The Effect of Perilla frutescens Extract on the Oxidative Stability of Model Food Emulsions
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Material
2.2. Reagents
2.3. Extraction
2.4. Total Phenol and Flavonoid Content
2.5. Antioxidant Capacity Determination
2.5.1. ABTS Assay
2.5.2. The Oxygen Radical Absorbance Capacity (ORAC) Assay
2.5.3. FRAP Assay
2.6. Determination of Cinnamic Acid Derivatives by High-Performance Liquid Chromatography (HPLC)
2.7. Oil-in-Water Emulsion System
2.7.1. Removal of Tocopherols from Sunflower Oil
2.7.2. Preparation of Emulsions and Storage Conditions
| Fatty acid name | Numerical symbol | Amount (%) |
|---|---|---|
| Saturated | 12.79 | |
| Palmitic acid | C16:0 | 6.99 ± 0.08 |
| Stearic acid | C18:0 | 4.16 ± 0.04 |
| Arachidic acid | C20:0 | 0.33 ± 0.01 |
| Behenic acid | C22:0 | 0.96 ± 0.02 |
| Lignoceric acid | C24:0 | 0.35 ± 0.02 |
| Unsaturated | 87.21 | |
| Oleic acid | C18:1 (n-9) | 34.51 ± 0.11 |
| Eicosenoic acid | C20:1 (n-9) | 0.32 ± 0.03 |
| Linolenic acid | C18:2 (n-6) | 52.38 ± 0.23 |
2.7.3. Measurement of Primary Oxidation by Peroxide Value (PV) and pH
2.7.4. Measurement of Secondary Oxidation by TBARs and Hexanal Methods
2.8. Statistical Analysis
3. Results and Discussion
3.1. Phenolic and Flavonoid Content of Extract
| Method | Amount detected a |
|---|---|
| Total phenol content (mg GAE/g DW) | 22.67 ± 0.52 |
| Total flavonoid content (mg CE/g DW) | 2.90 ± 0.07 |
| ABTS (mg TE/g DW) | 65.03 ± 2.98 |
| ORAC (mg TE/g DW) | 179.60 ± 6.02 |
| FRAP (mg TE/g DW) | 44.46 ± 1.55 |
3.2. In Vitro Antioxidant Activity of Extract
3.3. Quantitative Analysis of Cinnamic Acid Derivatives
| Rosmarinic acid | Caffeic acid | Solvent | Place of cultivation | Reference |
|---|---|---|---|---|
| 39.5 | ND | Water:acetone:hydrochloric (20:80:1) | Japan | Natsume et al. [39] |
| 3.4–10 | 0.05–1.2 | Water with 0.01 M H2SO4 | China and Japan | Meng et al. [36] |
| 0.21–3.76 | ND | 70% EtOH | Geochang, Korea | Hong and Kim [17] |
| 51.37–155.50 | ND | MeOH with ethyl acetate fraction | Geochang, Korea | Hong et al. [32] |
| 29.28–54.76 | 1.09–3.86 | MeOH with 1% TFA | Yeongnam, Korea | Kang and Lee [40] |
| 26.84 | 1.32 | Water at 100 °C | Miryang, Korea | Yang et al. [38] |
| 2.29 | 0.51 | 50% EtOH | Spain | This paper |
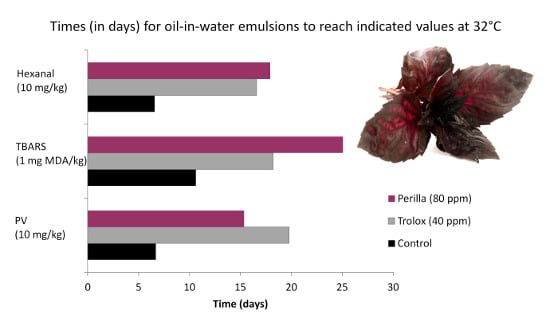
3.4. Antioxidant Activity of Extracts in Model Emulsion System




4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef]
- Waraho, T.; Cardenia, V.; Nishino, Y.; Seneviratne, K.N.; Rodriguez-Estrada, M.T.; McClements, D.J.; Decker, E.A. Antioxidant effects of mono- and diacylglycerols in non-stripped and stripped soybean oil-in-water emulsions. Food Res. Int. 2012, 48, 353–358. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Falguera, V.; Aliguer, N.; Falguera, M. An integrated approach to current trends in food consumption: Moving toward functional and organic products? Food Control 2012, 26, 274–228. [Google Scholar] [CrossRef]
- Skowyra, M.; Falguera, V.; Gallego, G.; Peiró, S.; Almajano, M.P. Antioxidant properties of aqueous and ethanolic extracts of tara (Caesalpinia spinosa) pods in vitro and in model food emulsions. J. Sci. Food Agric. 2013. [Google Scholar] [CrossRef]
- Wettasinghe, M.; Shahidi, F. Antioxidant and free radical-scavenging properties of ethanolic extracts of defatted borage (Borago offcinalis L.) seeds. Food Chem. 1999, 67, 399–414. [Google Scholar] [CrossRef]
- Santas, J.; Carbo, R.; Gordon, M.; Almajano, M. Comparison of the antioxidant activity of two Spanish onion varieties. Food Chem. 2008, 107, 1210–1216. [Google Scholar] [CrossRef]
- Decker, E.A.; Warner, K.; Richards, M.P.; Shahidi, F. Measuring antioxidant effectiveness in food. J. Agric. Food Chem. 2005, 53, 4303–4310. [Google Scholar] [CrossRef]
- Ramful, D.; Aumjaud, B.; Neergheen, V.S.; Soobrattee, M.; Googoolye, K.; Aruoma, O.I.; Bahorun, T. Polyphenolic content and antioxidant activity of Eugenia pollicina leaf extract in vitro and in model emulsion systems. Food Res. Int. 2011, 44, 1190–1196. [Google Scholar] [CrossRef]
- Almajano, M.P.; Gordon, M.H. Synergistic effect of BSA on antioxidant activities in model food emulsions. J. Am. Oil Chem. Soc. 2004, 81, 275–280. [Google Scholar] [CrossRef]
- Al-Bandak, G.; Dermesonlouglou, E.K.; Taoukis, P.S.; Oreopoulou, V. Antioxidant effect of Majorana syriaca extract in bulk corn oil and o/w emulsion after applying high hydrostatic pressure. Food Chem. 2011, 125, 1166–1170. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.J.; Skowyra, M.; Almajano, M.P. Antioxidant properties of three aromatic herbs (rosemary, thyme and lavender) in oil-in-water emulsions. J. Am. Oil Chem. Soc. 2013, 90, 1559–1568. [Google Scholar] [CrossRef]
- De Ciriano, M.G.-I.; Rehecho, S.; Calvo, M.I.; Cavero, R.Y.; Navarro, I.; Astiasarán, I.; Ansorena, D. Effect of lyophilized water extracts of Melissa officinalis on the stability of algae and linseed oil-in-water emulsion to be used as a functional ingredient in meat products. Meat Sci. 2010, 85, 373–377. [Google Scholar] [CrossRef]
- Poyato, C.; Navarro-Blasco, I.; Calvo, M.I.; Cavero, R.Y.; Astiasaran, I.; Ansorena, D. Oxidative stability of O/W and W/O/W emulsions: Effect of lipid composition and antioxidant polarity. Food Res. Int. 2013, 51, 132–140. [Google Scholar] [CrossRef]
- Lin, E.; Chou, H.; Kuo, P.; Huang, Y. Antioxidant and antiproliferative activities of methanolic extracts of Perilla frutescens. J. Med. Plant Res. 2010, 4, 477–483. [Google Scholar]
- Mao, Q.-Q.; Zhong, X.-M.; Li, Z.-Y.; Feng, C.-R.; Pan, A.-J.; Huang, Z. Herbal formula SYJN increases neurotrophin-3 and nerve growth factor expression in brain regions of rats exposed to chronic unpredictable stress. J. Ethnopharmacol. 2010, 131, 182–186. [Google Scholar] [CrossRef]
- Hong, E.; Kim, G.-H. Comparison of extraction conditions for phenolic, flavonoid content and determination of rosmarinic acid from Perilla frutescens var. acuta. Int. J. Food Sci. Technol. 2010, 45, 1353–1359. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, K.H.; Lee, M.-H.; Kim, H.-T.; Seo, W.D.; Kim, J.Y.; Ha, T.J. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013, 136, 843–852. [Google Scholar] [CrossRef]
- Eckert, G.P.; Franke, C.; Nöldner, M.; Rau, O.; Wurglics, M.; Schubert-Zsilavecz, M.; Müller, W.E. Plant derived omega-3-fatty acids protect mitochondrial function in the brain. Pharmacol. Res. 2010, 61, 234–241. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. FoodChem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Yoshida, H.; Kajimoto, G.; Emura, S. Antioxidant effects of d-tocopherols at different concentrations in oils during microwave heating. J. Am. Oil Chem. Soc. 1993, 70, 989–995. [Google Scholar] [CrossRef]
- Conde, E.; Moure, A.; Domínguez, H.; Gordon, M.H.; Parajó, J.C. Purified phenolics from hydrothermal treatments of biomass: Ability to protect sunflower bulk oil and model food emulsions from oxidation. J. Agric. Food Chem. 2011, 59, 9158–9165. [Google Scholar] [CrossRef]
- Frankel, E.N. Methods to Determine Extent of Oxidation. In Lipid Oxidation; The Oily Press: Dundee, UK, 1998; pp. 79–97. [Google Scholar]
- American Oil Chemists’ Society. AOCS Official Method Cd 8-53; Firestone, D., Ed.; Official Methods and Recommended Practices of the American Oil Chemists’ Society: Champaign, IL, USA, 1997. [Google Scholar]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Kee, K.T.; Koh, M.; Oong, L.X.; Ng, K. Screening culinary herbs for antioxidant and α-glucosidase inhibitory activities. Int. J. Food Sci. Technol. 2013, 48, 1884–1891. [Google Scholar] [CrossRef]
- Müller-Waldeck, F.; Sitzmann, J.; Schnitzler, W.H.; Grassmann, J. Determination of toxic perilla ketone, secondary plant metabolites and antioxidative capacity in five Perilla frutescens L. varieties. Food Chem. Toxicol. 2010, 48, 264–270. [Google Scholar] [CrossRef]
- Hong, E.; Park, K.H.; Kim, G.-H. Phenolic-enriched fractions from Perilla frutescens var. acuta: Determinating rosmarinic acid and antioxidant activity. J. Food Biochem. 2011, 35, 1637–1645. [Google Scholar] [CrossRef]
- Hossain, M.B.; Barry-Ryan, C.; Martin-Diana, A.B.; Brunton, N.P. Optimisation of accelerated solvent extraction of antioxidant compounds from rosemary (Rosmarinus officinalis L.), marjoram (Origanum majorana L.) and oregano (Origanum vulgare L.) using response surface methodology. Food Chem. 2011, 126, 339–346. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of cocoa (Theobroma cacao L.) co-products. Food Res. Int. 2012, 49, 39–45. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.F.; Gaydou, E.M.; Li, B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules 2009, 14, 133–140. [Google Scholar]
- Medina, I.; Undeland, I.; Larsson, K.; Storrø, I.; Rustad, T.; Jacobsen, C.; Gallardo, J.M. Activity of caffeic acid in different fish lipid matrices: A review. Food Chem. 2012, 131, 730–740. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Hong, C.-O.; Lee, G.P.; Kim, C.-T.; Lee, K.-W. The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage. Food Chem. Toxicol. 2013, 55, 92–99. [Google Scholar] [CrossRef]
- Natsume, M.; Muto, Y.; Fukuda, K.; Tokunaga, T.; Osakabe, N. Determination of rosmarinic acid and luteolin in Perilla frutescens Britton (Labiatae). J. Agric. Food Chem. 2006, 86, 897–901. [Google Scholar] [CrossRef]
- Kang, N.S.; Lee, J.H. Characterisation of phenolic phytochemicals and quality changes related to the harvest times from the leaves of Korean purple perilla (Perilla frutescens). Food Chem. 2011, 124, 556–562. [Google Scholar] [CrossRef]
- Lee, J. Caffeic acid derivatives in dried Lamiaceae and Echinacea purpurea products. J. Funct. Foods 2010, 2, 158–162. [Google Scholar] [CrossRef]
- Kiokias, S.; Varzakas, T.; Oreopoulou, V. In vitro activity of vitamins, flavonoids and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit. Rev. Food Sci. Nutr. 2008, 48, 78–93. [Google Scholar] [CrossRef]
- Kiokias, S.; Dimakou, C.; Oreopoulou, V. Activity of natural carotenoid preparations against the autoxidative deterioration of sunflower oil-in-water emulsions. Food Chem. 2009, 114, 1278–1284. [Google Scholar] [CrossRef]
- Kiokias, S.; Varzakas, T. Activity of flavonoids and β-carotene during the autooxidative deterioration of model food oil-in-water emulsions. Food Chem. 2014, 150, 280–286. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Pongsawatmanit, R.; Gordon, M.H. Antioxidant properties of Teaw (Cratoxylum formosum Dyer) extract in soybean oil and emulsions. J. Agric. Food Chem. 2006, 54, 2719–2725. [Google Scholar] [CrossRef]
- Roedig-Penman, A.; Gordon, M. Antioxidant properties of catechins and green tea extracts in model food emulsions. J. Agric. Food Chem. 1997, 8561, 4267–4270. [Google Scholar]
- Sun, Y.-E.; Wang, W.-D.; Chen, H.-W.; Li, C. Autoxidation of unsaturated lipids in food emulsion. Crit. Rev. Food Sci. Nutr. 2011, 51, 453–466. [Google Scholar] [CrossRef]
- Sørensen, A.-D.M.; Haahr, A.-M.; Becker, E.M.; Skibsted, L.H.; Bergenståhl, B.; Nilsson, L.; Jacobsen, C. Interactions between iron, phenolic compounds, emulsifiers, and pH in omega-3-enriched oil-in-water emulsions. J. Agric. Food Chem. 2008, 56, 1740–1750. [Google Scholar] [CrossRef]
- Zhou, L.; Elias, R.J. Antioxidant and pro-oxidant activity of (−)-epigallocatechin-3-gallate in food emulsions: Influence of pH and phenolic concentration. Food Chem. 2013, 138, 1503–1509. [Google Scholar] [CrossRef]
- Conde, E.; Gordon, M.H.; Moure, A.; Dominguez, H. Effects of caffeic acid and bovine serum albumin in reducing the rate of development of rancidity in oil-in-water and water-in-oil emulsions. Food Chem. 2011, 129, 1652–1659. [Google Scholar] [CrossRef]
- Dimakou, C.; Oreopoulou, V. Antioxidant activity of carotenoids against the oxidative destabilization of sunflower oil-in-water emulsions. LWT—Food Sci. Technol. 2012, 46, 393–400. [Google Scholar] [CrossRef]
- Feng, L.-J.; Yu, C.-H.; Ying, K.-J.; Hua, J.; Dai, X.-Y. Hypolipidemic and antioxidant effects of total flavonoids of Perilla frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res. Int. 2011, 44, 404–409. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Skowyra, M.; Falguera, V.; Azman, N.A.M.; Segovia, F.; Almajano, M.P. The Effect of Perilla frutescens Extract on the Oxidative Stability of Model Food Emulsions. Antioxidants 2014, 3, 38-54. https://doi.org/10.3390/antiox3010038
Skowyra M, Falguera V, Azman NAM, Segovia F, Almajano MP. The Effect of Perilla frutescens Extract on the Oxidative Stability of Model Food Emulsions. Antioxidants. 2014; 3(1):38-54. https://doi.org/10.3390/antiox3010038
Chicago/Turabian StyleSkowyra, Monika, Victor Falguera, Nurul A. M. Azman, Francisco Segovia, and Maria P. Almajano. 2014. "The Effect of Perilla frutescens Extract on the Oxidative Stability of Model Food Emulsions" Antioxidants 3, no. 1: 38-54. https://doi.org/10.3390/antiox3010038
APA StyleSkowyra, M., Falguera, V., Azman, N. A. M., Segovia, F., & Almajano, M. P. (2014). The Effect of Perilla frutescens Extract on the Oxidative Stability of Model Food Emulsions. Antioxidants, 3(1), 38-54. https://doi.org/10.3390/antiox3010038