μM) | 1. CML expression (↑) | Dunaliella salina extract | 1. RAGE (↓) 2. IL-6 and IL-8 (↓) 3. NRF2 (↑) | Havas et al., 2022 [114] |
| Human skin explants | Methylglyoxal (500 μM) | 1. Fibrillin-1 protein (↓) | Jasminum sambac cell extract, AGD | 1. Fibrillin-1 protein (↑) | Ceccacci et al., 2022 [108] |
| Human breast skin explants | Glycolaldehyde (25 mM) | 1. Autofluorescence (↑) | Fructosamine 3-kinase, fructosyl-amino acid oxidase | 1. Autofluorescence (↓) | De Decker et al., 2023 [115] |
| Hypertrophic scar tissue explants | Fructosamine 3-kinase | 1. Elongation rate (↑) | |||
| Skin explants | Glucose (1 M) | 1. Autofluorescence (↑) | N-Acetylhydroxyproline | 1. Autofluorescence (↓) | Knoblich et al., 2024 [95] |
| Model | Glycation Inducers | Induced Changes | Interventions | Outcomes | References |
|---|---|---|---|---|---|
| Wistar rats | Streptozotocin (50 mg kg−1, i.p.) | 1. Blood glucose (↑) 2. Glycosylated Hb (↑) 3. Skin collagen fluorescence (↑) | Rutin (1 g L−1) or AGD (1 g L−1) in drinking water | 1. Skin collagen fluorescence (↓) | Odetti et al., 1990 [117] |
| Emory mice prone to age-related cataract | Age (6.5–22 months) | 1. Plasma glucose (↑) 2. Tail tendon breakdown time (↑) 3. Dermatological lesions (↑) | Restricted diet with different compositions (high protein and low carbohydrates) | 1. Lifespan (↑) 2. Cataract grade (↓) 3. Plasma glucose (↓) 4. Glycohemoglobin (↓) 5. Tail tendon breakdown time (↓) 6. Dermatological lesions (↓) | Taylor et al., 1995 [118] |
| Brown-Norway rats | Age (4–25 months) | 1. CML and pentosidine in skin collagen (↑) | Caloric restriction | 1. CML, pentosidine, and fluorescence in collagen (↓) | Cefalu et al., 1995 [26] |
| Fischer 344 rats, | Age (6–24 months) | 1. Furosine and pentosidine in skin collagen (↑) | Diet restriction | 1. Furosine and pentosidine in skin collagen (↓) | Sell, 1997 [119] |
| C57BL/6NNia mice | Age (1–26 months) | ||||
| Sprague–Dawley rats | Age (2–24 months) | 1. Skin and aortic collagen-linked fluorescence (↑) 2. Glycated plasma protein and Hb (↓) | Diet restriction | 1. Glycated plasma proteins and fluorescent products in skin collagen of younger rats (↓) 2. Did not affect glycated Hb or aortic collagen fluorescence. | Novelli et al., 1998 [120] |
| BALB/c mice | Glucose (10% in feed) | 1. Blood glucose (-) 2. Fluorescent AGE levels in dorsal subcutaneous tissues (↑) 3. Calibers of arterial microvessels (↓) 4. Vascular lesion indices (↑) | AGD (0.25%) in drinking water | 1. Calibers of arterial microvessels (↑) 2. Vascular lesion indices (↓) | Yamada and Ohkubo, 1999 [121] |
| Broiler breeder chicks | Age (8–92 weeks) | 1. Skin pentosidine (↑) | Diet restriction (60% of control) and AGD (400 ppm) in feed | 1. Skin pentosidine (↓) | Iqbal et al., 1999 [122] |
| C57 BL/6J mice | Galactose (50 mg kg−1, s.c.) | 1. Serum AGE levels (↑) 2. Motor activity (↓) 3. Memory latency time (↓) 4. Memory error (↑) 5. Lymphocyte proliferation (↓) 6. IL-2 production (↓) | AGD (0.1%) in drinking water | 1. Serum AGE levels (↑) 2. Motor activity (↑) 3. Memory latency time (↑) 4. Memory error (↓) 5. Lymphocyte proliferation (↑) 6. IL-2 production (↑) | Song et al., 1999 [123] |
| Fischer 344 rats | Age (3–26 months), diets with different carbohydrate sources | 1. The source of dietary carbohydrates (cornstarch, sucrose, glucose, fructose, or a combination of glucose and fructose) had little effect on serum glycemic stress and AGE levels | Diet restriction | 1. Serum glucose and glycated Hb (↓) 2. Pentosidine in the collagen of a tail tendon (not skin or trachea) (↓) | Lingelbach et al., 2000 [124] |
| Broiler breeder chicks | Allopurinol (10 mg kg−1, p.o.) or hemin (10 mg kg−1, p.o.) | 1. Skin pentosidine (↑) | Diet restriction | 1. Skin pentosidine (↓) | Klandorf et al., 2001 [125] |
| Fisher 334 rats | Age (6–24 months) | 1. Plasma glucose (↑) 2. Tail tendon breakdown time (↑) 3. Glycation (furosine) and glycoxidation (pentosidine and CML) of skin collagen (↑) | AGD (1 g L−1) in drinking water | 1. There were no effects except for marginal effects on tail tendon breakdown time | Sell et al., 2001 [126] |
| C57BL/6 mice | Age (1, 4, 8–10.5 months) | 1. Skin furosine and pentosidine (↑) 2. Tail collagen fluorescence (↑) 3. Tail tendon breakdown time (↑) | Vitamins C and E, blueberry, and green tea extracts | 1. (Green tea extract) tail collagen fluorescence (↓) 2. (Green tea extract) tail tendon breakdown time (↓) | Rutter et al., 2003 [127] |
| Sprague–Dawley rats | Streptozotocin (45 mg kg−1) | 1. Crosslinking of skin collagen (↑) 2. Acid solubility of tail collagen (↓) | N-phenacylthiazolium and N-phenylacy-4,5-dimethylthiazolium halides, pyridoxamine | 1. There were no effects | Yang et al., 2003 [37] |
| Lewis rats | Streptozotocin (65 mg kg−1, i.v.) | 1. Red blood cell-immunoglobulin (Ig) G crosslink (↑) 2. Tail tendon collagen crosslink (↑) | ALT-711 (10 mg kg−1, oral) | 1. Red blood cell-IgG crosslink (↓) 2. Tail tendon collagen crosslink (↓) | Vasan et al., 2003 [36] |
| Fischer 344 rats | Aged 24 months | ALT-711 (5%, topical) | 1. Skin elasticity (↑) 2. Skin hydration (↑) | ||
| Squirrel and rhesus monkeys | Age | 1. Rate of furosine formation in skin collagen (↑?) 2. Rate of pentosidine formation in skin collagen (↑?) 3. No change for CML | Diet restriction (70% of control) | 1. Rate of furosine formation in skin collagen of rhesus monkeys (↓) 2. No change in pentosidine or CML level | Sell et al., 2003 [128] |
| Nude mice | Galactose (1000 mg kg−1, s.c.) | 1. Serum AGE levels (↑) 2. Skin SOD activity (↓) 3. Skin MDA level (↑) 4. Dermal thickness (↓) 5. Skin collagen (↓) 6. Skin CD31-positive vessels density (↓) 7. Skin VEGF expression (↓) | Adipose-derived stem cells (1 × 106 per injection) | 1. Serum AGE levels (↓) 2. Skin SOD activity (↑) 3. Skin MDA level (↓) 4. Dermal thickness ↑) 5. Skin collagen (↑) 6. Skin CD31-positive vessels density (↑) 7. Skin VEGF expression (↑) | Zhang et al., 2014 [129], Wang et al., 2016 [130] |
| Cormorants | Age (1–5 years) | 1. Hydroxyproline (↑) 2. Pentosidine (↑) | Dorr et al., 2017 [131] | ||
| ICR mice | Galactose (120 mg kg−1, i.p.) | 1. Skin AGE levels (↑) 2. Skin collagen type I, III (↓) 3. Skin hyaluronan (↓) 4. Skin H2O2 (↑) | Lactobacillus fermentum CQPC04-fermented soy milk (10 mL kg−1) | 1. Skin AGE levels (↓) 2. Skin collagen type I, III (↑) 3. Skin hyaluronan (↑) 4. Skin H2O2 (↓) | Zhou et al., 2021 [132] |
| DBA/2CrSlc mice | UV radiation | 1. Ear skin Cu/Zn-SOD expression and total SOD activity (↓) 2. Ear skin CAT expression and activity (↓) | Mycosporine-like amino acid-containing emulsions (0.25 μmol g−1) (50 mg per ear, topical) | 1. Skin Cu/Zn-SOD expression and total SOD activity (↑) 2. Skin CAT expression and activity (↑) 3. No changes in AGE levels of ear skin | Waditee-Sirisattha and Kageyama, 2021 [87] |
| Sprague–Dawley rats | Streptozotocin (35 mg kg−1, i.p.) | 1. Serum fructosamine and HbA1c (↑) 2. AGE levels in the kidneys and tail skin (↑) | Vanillic acid (1.5, 4.5, or 15 mg kg−1, i.p.) | 1. AGE levels in the kidneys and tail skin (↓) | Alhadid et al., 2022 [92] |
| HR-1 mice | UVB radiation | 1. Wrinkle area, length, and depth (↑) 2. Skin and epidermal thickness (↑) 3. Skin moisture (↓) 4. Skin collagen and hyaluronan (↓) 5. Skin levels of AGEs and RAGE (↑) | Schizonepeta tenuifolia extract (containing rosmarinic acid) | 1. Wrinkle area, length, and depth (↓) 2. Skin and epidermal thickness (↓) 3. Skin moisture (↑) 4. Skin collagen and hyaluronan (↑) 5. Skin levels of AGEs and RAGE (↓) | Gu et al., 2023 [133] |
| C57BL/6 mice | UV radiation | 1. Skin melanin (↑) 2. Skin ROS (↑) 3. Skin AGE levels (↑) | Idebenone-loaded nanoparticles | 1. Skin melanin (↓) 2. Skin ROS (↓) 3. Skin AGE levels (↓) | Xie et al., 2023 [134] |
| Hairless mice | Streptozotocin (45 mg kg−1, i.p.) | 1. Skin wrinkle index (↑) 2. Skin hydration (↓) 3. Skin elasticity (↓) 4. Blood and skin levels of AGEs, CML, RAGE (↑) 5. Skin collagen and hyaluronan (↓) 6. Skin MMP-9 (↑) 7. Skin SOD and CAT activity (↓) 8. Skin TNF-α, IL-6 (↑) | Goji berry, fig, and Korean mint extracts) | 1. Skin wrinkle index (↓) 2. Skin hydration (↑) 3. Skin elasticity (↑) 4. Blood and skin levels of AGEs and skin CML (↓) 5. Skin collagen (↑) 6. Skin IL-6 (↓) | Yoo et al., 2023 [135] |
| Swiss albino mice | Galactose (500 mg kg−1, i.g.) | 1. Skin TNF-α, IL-1β, and malondialdehyde (MDA) (↑) 2. Skin glutathione peroxidase (GPX) (↓) 3. Skin collagen type I, III (↓) | Dapagliflozin (1 mg kg−1) | 1. TNF-α, IL-1β, and MDA (↓) 2. Skin GPX (↑) 3. Skin collagen type I, III (↑) | Shihab et al., 2024 [136] |
| Study Format or Size | Test Materials | Treatments | Outcomes | References |
|---|---|---|---|---|
| A randomized, placebo-controlled study involving 22 subjects with type 2 diabetes | A dietary supplement containing 400 mg of vitamin E and 500 mg of vitamin C | The oral supplement was taken daily for a year. | 1. Neither the treatment nor placebo group had significant changes in AGE level. | Konen et al., 2000 [137] |
| A double-blinded, placebo-controlled trial involving 40 women | A cream containing 10% green tea extract, an oral supplement (300 mg) containing green tea extract | The cream was applied to the face and arms, and the oral supplement was taken twice a day for 8 weeks. | 1. No significant effects on the scoring of skin parameters by a physician and patients. 2. Histologically improved elastic tissue content of treated specimens. | Chiu et al., 2005 [138] |
| A single-center study enrolling 20 women with type II diabetes aged >50 | A topical product formulation containing blueberry extract and C-xyloside | Subjects used the product on their face, hands, and inner forearms twice daily for 12 weeks. | 1. Increased skin thickness and hydration. 2. Improved fine lines, firmness, radiance, skin tone, smoothness, creping, and overall appearance. | Draelos et al., 2009 [139] |
| A randomized, double-blinded, controlled study involving 42 subjects | A capsule containing carnosine, N-acetylcysteine, histidine, vitamin E, pantethine, methionine, and zinc picolinate | The capsule was taken orally every second day for 3 months, followed by a 1-month supplementation-free period. | 1. Improved skin appearance, fine lines, and shiny look. 2. Improved the skin surface parameters, contrast, and circular roughness. | Babizhayev et al., 2012 [140] |
| 21 adult volunteers | A cream containing 3% Argania plant extract, α-tocopheryl acetate, rutin, and ferulic acid | The cream was applied to the skin for 2 weeks. | 1. Decreased the UVA-induced chemiluminescence of the skin, a measure of free radical production. | Danoux et al., 2014 [75] |
| A single-center, randomized controlled trial on 57 post-menopausal women | An equol supplement containing 98% S-equol, 2% daidzein, 0.2% glycitein, and 0.1% genistein | The supplement (10 mg) was taken orally for 3 months. | 1. Improved climactic symptoms without effects on metabolic or aging-related biomarkers, including AGEs. 2. In certain populations (internal equol producers supplemented with external equol), AGE levels and visceral fat area were reduced. | Yoshikata et al., 2021 [141] |
| Twenty-two healthy women | An ampule containing hydrolyzed fish collagen (25% tripeptide) | The ampule was applied to the entire face twice daily for 4 weeks. | 1. Improved periorbital and glabellar skin wrinkles, skin surface elasticity, and dermal density. 2. Reduced skin AGE levels. | Lee et al., 2022 [104] |
| A randomized, double-blinded, split-face, placebo-controlled trial on 25 female volunteers | A gel cream containing Dunaliella salina extract (rich in colorless carotenoids, such as phytoene and phytofluene) | One side of the face received the active product, and the other received the placebo for 56 days. | 1. Reduced skin AGE levels. 2. Reduced histamine-stimulated microcircular blood flow. 3. Reduced periocular wrinkles and red spots. | Havas et al., 2022 [114] |
| Female subjects aged from 50 to 65 | A lotion containing 0.025% Cirsium japonicum flower extract | Topically applied to the face twice a day for 8 weeks. | 1. Decreased depth and volume of wrinkles. 2. Reduced maximum depth of the biggest wrinkle but not the total wrinkle area. 3. Increased skin elasticity. | Yoon et al., 2022 [90] |
| A double-blinded, randomized, controlled, single-center case study on 28 female subjects. | A serum containing sunflower sprout extract | The product was applied alone or in combination with a moisturizer lotion twice daily for 7 days. | 1. Improved facial conditions, such as radiance, smoothness (tactile), fine lines of crow’s feet, and overall eye appearance. | Barua et al., 2022 [106] |
| A randomized, double-blinded, placebo-controlled trial on 31 individuals | Fish-derived collagen peptides (rich in prolyl-hydroxyproline and hydroxyprolyl-glycine) | Ingested collagen peptide (5 g) or placebo (maltodextrin) daily for 12 weeks. | 1. Improved skin AGE levels and insulin resistance index (homeostasis model assessment ratio). | Koizumi et al., 2023 [142] |
| A split-face, placebo-controlled study involving 32 volunteers | An essence containing 0.5%, 1%, or 2% supramolecular carnosine | Topically applied to the face for 28 days. | 1. Reduced brown spots. 2. Increased lightness (L* value) and individual topology angle (ITAo) and decreased melanin index of the facial skin. | Bai et al., 2024 [110] |
| Single-blinded clinical trials with 9 and 34 participants | A serum that claims to be anti-aging | Topically applied to the face for 7 or 56 days. | 1. Reduced skin AGE levels. 2. Improved skin brightness and hydration, but not skin texture (elasticity, firmness). | Zhang et al., 2025 [143] |
| A randomized, double-blinded, placebo-controlled trial on 104 female participants | A capsule containing 300 mg of rosemary extract, 2 μg of biotin, and 0.45 mg of zinc gluconate | Subjects were instructed to take two capsules three times daily for weeks 1 to 4, two times daily for weeks 5 to 8, and one capsule two times daily for weeks 9 to 12. | 1. Decreased the levels of 4-hydroxynonenal-protein adducts in the skin compared to both baseline values and the placebo group at 12 weeks. 2. Reduced skin AGE levels compared to both baseline values and placebo controls at 12 weeks. | Guiotto et al., 2025 [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.Y.; Ha, N.G.; Lee, W.J.; Boo, Y.C. Synthetic and Natural Agents Targeting Advanced Glycation End-Products for Skin Anti-Aging: A Comprehensive Review of Experimental and Clinical Studies. Antioxidants 2025, 14, 498. https://doi.org/10.3390/antiox14040498
Choi JY, Ha NG, Lee WJ, Boo YC. Synthetic and Natural Agents Targeting Advanced Glycation End-Products for Skin Anti-Aging: A Comprehensive Review of Experimental and Clinical Studies. Antioxidants. 2025; 14(4):498. https://doi.org/10.3390/antiox14040498
Chicago/Turabian StyleChoi, Joon Yong, Nam Gyoung Ha, Weon Ju Lee, and Yong Chool Boo. 2025. "Synthetic and Natural Agents Targeting Advanced Glycation End-Products for Skin Anti-Aging: A Comprehensive Review of Experimental and Clinical Studies" Antioxidants 14, no. 4: 498. https://doi.org/10.3390/antiox14040498
APA StyleChoi, J. Y., Ha, N. G., Lee, W. J., & Boo, Y. C. (2025). Synthetic and Natural Agents Targeting Advanced Glycation End-Products for Skin Anti-Aging: A Comprehensive Review of Experimental and Clinical Studies. Antioxidants, 14(4), 498. https://doi.org/10.3390/antiox14040498