Oxidative Stress in the Newborn Period: Useful Biomarkers in the Clinical Setting
Abstract
:1. Introduction
2. Aerobic Metabolism, Reactive Oxygen Species, and Oxidative Stress
3. The Fetal-to-Neonatal Transition
3.1. Cardiocirculatory and Metabolic Changes during Birth and Postnatal Stabilization
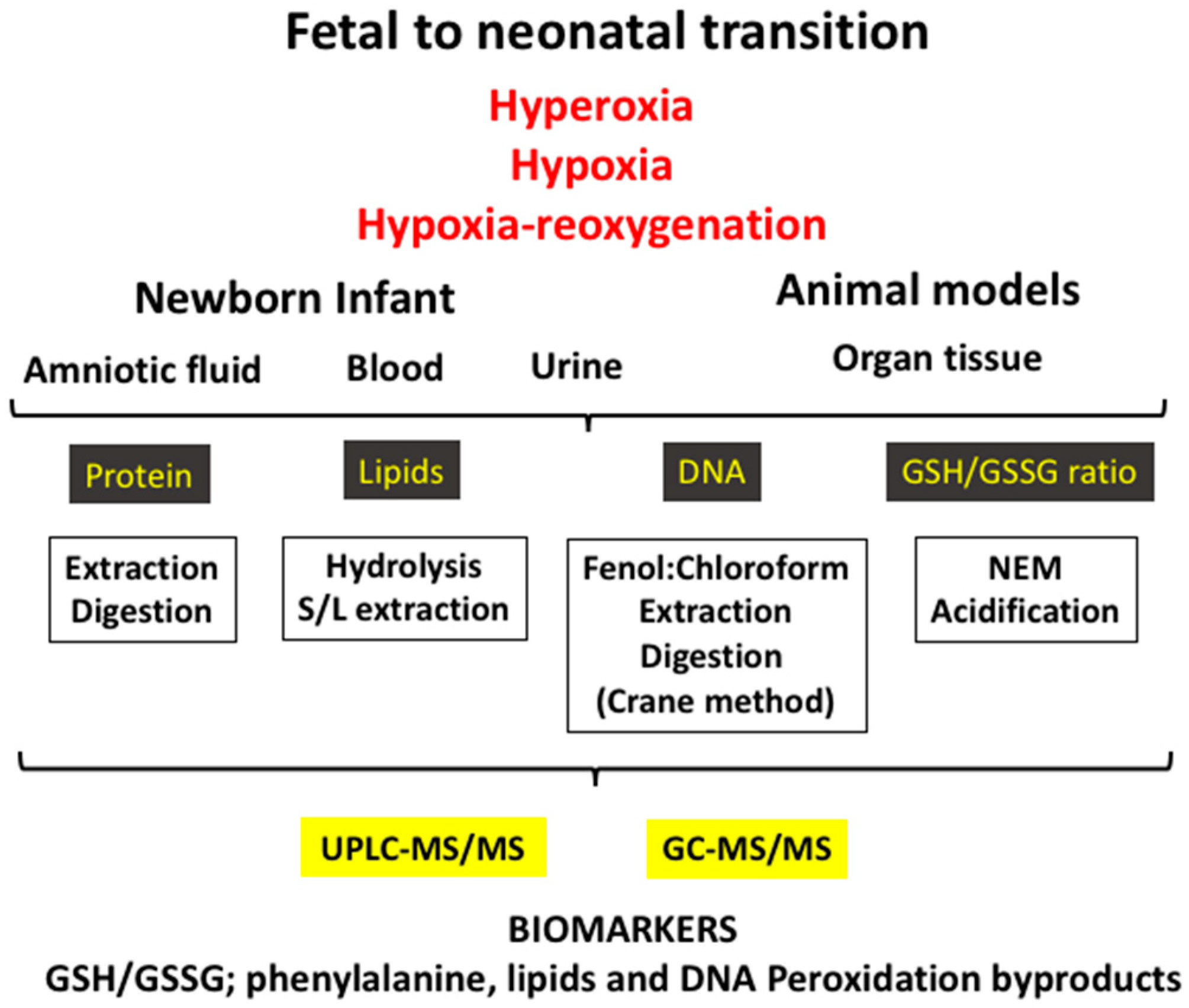
3.2. Experimental Models of Oxidative Stress in the Fetal-to-Neonatal Transition
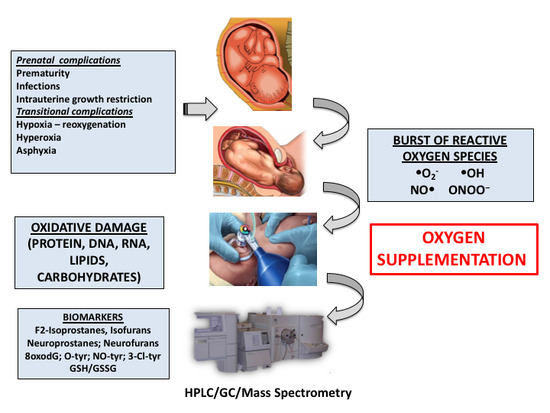
4. Pathologic Conditions during the Fetal-to-Neonatal Transition in Newborn Infants
4.1. Perinatal Asphyxia
4.2. Prematurity
5. Biomarkers of Oxidative Stress Employed in the Clinical Setting during the Neonatal Period
5.1. Ratio of Reduced Glutathione to Oxidized Glutathione (GSH/GSSG)
5.2. Protein Oxidation
5.3. DNA Oxidation
5.4. Lipid Peroxidation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morton, S.U.; Brodsky, D. Fetal Physiology and the Transition to Extrauterine Life. Clin. Perinatol. 2016, 43, 395–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vento, M.; Moro, M.; Escrig, R.; Arruza, L.; Villar, G.; Izquierdo, I.; Roberts, L.J.; Arduini, A.; Escobar, J.J.; Sastre, J.; et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009, 124, e439–e449. [Google Scholar] [CrossRef] [PubMed]
- Negro, S.; Benders, M.J.N.L.; Tataranno, M.L.; Coviello, C.; de Vries, L.S.; van Bel, F.; Groenendaal, F.; Longini, M.; Proietti, F.; Belvisi, E.; et al. Oxygen and oxidative stress in the perinatal period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef]
- Campbell, I. Intermediary metabolism. Anaesth. Intensiv. Care Med. 2017, 18, 147–149. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Beharry, K.D.; Cai, C.L.; Valencia, G.B.; Valencia, A.M.; Lazzaro, D.R.; Bany-Mohammed, F.; Aranda, J.V. Neonatal Intermittent Hypoxia, Reactive Oxygen Species, and Oxygen-Induced Retinopathy. React. Oxyg. Species 2017, 3, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Bao, Y.; Korthuis, R.J. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs. preconditioning. Redox Biol. 2014, 2, 702–714. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Chiao, P.J.; Achanta, G.; Arlinghaus, R.B.; Liu, J.; et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Bubb, K.J.; Birgisdottir, A.B.; Tang, O.; Hansen, T.; Figtree, G.A. Redox modification of caveolar proteins in the cardiovascular system-role in cellular signaling and disease. Free Radic. Biol. Med. 2017, 109, 61–74. [Google Scholar] [CrossRef]
- Jones, D.P. Redox sensing: Orthogonal control in cell cycle and apoptosis signalling. J. Intern. Med. 2010, 268, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Sies, H. The Redox Code. Antioxid. Redox Signal. 2015, 23, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Baik, N.; Urlesberger, B.; Schwaberger, B.; Schmölzer, G.M.; Mileder, L.; Avian, A.; Pichler, G. Reference Ranges for Cerebral Tissue Oxygen Saturation Index in Term Neonates during Immediate Neonatal Transition after Birth. Neonatology 2015, 108, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Yigit, M.B.; Kowalski, W.J.; Hutchon, D.J.R.; Pekkan, K. Transition from fetal to neonatal circulation: Modeling the effect of umbilical cord clamping. J. Biomech. 2015, 48, 1662–1670. [Google Scholar] [CrossRef] [Green Version]
- Dawson, J.A.; Kamlin, C.O.F.; Vento, M.; Wong, C.; Cole, T.J.; Donath, S.M.; Davis, P.G.; Morley, C.J. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010, 125, e1340–e1347. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. RBE 2012, 10, 49. [Google Scholar] [CrossRef]
- Shim, S.-Y.; Kim, H.-S. Oxidative stress and the antioxidant enzyme system in the developing brain. Korean J. Pediatr. 2013, 56, 107–111. [Google Scholar] [CrossRef]
- Vento, M.; Aguar, M.; Escobar, J.; Arduini, A.; Escrig, R.; Brugada, M.; Izquierdo, I.; Asensi, M.A.; Sastre, J.; Saenz, P. Antenatal steroids and antioxidant enzyme activity in preterm infants: Influence of gender and timing. Antioxid. Redox Signal. 2009, 11, 2945–2955. [Google Scholar] [CrossRef]
- Viña, J.; Vento, M.; García-Sala, F.; Puertes, I.R.; Gascó, E.; Sastre, J.; Asensi, M.; Pallardó, F.V. L-cysteine and glutathione metabolism are impaired in premature infants due to cystathionase deficiency. Am. J. Clin. Nutr. 1995, 61, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Pallardo, F.V.; Sastre, J.; Asensi, M.; Rodrigo, F.; Estrela, J.M.; Viña, J. Physiological changes in glutathione metabolism in foetal and newborn rat liver. Biochem. J. 1991, 274, 891–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltepe, E.; Saugstad, O.D. Oxygen in health and disease: Regulation of oxygen homeostasis—Clinical implications. Pediatr. Res. 2009, 65, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Asensi, M.; Rodrigo, F.; Pallardó, F.V.; Vento, M.; Viña, J. Antioxidant administration to the mother prevents oxidative stress associated with birth in the neonatal rat. Life Sci. 1994, 54, 2055–2059. [Google Scholar] [CrossRef]
- Gelfand, S.L.; Vento, M.; Sastre, J.; Lust, W.D.; Smith, M.A.; Perry, G.; Walsh, M.; Martin, R. A new model of oxidative stress in rat pups. Neonatology 2008, 94, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Presti, A.L.; Kishkurno, S.V.; Slinko, S.K.; Randis, T.M.; Ratner, V.I.; Polin, R.A.; Ten, V.S. Reoxygenation with 100% oxygen versus room air: Late neuroanatomical and neurofunctional outcome in neonatal mice with hypoxic-ischemic brain injury. Pediatr. Res. 2006, 60, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Bookatz, G.B.; Mayer, C.A.; Wilson, C.G.; Vento, M.; Gelfand, S.L.; Haxhiu, M.A.; Martin, R.J. Effect of supplemental oxygen on reinitiation of breathing after neonatal resuscitation in rat pups. Pediatr. Res. 2007, 61, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D. Oxygen and oxidative stress in bronchopulmonary dysplasia. J. Perinat. Med. 2010, 38, 571–577. [Google Scholar] [CrossRef]
- Bhandari, V. Hyperoxia-derived lung damage in preterm infants. Semin. Fetal Neonatal Med. 2010, 15, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Yee, M.; Cohen, E.D.; Domm, W.; Porter, G.A.; McDavid, A.N.; O’Reilly, M.A. Neonatal hyperoxia depletes pulmonary vein cardiomyocytes in adult mice via mitochondrial oxidation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L846–L859. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cuevas, I.; Aupi, M.; Asensi, M.A.; Vento, M.; Ortega, Á.; Escobar, J. 7,8-hydroxy-2′-deoxyguanosine/2′-deoxiguanosine ratio determined in hydrolysates of brain DNA by ultrachromatrography coupled to tandem mass spectrometry. Talanta 2017, 170, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D.; Sejersted, Y.; Solberg, R.; Wollen, E.J.; Bjørås, M. Oxygenation of the Newborn: A Molecular Approach. Neonatology 2012, 101, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Merchant, N.; Azzopardi, D. Early predictors of outcome in infants treated with hypothermia for hypoxic-ischaemic encephalopathy. Dev. Med. Child Neurol. 2015, 57 (Suppl. 3), 8–16. [Google Scholar] [CrossRef] [Green Version]
- Allen, K.A.; Brandon, D.H. Hypoxic Ischemic Encephalopathy: Pathophysiology and Experimental Treatments. Newborn Infant Nurs. Rev. NAINR 2011, 11, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odeh, M. The role of reperfusion-induced injury in the pathogenesis of the crush syndrome. N. Engl. J. Med. 1991, 324, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, G.; Groenendaal, F. Anti-oxidant strategies. Semin. Fetal Neonatal Med. 2007, 12, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cuevas, I.; Cernada, M.; Nuñez, A.; Escobar, J.; Kuligowski, J.; Chafer-Pericas, C.; Vento, M. Oxygen Supplementation to Stabilize Preterm Infants in the Fetal to Neonatal Transition: No Satisfactory Answer. Front. Pediatr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D. Hypoxanthine as a measurement of hypoxia. Pediatr. Res. 1975, 9, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D.; Aasen, A.O. Plasma hypoxanthine concentrations in pigs. A prognostic aid in hypoxia. Eur. Surg. Res. Eur. Chir. Forsch. Rech. Chir. Eur. 1980, 12, 123–129. [Google Scholar] [CrossRef]
- Solberg, R.; Kuligowski, J.; Pankratov, L.; Escobar, J.; Quintás, G.; Lliso, I.; Sánchez-Illana, Á.; Saugstad, O.D.; Vento, M. Changes of the plasma metabolome of newly born piglets subjected to postnatal hypoxia and resuscitation with air. Pediatr. Res. 2016, 80, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Illana, Á.; Solberg, R.; Lliso, I.; Pankratov, L.; Quintás, G.; Saugstad, O.D.; Vento, M.; Kuligowski, J. Assessment of phospholipid synthesis related biomarkers for perinatal asphyxia: A piglet study. Sci. Rep. 2017, 7, 40315. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, J.; Solberg, R.; Sánchez-Illana, Á.; Pankratov, L.; Parra-Llorca, A.; Quintás, G.; Saugstad, O.D.; Vento, M. Plasma metabolite score correlates with Hypoxia time in a newly born piglet model for asphyxia. Redox Biol. 2017, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sahni, P.V.; Zhang, J.; Sosunov, S.; Galkin, A.; Niatsetskaya, Z.; Starkov, A.; Brookes, P.S.; Ten, V.S. Krebs cycle metabolites and preferential succinate oxidation following neonatal hypoxic-ischemic brain injury in mice. Pediatr. Res. 2018, 83, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Illana, Á.; Núñez-Ramiro, A.; Cernada, M.; Parra-Llorca, A.; Valverde, E.; Blanco, D.; Moral-Pumarega, M.T.; Cabañas, F.; Boix, H.; Pavón, A.; et al. Evolution of Energy Related Metabolites in Plasma from Newborns with Hypoxic-Ischemic Encephalopathy during Hypothermia Treatment. Sci. Rep. 2017, 7, 17039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vento, M.; Asensi, M.; Sastre, J.; García-Sala, F.; Pallardó, F.V.; Viña, J. Resuscitation with Room Air Instead of 100% Oxygen Prevents Oxidative Stress in Moderately Asphyxiated Term Neonates. Pediatrics 2001, 107, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Vento, M.; Asensi, M.; Sastre, J.; Lloret, A.; García-Sala, F.; Viña, J. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J. Pediatr. 2003, 142, 240–246. [Google Scholar] [CrossRef]
- Vento, M.; Sastre, J.; Asensi, M.A.; Viña, J. Room-air resuscitation causes less damage to heart and kidney than 100% oxygen. Am. J. Respir. Crit. Care Med. 2005, 172, 1393–1398. [Google Scholar] [CrossRef]
- Saugstad, O.D.; Ramji, S.; Soll, R.F.; Vento, M. Resuscitation of newborn infants with 21% or 100% oxygen: An updated systematic review and meta-analysis. Neonatology 2008, 94, 176–182. [Google Scholar] [CrossRef]
- Perlman, J.M.; Wyllie, J.; Kattwinkel, J.; Wyckoff, M.H.; Aziz, K.; Guinsburg, R.; Kim, H.-S.; Liley, H.G.; Mildenhall, L.; Simon, W.M.; et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Pediatrics 2015, 136 (Suppl. 2), S120–S166. [Google Scholar] [CrossRef]
- Vento, M. Oxygen supplementation in the neonatal period: Changing the paradigm. Neonatology 2014, 105, 323–331. [Google Scholar] [CrossRef]
- Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef]
- Ezaki, S.; Suzuki, K.; Kurishima, C.; Miura, M.; Weilin, W.; Hoshi, R.; Tanitsu, S.; Tomita, Y.; Takayama, C.; Wada, M.; et al. Resuscitation of preterm infants with reduced oxygen results in less oxidative stress than resuscitation with 100% oxygen. J. Clin. Biochem. Nutr. 2009, 44, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Tataranno, M.L.; Oei, J.L.; Perrone, S.; Wright, I.M.; Smyth, J.P.; Lui, K.; Tarnow-Mordi, W.O.; Longini, M.; Proietti, F.; Negro, S.; et al. Resuscitating preterm infants with 100% oxygen is associated with higher oxidative stress than room air. Acta Paediatr. 2015, 104, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, V.S.; Chalak, L.F.; Sparks, J.E.; Allen, J.R.; Savani, R.C.; Wyckoff, M.H. Resuscitation of preterm neonates with limited versus high oxygen strategy. Pediatrics 2013, 132, e1488–e1496. [Google Scholar] [CrossRef] [PubMed]
- Rook, D.; Schierbeek, H.; Vento, M.; Vlaardingerbroek, H.; van der Eijk, A.C.; Longini, M.; Buonocore, G.; Escobar, J.; van Goudoever, J.B.; Vermeulen, M.J. Resuscitation of preterm infants with different inspired oxygen fractions. J. Pediatr. 2014, 164, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Aguar, M.; Izquierdo, M.; Brugada, M. Preterm babies randomly assigned to be blindly resuscitated with higher (60%) vs. lower (30%) initial FIO2: Effects on oxidative stress and mortality. In Proceedings of the EAPS, Vancouver, BC, Canada, 3–6 May 2014. 3843.540. [Google Scholar]
- Escobar, J.; Sánchez-Illana, Á.; Kuligowski, J.; Torres-Cuevas, I.; Solberg, R.; Garberg, H.T.; Huun, M.U.; Saugstad, O.D.; Vento, M.; Cháfer-Pericás, C. Development of a reliable method based on ultra-performance liquid chromatography coupled to tandem mass spectrometry to measure thiol-associated oxidative stress in whole blood samples. J. Pharm. Biomed. Anal. 2016, 123, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Illana, Á.; Mayr, F.; Cuesta-García, D.; Piñeiro-Ramos, J.D.; Cantarero, A.; Guardia, M.D.L.; Vento, M.; Lendl, B.; Quintás, G.; Kuligowski, J. On-Capillary Surface-Enhanced Raman Spectroscopy: Determination of Glutathione in Whole Blood Microsamples. Anal. Chem. 2018, 90, 9093–9100. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Analytical methods for 3-nitrotyrosine quantification in biological samples: The unique role of tandem mass spectrometry. Amino Acids 2012, 42, 45–63. [Google Scholar] [CrossRef]
- Franco, M.C.; Estévez, A.G. Tyrosine nitration as mediator of cell death. Cell. Mol. Life Sci. CMLS 2014, 71, 3939–3950. [Google Scholar] [CrossRef]
- Torres-Cuevas, I.; Kuligowski, J.; Cárcel, M.; Cháfer-Pericás, C.; Asensi, M.; Solberg, R.; Cubells, E.; Nuñez, A.; Saugstad, O.D.; Vento, M.; et al. Protein-bound tyrosine oxidation, nitration and chlorination by-products assessed by ultraperformance liquid chromatography coupled to tandem mass spectrometry. Anal. Chim. Acta 2016, 913, 104–110. [Google Scholar] [CrossRef]
- Solberg, R.; Andresen, J.H.; Escrig, R.; Vento, M.; Saugstad, O.D. Resuscitation of hypoxic newborn piglets with oxygen induces a dose-dependent increase in markers of oxidation. Pediatr. Res. 2007, 62, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Ledo, A.; Arduini, A.; Asensi, M.A.; Sastre, J.; Escrig, R.; Brugada, M.; Aguar, M.; Saenz, P.; Vento, M. Human milk enhances antioxidant defenses against hydroxyl radical aggression in preterm infants. Am. J. Clin. Nutr. 2009, 89, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Escobar, J.; Teramo, K.; Stefanovic, V.; Andersson, S.; Asensi, M.A.; Arduini, A.; Cubells, E.; Sastre, J.; Vento, M. Amniotic fluid oxidative and nitrosative stress biomarkers correlate with fetal chronic hypoxia in diabetic pregnancies. Neonatology 2013, 103, 193–198. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef] [PubMed]
- Ambroz, A.; Vlkova, V.; Rossner, P.; Rossnerova, A.; Svecova, V.; Milcova, A.; Pulkrabova, J.; Hajslova, J.; Veleminsky, M.; Solansky, I.; et al. Impact of air pollution on oxidative DNA damage and lipid peroxidation in mothers and their newborns. Int. J. Hyg. Environ. Health 2016, 219, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Loft, S.; Danielsen, P.; Løhr, M.; Jantzen, K.; Hemmingsen, J.G.; Roursgaard, M.; Karotki, D.G.; Møller, P. Urinary excretion of 8-oxo-7,8-dihydroguanine as biomarker of oxidative damage to DNA. Arch. Biochem. Biophys. 2012, 518, 142–150. [Google Scholar] [CrossRef]
- Barregard, L.; Møller, P.; Henriksen, T.; Mistry, V.; Koppen, G.; Rossner, P.; Sram, R.J.; Weimann, A.; Poulsen, H.E.; Nataf, R.; et al. Human and methodological sources of variability in the measurement of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine. Antioxid. Redox Signal. 2013, 18, 2377–2391. [Google Scholar] [CrossRef]
- Kuligowski, J.; Escobar, J.; Quintás, G.; Lliso, I.; Torres-Cuevas, I.; Nuñez, A.; Cubells, E.; Rook, D.; van Goudoever, J.B.; Vento, M. Analysis of lipid peroxidation biomarkers in extremely low gestational age neonate urines by UPLC-MS/MS. Anal. Bioanal. Chem. 2014, 406, 4345–4356. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol. Aspects Med. 2018, 64, 1–17. [Google Scholar] [CrossRef]
- Milne, G.L.; Dai, Q.; Roberts, L.J. The isoprostanes—25 years later. Biochim. Biophys. Acta 2015, 1851, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Solberg, R.; Longini, M.; Proietti, F.; Vezzosi, P.; Saugstad, O.D.; Buonocore, G. Resuscitation with supplementary oxygen induces oxidative injury in the cerebral cortex. Free Radic. Biol. Med. 2012, 53, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Galano, J.-M.; Lee, Y.Y.; Oger, C.; Vigor, C.; Vercauteren, J.; Durand, T.; Giera, M.; Lee, J.C. Isoprostanes, neuroprostanes and phytoprostanes: An overview of 25 years of research in chemistry and biology. Prog. Lipid Res. 2017, 68, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, J.; Aguar, M.; Rook, D.; Lliso, I.; Torres-Cuevas, I.; Escobar, J.; Quintás, G.; Brugada, M.; Sánchez-Illana, Á.; van Goudoever, J.B.; et al. Urinary Lipid Peroxidation Byproducts: Are They Relevant for Predicting Neonatal Morbidity in Preterm Infants? Antioxid. Redox Signal. 2015, 23, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Cháfer-Pericás, C.; Torres-Cuevas, I.; Sanchez-Illana, A.; Escobar, J.; Kuligowski, J.; Solberg, R.; Garberg, H.T.; Huun, M.U.; Saugstad, O.D.; Vento, M. Development of a reliable analytical method to determine lipid peroxidation biomarkers in newborn plasma samples. Talanta 2016, 153, 152–157. [Google Scholar] [CrossRef]
- Cháfer-Pericas, C.; Cernada, M.; Rahkonen, L.; Stefanovic, V.; Andersson, S.; Vento, M. Preliminary case control study to establish the correlation between novel peroxidation biomarkers in cord serum and the severity of hypoxic ischemic encephalopathy. Free Radic. Biol. Med. 2016, 97, 244–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barden, A.E.; Corcoran, T.B.; Mas, E.; Durand, T.; Galano, J.-M.; Roberts, L.J.; Paech, M.; Muchatuta, N.A.; Phillips, M.; Mori, T.A. Is There a Role for Isofurans and Neuroprostanes in Pre-Eclampsia and Normal Pregnancy? Antioxid. Redox Signal. 2012, 16, 165–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Illana, Á.; Thayyil, S.; Montaldo, P.; Jenkins, D.; Quintás, G.; Oger, C.; Galano, J.-M.; Vigor, C.; Durand, T.; Vento, M.; et al. Novel free-radical mediated lipid peroxidation biomarkers in newborn plasma. Anal. Chim. Acta 2017, 996, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Peña-Bautista, C.; Carrascosa-Marco, P.; Oger, C.; Vigor, C.; Galano, J.; Durand, T.; Baquero, M.; López-Nogueroles, M.; Vento, M.; García-Blanco, A.C.; et al. Validated analytical method to determine new salivary lipid peroxidation compounds as potential neurodegenerative biomarkers. J. Pharm. Biomed. Anal. 2018, 164, 742–749. [Google Scholar] [CrossRef] [PubMed]

| Oxidative Biomarker | Target Biomolecule | Modification | Biological Sampling |
|---|---|---|---|
| GSH/GSSG Cysteine/Cystine Homocysteine/Homocystine | Antioxidant | Redox status | Umbilical cord blood/whole blood/tissue |
| o-Tyr/Phe ratio m-Tyr/Phe ratio 3NO2-Tyr/p-Tyr ratio 3Cl-Tyr/p-Tyr ratio | Protein | Tyrosine Hydroxylation Tyrosine nitration Tyrosine chlorination | Urine/plasma/milk/tissue |
| 8oxodG (8oxodG/2dG ratio) | DNA | Hydroxylation DNA nucleotides | Urine/plasma/amniotic fluid/tissue |
| IsoP | Lipids | AA peroxidation | Urine/plasma/amniotic fluid/tissue |
| Dihomo-IsoP | Lipids | AdA peroxidation | Urine/plasma/tissue |
| IsoF | Lipids | AA peroxidation | Urine/plasma/tissue |
| NeuroP | Lipids | DHA peroxidation | Urine/plasma/tissue |
| NeuroF | Lipids | DHA peroxidation | Urine/plasma/tissue |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán, I.; Piñero-Ramos, J.D.; Lara, I.; Parra-Llorca, A.; Torres-Cuevas, I.; Vento, M. Oxidative Stress in the Newborn Period: Useful Biomarkers in the Clinical Setting. Antioxidants 2018, 7, 193. https://doi.org/10.3390/antiox7120193
Millán I, Piñero-Ramos JD, Lara I, Parra-Llorca A, Torres-Cuevas I, Vento M. Oxidative Stress in the Newborn Period: Useful Biomarkers in the Clinical Setting. Antioxidants. 2018; 7(12):193. https://doi.org/10.3390/antiox7120193
Chicago/Turabian StyleMillán, Iván, José David Piñero-Ramos, Inmaculada Lara, Anna Parra-Llorca, Isabel Torres-Cuevas, and Máximo Vento. 2018. "Oxidative Stress in the Newborn Period: Useful Biomarkers in the Clinical Setting" Antioxidants 7, no. 12: 193. https://doi.org/10.3390/antiox7120193
APA StyleMillán, I., Piñero-Ramos, J. D., Lara, I., Parra-Llorca, A., Torres-Cuevas, I., & Vento, M. (2018). Oxidative Stress in the Newborn Period: Useful Biomarkers in the Clinical Setting. Antioxidants, 7(12), 193. https://doi.org/10.3390/antiox7120193