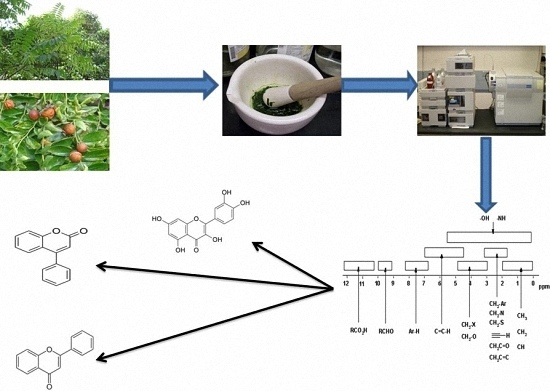
Antioxidant and Antiglycating Constituents from Leaves of Ziziphus oxyphylla and Cedrela serrata
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Biological Assays
2.4.1. DPPH Scavenging Activity
2.4.2. Superoxide-Radical Scavenging Activity by PMS-NADH System
2.4.3. Total Antioxidant Activity (ABTS+ Assay)
2.4.4. Anti-Glycation Assay
2.5. Statistical Analysis
3. Results
Identification of Isolated Compounds
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akhtar, N.; Rashid, A.; Murad, W.; Bergmeier, E. Diversity and use of ethno-medicinal plants in the region of Swat, North Pakistan. J. Ethnobiol. Ethnomed. 2013, 9, 25–37. [Google Scholar] [PubMed]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; Lalit Mohan Basu Pub.: Allahabad, India, 1984. [Google Scholar]
- Kaleem, W.A.; Muhammad, N.; Qayum, M.; Khan, H.; Khan, A.; Aliberti, L.; Feo, V.D. Antinociceptive activity of cyclopeptide alkaloids isolated from Ziziphus oxyphylla Edgew (Rhamnaceae). Fitoterapia 2013, 91, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Sher, H. Ethno-ecological evaluation of some medicinal and aromatic plants of Kot Malak, Pakistan. Sci. Res. Essays 2011, 6, 2164–2173. [Google Scholar]
- Nisar, M.; Adzu, B.; Inamullah, K.; Bashir, A.; Ihsan, A.; Gilani, A.H. Antinociceptive and antipyretic activities of the Ziziphus oxyphylla Edgew leaves. Phytother. Res. 2007, 2, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, S.A.; Qureshi, M.A.; Ahmed, G.; Khan, M.A.; Hussain, M.; Ghulam, M.G. Ethnobotany of some useful plants of Poonch Valley Azad Kashmir. J. Med. Plants Res. 2011, 5, 6140–6151. [Google Scholar]
- Rahman, I.U.; Khan, M.A.; Arfan, M.; Akhtar, G.; Khan, L.; Viqar-Uddin, A. A new 14-membered cyclopeptide alkaloid from Ziziphus oxyphylla. Nat. Prod. Res. 2007, 21, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.I.; Adhikari, A.; Rasheed, S.; Marasini, B.; Hussain, N.; Kaleem, W.A.; Rahman, A.U. Cyclopeptide alkaloids of Ziziphus oxyphylla Edgw as novel inhibitors of a-glucosidase enzyme and protein glycation. Phytochem. Lett. 2011, 4, 404–406. [Google Scholar] [CrossRef]
- Kaleem, W.A.; Nisar, M.; Qayum, M.; Zia-Ul-Haq, M.; Adhikari, A.; Feo, V.D. New 14-membered cyclopeptide alkaloids from Zizyphus oxyphylla Edgw. Int. J. Mol. Sci. 2012, 13, 11520–11529. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Upadhyay, A.; Ahmad, M.; Pieters, L. Antioxidant, antliglycation and antimicrobial activities of Ziziphus oxyphylla and Cedrela serrata extracts. Eur. J. Med. Plants 2013, 3, 520–529. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, M.; Jahan, N. Phytochemical screening and Antioxidant activity of the two plants Ziziphus oxyphylla Edgew and cedrela serrata Royle. Pak. J. Pharm. Sci. 2014, 27, 1477–1482. [Google Scholar] [PubMed]
- Jan, G.; Khan, M.A.; Khan, A.; Jan, F.G.; Khan, R.; Ahmad, M.; Rehman, A.U.; Danish, M.; Asif, M.; Khan, S.; et al. An ethnobotanical survey on fuel wood and timber plant species of Kaghan Valley, Khyber pakhtoonkhwa Province, Pakistan. Afr. J. Biotechnol. 2011, 10, 19075–19083. [Google Scholar]
- Awan, M.R.; Iqbal, Z.; Shah, S.M.; Jamal, Z.; Jan, G.; Afzal, M.; Majid, A.; Gul, A. Studies on traditional knowledge of economically important plants of Kaghan Valley, Mansehra District, Pakistan. J. Med. Plants Res. 2011, 5, 3958–3967. [Google Scholar]
- Perveen, F.; Zaib, S.; Irshad, S.; Hassan, M. Antioxidant and DNA protection activities of the hill toon Cedrela serrata (Royle) leaves extract and its fractions. J. Nat. Prod. 2012, 5, 207–213. [Google Scholar]
- Bebrevska, L.; Foubert, K.; Hermans, N.; Chatterjee, S.; van Marck, E.; de Meyer, G.; Vlietinck, A.; Pieters, L.; Apers, S. In vivo antioxidative activity of a quantified Pueraria lobata root extract. J. Ethnopharmacol. 2010, 127, 112–117. [Google Scholar] [PubMed]
- Upadhyay, A.; Tuenter, E.; Ahmad, R.; Amin, A.; Exarchou, V.; Apers, S.; Hermans, N.; Pieters, L. Kavalactones, a novel class of protein glycation and lipid peroxidation inhibitors. Planta Med. 2014, 80, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Tuenter, E.; Amin, A.; Exarchou, V.; Hermans, N.; Apers, S.; Pieters, L. 5-O-Demethylnobiletin, a polymethoxylated flavonoid, from Citrus depressa Hayata peel prevents protein glycation. J. Funct. Foods 2014, 11, 243–249. [Google Scholar] [CrossRef]
- Upadhyay, A.; Chompoo, J.; Araki, N.; Tawata, S. Antioxidant, antimicrobial, 15-LOX, and AGEs inhibitions by pineapple stem waste. J. Food Sci. 2011, 71, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.; Higa, T.; Khanh, T.D.; Upadhyay, A.; Tawata, S. Antioxidant phenolic compounds from Smilax sebeana Miq. LWT—Food Sci. Technol. 2012, 44, 1681–1686. [Google Scholar] [CrossRef]
- Ao, C.; Li, A.; Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control 2008, 19, 940–948. [Google Scholar] [CrossRef]
- Chompoo, J.; Upadhyay, A.; Kishimoto, W.; Makise, T.; Tawata, S. Advanced glycation end product inhibitors from Alpinia zerumbet rhizomes. Food Chem. 2011, 129, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Kim, SM.; Kang, K.; Jho, E.H.; Jung, Y.J.; Nho, C.N.; Um, B.H.; Pan, C.H. Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert-butyl hyperoxide. Phytother. Res. 2011, 25, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.; Wolbis, M. Flavonoids from some species of Genus Scopolia Jacq. Acta Pol Pharm 2002, 59, 275–280. [Google Scholar] [PubMed]
- Sikorska, M.; Matlawska, I. Kaempferol, isorhamnetin and their glycosides in the flowers of Asclepias syriaca L. Acta Pol. Pharm. 2001, 58, 269–272. [Google Scholar] [PubMed]
- Brasseur, T.; Angenot, L. Six flavonol glucosides from leaves of Strychnos variabilis. Phytochemistry 1988, 27, 1487–1490. [Google Scholar] [CrossRef]
- Wan, C.; Yuan, T.; Cirello, A.L.; Seeram, N.P. Antioxidant and α-glucosidase inhibitory phenolics isolated from highbush. Food Chem. 2012, 135, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.R.; Foo, L.Y. Identification and quantification of major polyphenols in apple pomace. Food Chem. 1997, 59, 187–194. [Google Scholar] [CrossRef]
- Budzianowski, J. Kaempferol glycosides from Hosta ventricosa. Phytochemistry 1990, 29, 3643–3647. [Google Scholar] [CrossRef]
- Shen, C.J.; Chen, C.K.; Lee, S.S. Polar constituents from Sageretiathea leaf characterized by HPLC-SPE-NMR assisted approaches. J. Chin. Chem. Soc. 2009, 56, 1002–1009. [Google Scholar] [CrossRef]
- Lam, S.H.; Chen, C.K.; Wang, J.S.; Lee, S.S. Investigation of flavonoids glycosides from Neolitsea sericea var. aurata via the general method and HPLC-SPE-NMR. J. Chin. Chem. Soc. 2008, 55, 449–455. [Google Scholar]
- Jakus, V.; Hrnciarova, H.; Carsky, J.; Krahulec, B.; Rietbrock, N. Inhibition of nonenzymatic protein glycation and lipid peroxidation by drugs with antioxidant activity. Life Sci. 1999, 65, 1991–1993. [Google Scholar] [CrossRef]




| Time (min) | Solvents | %2nd Solvent |
|---|---|---|
| 0 | AB | 0 |
| 45 | AB | 100 |
| 0.5 | BC | 0 |
| 30 | BC | 50 |
| ZE3 | ZE4 | CE3 | |||
|---|---|---|---|---|---|
| Time | %B | Time | %B | Time | %B |
| 0 | 30 | 0 | 30 | 0 | 30 |
| 40 | 50 | 45 | 50 | 10 | 40 |
| 44 | 100 | 49 | 100 | 20 | 40 |
| 50 | 100 | 54 | 100 | 28 | 45 |
| 52 | 30 | 56 | 30 | 30 | 100 |
| 57 | 30 | 60 | 30 | 35 | 100 |
| 37 | 30 | ||||
| 42 | 30 | ||||
| n-Hexane | Chloroform | Ethyl acetate | n-Butanol | Aq. MeOH | |
|---|---|---|---|---|---|
| Z. oxyphylla | 85.0 ± 1.2 | 4740 ± 24 | 3.0 ± 0.1 | 42.1 ± 0.4 | 64.2 ± 1.1 |
| C. serrata | 899.7 ± 9.4 | 473.0 ± 2.4 | 6.9 ± 0.8 | 510.5 ± 23.6 | 353.4 ± 12.1 |
| E1 | E2 | E3 | E4 | |
|---|---|---|---|---|
| Z. oxyphylla | 1.43 ± 0.10 | 0.46 ± 0.09 | 0.08 ± 0.01 | 0.43 ± 0.08 |
| C. serrata | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.13 ± 0.03 | 0.14 ± 0.02 |
| 1 | 2 | 3 | 5 | 6 | 7 | |
|---|---|---|---|---|---|---|
| 2 | 157.1 | 158.1 | 157 | 157 | 158.0 | 156.42 |
| 3 | 134.2 | 133.8 | 135 | 135 | 135.8 | 133.20 |
| 4 | 178.3 | 178 | 178.7 | 178.7 | 179.65 | 177.53 |
| 5 | 161.7 | 161.8 | 161 | 161 | 163.2 | 161.24 |
| 6 | 98.5 | 98.5 | 98 | 98 | 99.50 | 98.85 |
| 7 | 164.6 | 164.8 | 164.4 | 164.4 | 164.70 | 164.33 |
| 8 | 93.3 | 93.4 | 94 | 94 | 94.5 | 93.76 |
| 9 | 157.6 | 157.7 | 157 | 157 | 158.40 | 156.42 |
| 10 | 104.2 | 104.1 | 104 | 104 | 105.8 | 104.01 |
| 1' | 121.3 | 121.4 | - | - | 122.6 | 120.89 |
| 2' | 131.0 | 130.9 | 116.3 | 116.3 | 116.1 | 130.96 |
| 3' | 114.7 | 114.6 | 144 | 144 | 145.8 | 115.21 |
| 4' | 160.2 | 160.1 | 148.6 | 148.6 | 149.0 | 160.10 |
| 5' | 114.7 | 114.6 | 114.6 | 114.6 | 116.7 | 115.21 |
| 6' | 131.0 | 130.9 | 121.4 | 121.4 | 122.9 | 130.96 |
| Glu-1'' | - | 102.7 | 103.8 | - | 100.87 | |
| 2'' | - | 71.6 | 71.6 | - | 74.24 | |
| 3'' | - | 75.6 | 75.6 | - | 77.58 | |
| 4'' | - | 68.4 | 68.4 | - | 69.89 | |
| 5'' | - | 73.5 | 73.5 | - | 76.46 | |
| 6'' | - | 60.4 | 60.4 | - | 60.83 | |
| Gal-1'' | 103.5 | 103.3 | - | - | - | - |
| 2'' | 71.6 | 71.8 | - | - | - | - |
| 3'' | 73.6 | 72.0- | - | - | - | - |
| 4'' | 68.6 | 69.8 | - | - | - | - |
| 5'' | 75.7 | 72.5 | - | - | - | - |
| 6'' | 60.6 | 65.5 | - | - | - | - |
| Rha-1'' | - | 100.5 | - | - | 102.7 | - |
| 2'' | - | 70.6 | - | - | 71.5 | - |
| 3'' | - | 70.6 | - | - | 72.1 | - |
| 4'' | - | 72.2 | - | - | 73.0 | - |
| 5'' | - | 68.4 | - | - | 71.3 | - |
| 6'' | - | 17.0 | - | - | 17.8 | - |
| O-coumaric | - | - | - | - | - | - |
| 1'' | - | 125.2 | - | - | - | - |
| 2'' | - | 129.9 | - | - | - | - |
| 3'' | - | 115.3 | - | - | - | - |
| 4'' | - | 159.8 | - | - | - | - |
| 5'' | - | 115.3 | - | - | - | - |
| 6'' | - | 129.9 | - | - | - | - |
| 7'' | - | 145.7 | - | - | - | - |
| 8'' | - | 113.4 | - | - | - | - |
| 9'' | - | 167 | - | - | - | - |
| DPPH | Superoxide | ABTS | AGEs | |
|---|---|---|---|---|
| (IC50, µg/mL) | (IC50, µg/mL) | (IC50, µg/mL) | (IC50, µg/mL) | |
| 1 | 17.8 ± 1.1 d,* | 910 ± 14 h | 230 ± 7 c | 559 ± 20 a,b,c |
| 2 | 30.5 ± 1.9 e | 512 ± 8 f | 320 ± 9 d | 530 ± 19 a,b |
| 3 | 10.8 ± 0.7 c | 400 ± 6 c | 170 ± 5 a | 556 ± 19 a,b,c |
| 4 | 19.8 ± 1.2 d | 410 ± 6 c | 200 ± 6 b | 574 ± 20 b,c |
| 5 | 18.9 ± 1.1 d | 431 ± 7 d | 180 ± 5 a | 548 ± 19 a,b,c |
| 6 | 19.2 ± 1.2 d | 381 ± 6 b | 170 ± 5 a | 554 ± 19 a,b,c |
| 7 | 40.4 ± 2.5 f | 482 ± 7 e | 240 ± 7 c | 818 ± 29 d |
| M1 | 5.3 ± 0.3 a,b | 207 ± 3 a | 170 ± 5 a | 586 ± 21 b,c |
| M2 | 8.0 ± 0.5 b | 713 ± 11 g | 170 ± 5 a | 589 ± 21 c |
| M3 | 4.4 ± 0.3 a | 200 ± 3 a | 170 ± 5 a | 541 ± 19 a, b, c |
| M4 | 13.4 ± 0.8 c | 410 ± 6 c | 170 ± 5 a | 593 ± 21 c |
| Quercetin | 3.6 ± 0.6 a | 374 ± 6 b | 180 ± 5 a | not tested |
| Aminoguanidine | not tested | not tested | not tested | 510 ± 18 a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.; Ahmad, N.; Naqvi, A.A.; Exarchou, V.; Upadhyay, A.; Tuenter, E.; Foubert, K.; Apers, S.; Hermans, N.; Pieters, L. Antioxidant and Antiglycating Constituents from Leaves of Ziziphus oxyphylla and Cedrela serrata. Antioxidants 2016, 5, 9. https://doi.org/10.3390/antiox5010009
Ahmad R, Ahmad N, Naqvi AA, Exarchou V, Upadhyay A, Tuenter E, Foubert K, Apers S, Hermans N, Pieters L. Antioxidant and Antiglycating Constituents from Leaves of Ziziphus oxyphylla and Cedrela serrata. Antioxidants. 2016; 5(1):9. https://doi.org/10.3390/antiox5010009
Chicago/Turabian StyleAhmad, Rizwan, Niyaz Ahmad, Atta Abbas Naqvi, Vassiliki Exarchou, Atul Upadhyay, Emmy Tuenter, Kenn Foubert, Sandra Apers, Nina Hermans, and Luc Pieters. 2016. "Antioxidant and Antiglycating Constituents from Leaves of Ziziphus oxyphylla and Cedrela serrata" Antioxidants 5, no. 1: 9. https://doi.org/10.3390/antiox5010009
APA StyleAhmad, R., Ahmad, N., Naqvi, A. A., Exarchou, V., Upadhyay, A., Tuenter, E., Foubert, K., Apers, S., Hermans, N., & Pieters, L. (2016). Antioxidant and Antiglycating Constituents from Leaves of Ziziphus oxyphylla and Cedrela serrata. Antioxidants, 5(1), 9. https://doi.org/10.3390/antiox5010009