Gingival Inflammation Modulates NLRP3 Inflammasome Signalling in Peripheral Blood Mononuclear Cells of PCOS Patients: A Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Anthropometric and Biochemical Determinants
2.4. Periodontal Parameters
2.5. Isolation of PBMCs
2.6. Flow Cytometry Assay
2.7. Protein Expression Analysis
2.8. RNA Extraction and Gene Expression Analysis
2.9. Evaluation of Systemic IL1β Levels
2.10. Statistical Analysis
3. Results
3.1. Study Population and Group Characteristics
3.2. Proinflammatory Mediators
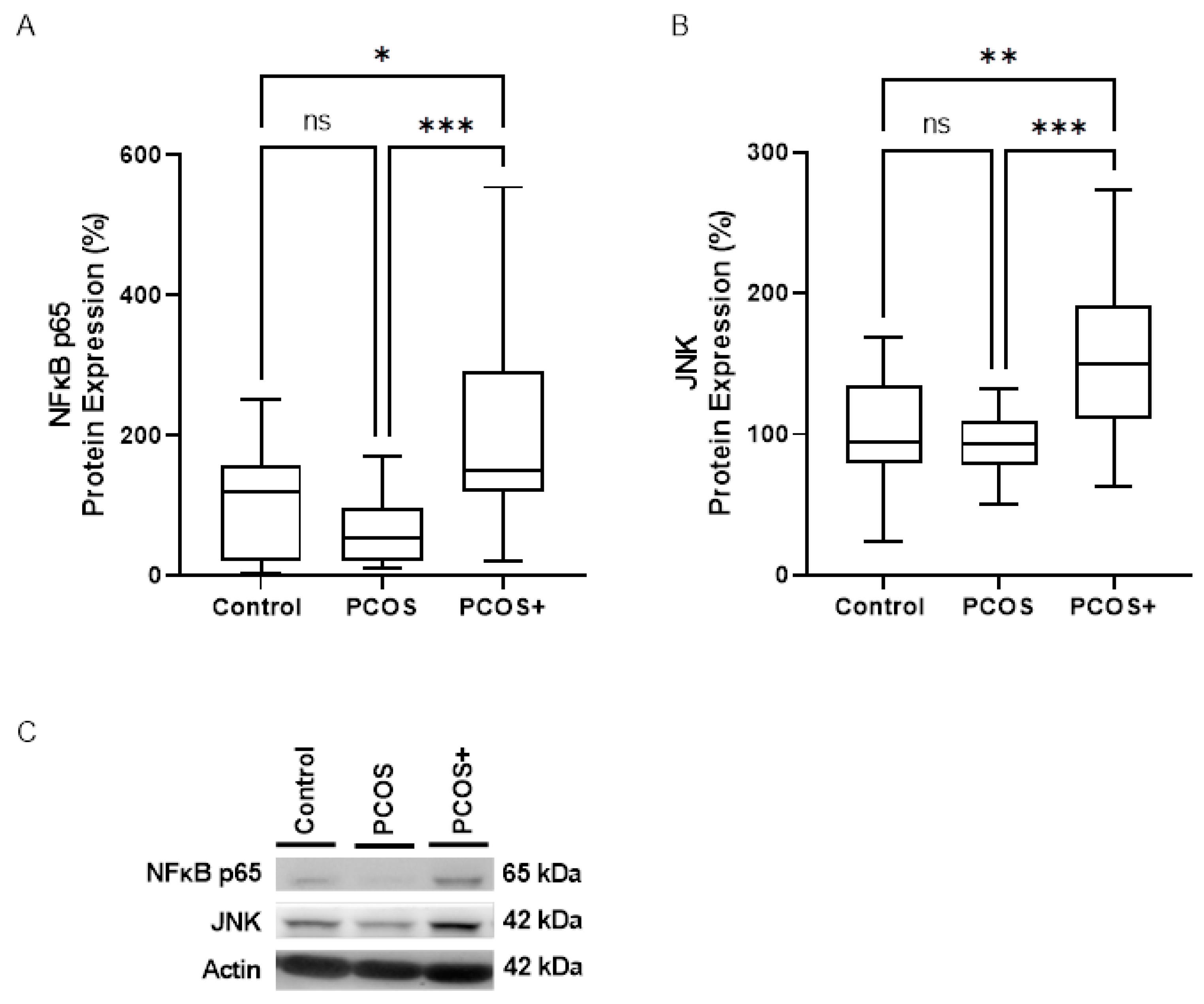
3.3. NLRP3 Inflammasome Assembly Engagement
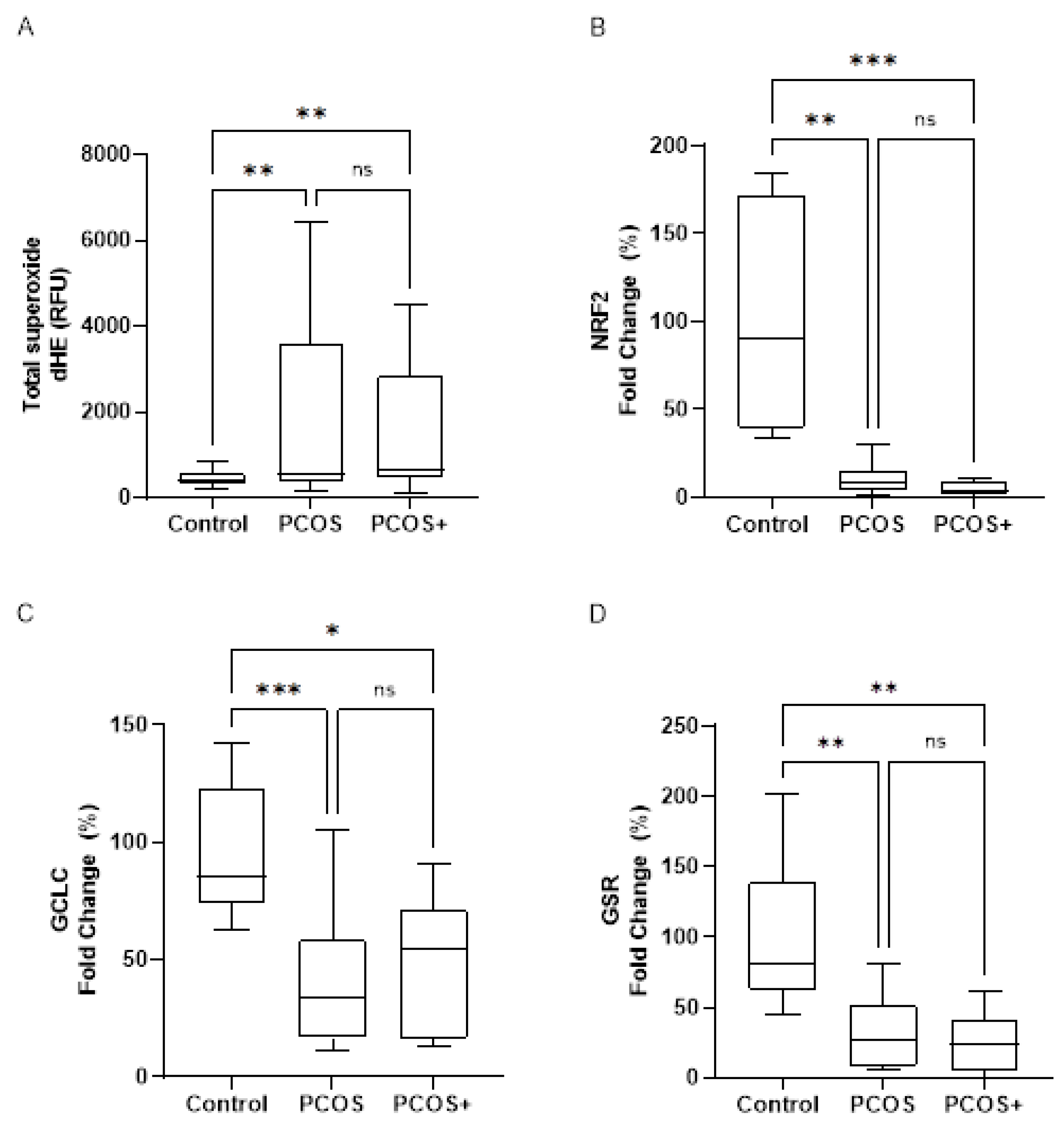
3.4. Oxidative Stress and Antioxidant Response
3.5. Correlations Between Gingival Inflammation and Molecular Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASC | Apoptosis-associated Speck-like protein containing a CARD |
| BOP | Bleeding on probing |
| BMI | Body Mass Index |
| CAL | Clinical Attachment Level |
| DAMPs | Damage-associated molecular patterns |
| dHE | Dihydroethidium |
| GCLC | Glutamate–Cysteine Ligase Catalytic subunit |
| GSR | Glutathione Reductase |
| hsCRP | High-sensitivity C-reactive protein |
| IL1β | Interleukin 1β |
| IR | Insulin resistance |
| JNK | c-Jun N-terminal kinase |
| LPS | Lipopolysaccharide |
| NFκB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | Nucleotide-binding oligomerization domain (NOD), leucine-rich repeats (LRR) and pyrin domain-containing protein 3 |
| NRF2 | Nuclear factor erythroid 2–related factor 2 |
| PAMPs | Pathogen-associated molecular patterns |
| PBMCs | Peripheral blood mononuclear cells |
| PCOS | Polycystic ovary syndrome |
| PD | Periodontal disease |
| PPD | Probing Pockets Depth |
| ROS | Reactive Oxygen Species |
| TLR | Toll-like receptor |
| TNFα | Tumor necrosis factor alpha |
References
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.E.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic Ovary Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, D.A. Polycystic Ovary Syndrome. N. Engl. J. Med. 2005, 352, 1223–1236. [Google Scholar] [CrossRef] [PubMed]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome (PCOS). Human. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Victor, V.M.; Rocha, M.; Bañuls, C.; Alvarez, A.; De Pablo, C.; Sanchez-Serrano, M.; Gomez, M.; Hernandez-Mijares, A. Induction of Oxidative Stress and Human Leukocyte/Endothelial Cell Interactions in Polycystic Ovary Syndrome Patients with Insulin Resistance. J. Clin. Endocrinol. Metab. 2011, 96, 3115–3122. [Google Scholar] [CrossRef]
- Ojeda-Ojeda, M.; Murri, M.; Insenser, M.; Escobar-Morreale, H.F. Mediators of Low-Grade Chronic Inflammation in Polycystic Ovary Syndrome (PCOS). Curr. Pharm. Des. 2013, 19, 5775–5791. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Cui, J.; Goodarzi, M.O. Polycystic Ovary Syndrome and Risk of Type 2 Diabetes, Coronary Heart Disease, and Stroke. Diabetes 2021, 70, 627–637. [Google Scholar] [CrossRef]
- Sathish, A.K.; Varghese, J.; Fernandes, A.J. The Impact of Sex Hormones on the Periodontium During a Woman’s Lifetime: A Concise-Review Update. Curr. Oral Health Rep. 2022, 9, 146–156. [Google Scholar] [CrossRef]
- Dursun, E.; Akalın, F.A.; Güncü, G.N.; Çınar, N.; Aksoy, D.Y.; Tözüm, T.F.; Kılınc, K.; Yıldız, B.O. Periodontal Disease in Polycystic Ovary Syndrome. Fertil. Steril. 2011, 95, 320–323. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Malignaggi, V.R.; Kellesarian, T.V.; Al-Kheraif, A.A.; Alwageet, M.M.; Malmstrom, H.; Romanos, G.E.; Javed, F. Association between Periodontal Disease and Polycystic Ovary Syndrome: A Systematic Review. Int. J. Impot. Res. 2017, 29, 89–95. [Google Scholar] [CrossRef]
- Márquez-Arrico, C.F.; Silvestre-Rangil, J.; Gutiérrez-Castillo, L.; Martinez-Herrera, M.; Silvestre, F.J.; Rocha, M. Association between Periodontal Diseases and Polycystic Ovary Syndrome: A Systematic Review. J. Clin. Med. 2020, 9, 1586. [Google Scholar] [CrossRef]
- Machado, V.; Escalda, C.; Proença, L.; Mendes, J.J.; Botelho, J. Is There a Bidirectional Association between Polycystic Ovarian Syndrome and Periodontitis? A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1961. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Mancini, L.; Torge, D.; Cristiano, L.; Mattei, A.; Varvara, G.; Macchiarelli, G.; Marchetti, E.; Bernardi, S. Bio-Morphological Reaction of Human Periodontal Ligament Fibroblasts to Different Types of Dentinal Derivates: In Vitro Study. Int. J. Mol. Sci. 2021, 22, 8681. [Google Scholar] [CrossRef] [PubMed]
- Timonen, P.; Suominen-Taipale, L.; Jula, A.; Niskanen, M.; Knuuttila, M.; Ylöstalo, P. Insulin Sensitivity and Periodontal Infection in a Non-Diabetic, Non-Smoking Adult Population: Insulin Sensitivity and Periodontitis. J. Clin. Periodontol. 2011, 38, 17–24. [Google Scholar] [CrossRef]
- Demmer, R.T.; Squillaro, A.; Papapanou, P.N.; Rosenbaum, M.; Friedewald, W.T.; Jacobs, D.R.; Desvarieux, M. Periodontal Infection, Systemic Inflammation, and Insulin Resistance. Diabetes Care 2012, 35, 2235–2242. [Google Scholar] [CrossRef]
- Martinez-Herrera, M.; Silvestre, F.J.; Silvestre-Rangil, J.; Bañuls, C.; Rocha, M.; Hernández-Mijares, A. Involvement of Insulin Resistance in Normoglycaemic Obese Patients with Periodontitis: A Cross-sectional Study. J. Clin. Periodontol. 2017, 44, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal Diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus Report of Workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef]
- Almerich-Silla, J.M.; Montiel-Company, J.M.; Pastor, S.; Serrano, F.; Puig-Silla, M.; Dasí, F. Oxidative Stress Parameters in Saliva and Its Association with Periodontal Disease and Types of Bacteria. Dis. Markers 2015, 2015, 653537. [Google Scholar] [CrossRef]
- Meyle, J.; Chapple, I. Molecular Aspects of the Pathogenesis of Periodontitis. Periodontology 2000 2015, 69, 7–17. [Google Scholar] [CrossRef]
- Dou, Y.; Xin, J.; Zhou, P.; Tang, J.; Xie, H.; Fan, W.; Zhang, Z.; Wu, D. Bidirectional Association between Polycystic Ovary Syndrome and Periodontal Diseases. Front. Endocrinol. 2023, 14, 1008675. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of Assembly, Regulation and Signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zhang, G.; Wu, Y.; Xiong, Y. Inflammasome Complexes: Crucial Mediators in Osteoimmunology and Bone Diseases. Int. Immunopharmacol. 2022, 110, 109072. [Google Scholar] [CrossRef]
- Olona, A.; Leishman, S.; Anand, P.K. The NLRP3 Inflammasome: Regulation by Metabolic Signals. Trends Immunol. 2022, 43, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Haasken, S.; Cassel, S.L. Mechanism of NLRP3 Inflammasome Activation. Ann. N. Y. Acad. Sci. 2014, 1319, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Pétrilli, V.; Papin, S.; Dostert, C.; Mayor, A.; Martinon, F.; Tschopp, J. Activation of the NALP3 Inflammasome Is Triggered by Low Intracellular Potassium Concentration. Cell Death Differ. 2007, 14, 1583–1589. [Google Scholar] [CrossRef]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef]
- Okada, M.; Matsuzawa, A.; Yoshimura, A.; Ichijo, H. The Lysosome Rupture-Activated TAK1-JNK Pathway Regulates NLRP3 Inflammasome Activation. J. Biol. Chem. 2014, 289, 32926–32936. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-Interacting Protein Links Oxidative Stress to Inflammasome Activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.-K. An Update on the Regulatory Mechanisms of NLRP3 Inflammasome Activation. Cell Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- Xue, F.; Shu, R.; Xie, Y. The Expression of NLRP3, NLRP1 and AIM2 in the Gingival Tissue of Periodontitis Patients: RT-PCR Study and Immunohistochemistry. Arch. Oral Biol. 2015, 60, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.J.; Spangler, C.C.; Yusaf, N.K. Barriers to Dental Care Access for Patients with Special Needs in an Affluent Metropolitan Community. Spec. Care Dent. 2015, 35, 190–196. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Williams, R.C. Periodontitis Activates the NLRP3 Inflammasome in Serum and Saliva. J. Periodontol. 2022, 93, 135–145. [Google Scholar] [CrossRef]
- Sen, P.; Kemppainen, E.; Orešič, M. Perspectives on Systems Modeling of Human Peripheral Blood Mononuclear Cells. Front. Mol. Biosci. 2018, 4, 96. [Google Scholar] [CrossRef]
- Márquez-Arrico, C.F.; Silvestre, F.J.; Fernández-Reyes, M.; López-Domènech, S.; Hermenejildo, J.; Abad-Jiménez, Z.; Silvestre-Rangil, J.; Fernández-Collazo, P.; Morillas, C.; Montiel-Company, J.M.; et al. Gingival Inflammation and Leukocyte–Endothelium Cell Interactions in Women with Polycystic Ovary Syndrome. J. Periodontol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.C.; Mealey, B.L.; Van Dyke, T.E.; Bartold, P.M.; Dommisch, H.; Eickholz, P.; Geisinger, M.L.; Genco, R.J.; Glogauer, M.; Goldstein, M.; et al. Periodontal Health and Gingival Diseases and Conditions on an Intact and a Reduced Periodontium: Consensus Report of Workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S68–S77. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal Disease in Pregnancy II. Correlation Between Oral Hygiene and Periodontal Condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The Plaque Control Record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Ramfjord, S.P. The Periodontal Disease Index (PDI). J. Periodontol. 1967, 38, 602–610. [Google Scholar] [CrossRef]
- Hajishengallis, G. The Inflammophilic Character of the Periodontitis-associated Microbiota. Mol. Oral Microbiol. 2014, 29, 248–257. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Berglundh, T.; Sculean, A.; Tonetti, M.S. EFP Workshop Participants and Methodological Consultants Treatment of Stage I–III Periodontitis—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2020, 47, 4–60. [Google Scholar] [CrossRef]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 Inflammasome in Inflammatory Diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Isaza-Guzmán, D.M.; Medina-Piedrahíta, V.M.; Gutiérrez-Henao, C.; Tobón-Arroyave, S.I. Salivary Levels of NLRP3 Inflammasome-Related Proteins as Potential Biomarkers of Periodontal Clinical Status. J. Periodontol. 2017, 88, 1329–1338. [Google Scholar] [CrossRef]
- McKee, C.M.; Coll, R.C. NLRP3 Inflammasome Priming: A Riddle Wrapped in a Mystery inside an Enigma. J. Leukoc. Biol. 2020, 108, 937–952. [Google Scholar] [CrossRef]
- Song, N.; Liu, Z.-S.; Xue, W.; Bai, Z.-F.; Wang, Q.-Y.; Dai, J.; Liu, X.; Huang, Y.-J.; Cai, H.; Zhan, X.-Y.; et al. NLRP3 Phosphorylation Is an Essential Priming Event for Inflammasome Activation. Mol. Cell 2017, 68, 185–197.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, R.-H.; Wang, K.-H. Formononetin Ameliorates Polycystic Ovary Syndrome through Suppressing NLRP3 Inflammasome. Mol. Med. 2025, 31, 27. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Li, Z.; Fan, H.; Yan, X.; Liu, X.; Xuan, J.; Feng, D.; Wei, X. The Release of Peripheral Immune Inflammatory Cytokines Promote an Inflammatory Cascade in PCOS Patients via Altering the Follicular Microenvironment. Front. Immunol. 2021, 12, 685724. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.S.B.; Ku, J.; Anders, H.-J. Cell Type-Specific Roles of NLRP3, Inflammasome-Dependent and -Independent, in Host Defense, Sterile Necroinflammation, Tissue Repair, and Fibrosis. Front. Immunol. 2023, 14, 1214289. [Google Scholar] [CrossRef]
- Yao, J.; Sterling, K.; Wang, Z.; Zhang, Y.; Song, W. The Role of Inflammasomes in Human Diseases and Their Potential as Therapeutic Targets. Signal Transduct. Target. Ther. 2024, 9, 10. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- González, F.; Rote, N.S.; Minium, J.; Kirwan, J.P. Reactive Oxygen Species-Induced Oxidative Stress in the Development of Insulin Resistance and Hyperandrogenism in Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Z.; Zhao, S.; Cheng, L.; Man, Y.; Gao, X.; Zhao, H. Oxidative Stress Markers in the Follicular Fluid of Patients with Polycystic Ovary Syndrome Correlate with a Decrease in Embryo Quality. J. Assist. Reprod. Genet. 2021, 38, 471–477. [Google Scholar] [CrossRef]
- Murri, M.; Luque-Ramírez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating Markers of Oxidative Stress and Polycystic Ovary Syndrome (PCOS): A Systematic Review and Meta-Analysis. Human. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Martinez De Marañon, A.; Rios-Navarro, C.; Alvarez, A.; Gomez, M.; Rocha, M.; Hernández-Mijares, A. Insulin Resistance in PCOS Patients Enhances Oxidative Stress and Leukocyte Adhesion: Role of Myeloperoxidase. PLoS ONE 2016, 11, e0151960. [Google Scholar] [CrossRef]
- Li, S.; Yang, W.; Li, A.; Zhang, L.; Guo, L. Protective Effect of Nrf2 in Periodontitis—A Preclinical Systematic Review and Meta-Analysis. Arch. Oral Biol. 2023, 151, 105713. [Google Scholar] [CrossRef]
- Sima, C.; Aboodi, G.M.; Lakschevitz, F.S.; Sun, C.; Goldberg, M.B.; Glogauer, M. Nuclear Factor Erythroid 2-Related Factor 2 Down-Regulation in Oral Neutrophils Is Associated with Periodontal Oxidative Damage and Severe Chronic Periodontitis. Am. J. Pathol. 2016, 186, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, J.T.; Girnary, M.S.; Moss, K.; Monaghan, E.T.; Egnatz, G.J.; Jiao, Y.; Zhang, S.; Beck, J.; Swanson, K.V. Role of Inflammasomes in the Pathogenesis of Periodontal Disease and Therapeutics. Periodontology 2000 2020, 82, 93–114. [Google Scholar] [CrossRef]
- Wang, D.; Weng, Y.; Zhang, Y.; Wang, R.; Wang, T.; Zhou, J.; Shen, S.; Wang, H.; Wang, Y. Exposure to Hyperandrogen Drives Ovarian Dysfunction and Fibrosis by Activating the NLRP3 Inflammasome in Mice. Sci. Total Environ. 2020, 745, 141049. [Google Scholar] [CrossRef]


| Control | PCOS | PCOS+ | |
|---|---|---|---|
| BOP (%) | 1.935 ± 2.336 | 3.211 ± 2.644 | 13.162 ± 4.184 *# |
| Plaque index | 0.423 ± 0.408 | 0.598 ± 0.472 | 0.843 ± 0.608 *# |
| Calculus index | 0.021 ± 0.053 | 0.022 ± 0.066 | 0.165 ± 0.436 *# |
| Dependent Variable | Independent Variable | B | Standard Error | Beta Coefficient | Adjust R2 | p-Value |
|---|---|---|---|---|---|---|
| BOP | 0.348 | <0.001 | ||||
| JNK | 0.030 | 0.012 | 0.275 | 0.012 | ||
| ASC | 0.027 | 0.010 | 0.297 | <0.001 | ||
| Procaspase-1 | 0.020 | 0.007 | 0.284 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez-Arrico, C.F.; Pelechá-Salvador, M.; Fernández-Reyes, M.; Silvestre, F.J.; Perea-Galera, L.; Hermenejildo, J.; Abad-Jiménez, Z.; Silvestre-Rangil, J.; Morillas, C.; Víctor, V.M.; et al. Gingival Inflammation Modulates NLRP3 Inflammasome Signalling in Peripheral Blood Mononuclear Cells of PCOS Patients: A Case-Control Study. Antioxidants 2025, 14, 507. https://doi.org/10.3390/antiox14050507
Márquez-Arrico CF, Pelechá-Salvador M, Fernández-Reyes M, Silvestre FJ, Perea-Galera L, Hermenejildo J, Abad-Jiménez Z, Silvestre-Rangil J, Morillas C, Víctor VM, et al. Gingival Inflammation Modulates NLRP3 Inflammasome Signalling in Peripheral Blood Mononuclear Cells of PCOS Patients: A Case-Control Study. Antioxidants. 2025; 14(5):507. https://doi.org/10.3390/antiox14050507
Chicago/Turabian StyleMárquez-Arrico, Cecilia Fabiana, María Pelechá-Salvador, Meylin Fernández-Reyes, Francisco Javier Silvestre, Laura Perea-Galera, Jonathan Hermenejildo, Zaida Abad-Jiménez, Javier Silvestre-Rangil, Carlos Morillas, Víctor M. Víctor, and et al. 2025. "Gingival Inflammation Modulates NLRP3 Inflammasome Signalling in Peripheral Blood Mononuclear Cells of PCOS Patients: A Case-Control Study" Antioxidants 14, no. 5: 507. https://doi.org/10.3390/antiox14050507
APA StyleMárquez-Arrico, C. F., Pelechá-Salvador, M., Fernández-Reyes, M., Silvestre, F. J., Perea-Galera, L., Hermenejildo, J., Abad-Jiménez, Z., Silvestre-Rangil, J., Morillas, C., Víctor, V. M., López-Domènech, S., & Rocha, M. (2025). Gingival Inflammation Modulates NLRP3 Inflammasome Signalling in Peripheral Blood Mononuclear Cells of PCOS Patients: A Case-Control Study. Antioxidants, 14(5), 507. https://doi.org/10.3390/antiox14050507











