Polyphenolic Fraction from Olive Mill Wastewater: Scale-Up and in Vitro Studies for Ophthalmic Nutraceutical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. OMWW Pretreatment
2.3. Adsorption/Desorption Treatment
2.4. Determination of Total Phenol Content
2.5. Determination of Total Carbohydrates
2.6. Determination of the Pollutant Load
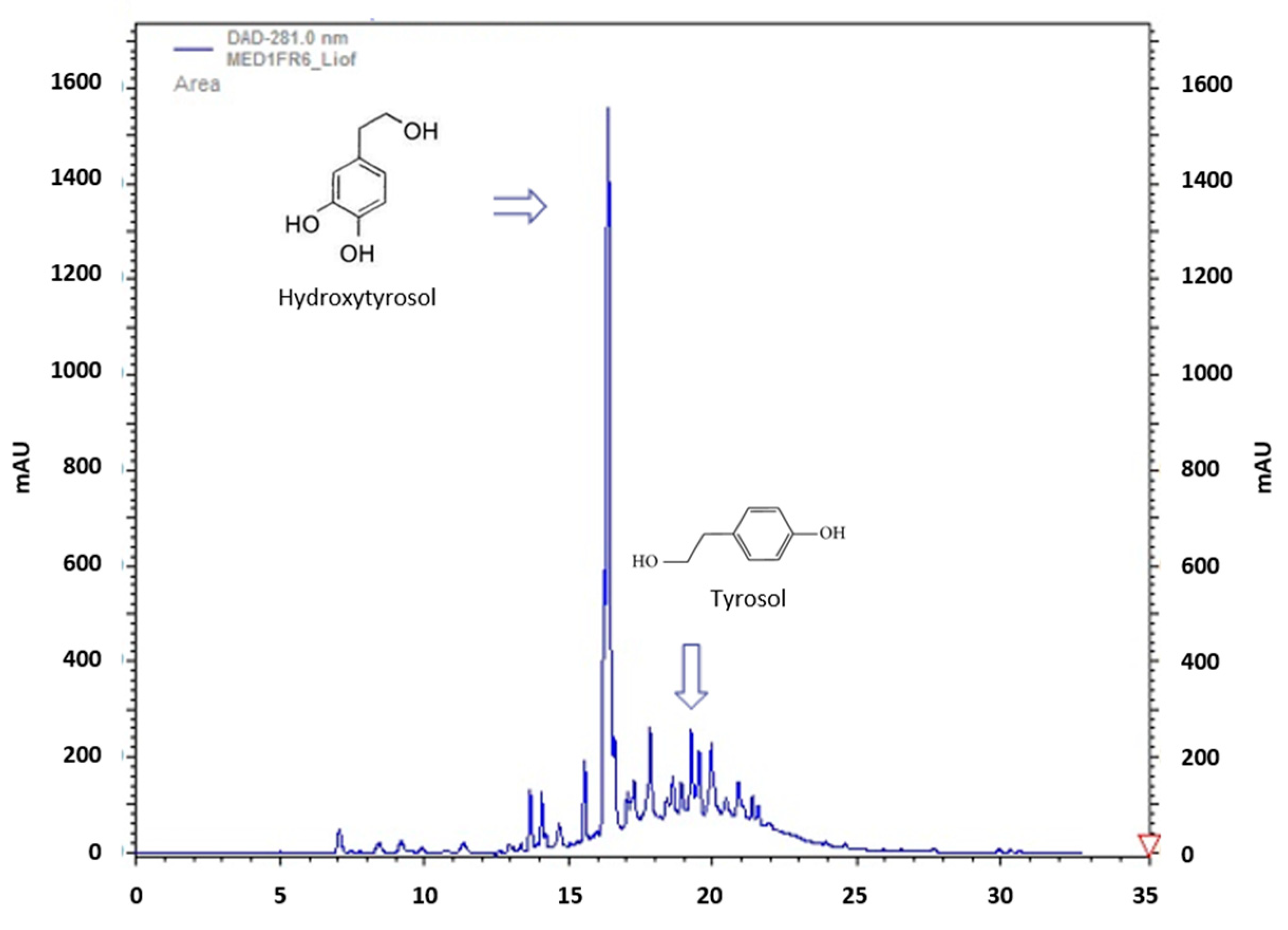
2.7. Chromatographic Analysis of Polyphenols
2.8. H-NMR Analysis
2.9. Adsorption Equilibrium Tests
2.10. Adsorption Kinetic Tests
2.11. Cycling Efficiency Tests
2.12. Process Scale-Up
2.13. In Vitro Study
2.13.1. Cell Cultures and Treatments
2.13.2. MTT Assay
2.13.3. Lactic Dehydrogenase Release
2.13.4. Alkaline Comet Assay
2.13.5. Reactive Oxygen Species (ROS) Determination
2.13.6. Protective Effect against Oxidative Stress
2.13.7. Protective Effect against Inflammation
2.14. Studies for Ophthalmic Nutraceutical Application
2.14.1. Ophthalmic Formulation and Stability Study
2.14.2. Ocular Irritation Test
2.15. Statistical Analysis
3. Results and Discussion
3.1. Selective Recovery of Polyphenolic Fraction from OMWW
3.2. Adsorption Equilibrium and Kinetic Tests
3.3. Cycling Efficiency Tests
3.4. In Vitro Study and Ophthalmic Nutraceutical Application
3.5. Scaling up and Pilot Plant Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deeb, A.A.; Fayyad, M.K.; Alawi, M.A. Separation of Polyphenols from Jordanian Olive Oil Mill Wastewater. Chromatogr. Res. Int. 2012, 2012, 812127. [Google Scholar] [CrossRef]
- Kapellakis, I.E.; Tsagarakis, K.P.; Crowther, J.C. Olive oil history, production and by-product management. Rev. Environ. Sci. Biol. 2008, 7, 1–26. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Allouche, N.; Fki, I.; Sayadi, S. Toward a high yield recovery of antioxidants and purified hydroxytyrosol from olive mill wastewaters. J. Agric. Food Chem. 2004, 52, 267–273. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Kontogianni, V.G.; Kotsiou, K.; Linsalata, V.; Logrieco, A.F.; Tasioula-Margari, M.; Cardinali, A. Polyphenolic characterization of Olive Mill WasteWaters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res. Int. 2014, 65, 301–310. [Google Scholar] [CrossRef]
- Fiorentino, A.; Gentili, A.; Isidori, M.; Lavorgna, M.; Parrella, A.; Temussi, F. Olive oil mill wastewater treatment using a chemical and biological approach. J. Agric. Food Chem. 2004, 52, 5151–5154. [Google Scholar] [CrossRef] [PubMed]
- Günay, A.; Çetin, M. Determination of aerobic biodegradation kinetics of olive oil mill wastewater. Int. Biodeter. Biodegr. 2013, 85, 237–242. [Google Scholar] [CrossRef]
- Papadimitriou, E.K.; Chatjipavlidis, I.; Balis, C. Application of composting to olive mill wastewater treatment. Environ. Technol. 1997, 18, 101–107. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Smiljković, M.; Dias, M.I.; Stojković, D.; Barros, L.; Bukvički, D.; Ferreira, I.C.F.R.; Soković, M. Characterization of phenolic compounds in tincture of edible Nepeta nuda: Development of antimicrobial mouthwash. Food Funct. 2018, 9, 5417–5425. [Google Scholar] [CrossRef] [PubMed]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Grasso, S.; Siracusa, L.; Spatafora, C.; Renis, M.; Tringali, C. Hydroxytyrosol lipophilic analogues: Enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorg. Chem. 2007, 35, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ramiro, I.; Martín, M.A.; Ramos, S.; Bravo, L.; Goya, L. Olive oil hydroxytyrosol reduces toxicity evoked by acrylamide in human Caco-2 cells by preventing oxidative stress. Toxicology 2011, 288, 43–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiuri, M.C.; De Stefano, D.; Di Meglio, P.; Irace, C.; Savarese, M.; Sacchi, R.; Cinelli, M.P.; Carnuccio, R. Hydroxytyrosol, a phenolic compound from virgin olive oil, prevents macrophage activation. Naunyn Schmiedebergs Arch. Pharmacol. 2005, 371, 457–465. [Google Scholar] [CrossRef]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol Exerts Anti-Inflammatory and Anti-Oxidant Activities in a Mouse Model of Systemic Inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, K.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef]
- Bucolo, C.; Fidilio, A.; Platania, C.B.M.; Geraci, F.; Lazzara, F.; Drago, F. Antioxidant and Osmoprotecting Activity of Taurine in Dry Eye Models. J. Ocul. Pharmacol. Ther. 2018, 34, 188–194. [Google Scholar] [CrossRef]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Invest. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, T.; Li, W.; Sun, X. Inhibiting effects of dietary polyphenols on chronic eye diseases. J. Funct. Foods 2017, 39, 186–197. [Google Scholar] [CrossRef]
- Khoufi, S.; Hamza, M.; Sayadi, S. Enzymatic hydrolysis of olive wastewater for hydroxytyrosol enrichment. Bioresour. Technol. 2011, 102, 9050–9058. [Google Scholar] [CrossRef]
- Leouifoudi, I.; Zyad, A.; Amechrouq, A.; Oukerrou, M.A.; Mouse, H.A.; Mbarki, M. Identification and characterisation of phenolic compounds extracted from Moroccan olive mill wastewater. Food Sci. Technol. 2014, 34, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive Mill Waste Extracts: Polyphenols Content, Antioxidant, and Antimicrobial Activities. Adv. Pharmacol. Sci. 2015, 2015, 714138. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J. Hazard. Mater. 2015, 21, 69–76. [Google Scholar] [CrossRef]
- Fava, G.; Di Mauro, M.D.; Spampinato, M.; Biondi, D.; Gambera, G.; Centonze, G.; Maggiore, R.; D’Antona, N. Hydroxytyrosol Recovery from Olive Mill Wastewater: Process Optimization and Development of a Pilot Plant. Clean Soil Air Water 2017, 45, 1600042. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and Analysis of Biophenols Recovered from Olive Mill Waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Giardina, R.C.; Fava, G.; Mirabella, E.F.; Acquaviva, R.; Renis, M.; D’Antona, N. Polyphenolic profile and antioxidant activity of olive mill wastewater from two Sicilian olive cultivars: Cerasuola and Nocellara etnea. Eur. Food Res. Technol. 2017, 243, 1895–1903. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Tomasello, B.; Giardina, R.C.; Dattilo, S.; Mazzei, V.; Sinatra, F.; Caruso, M.; D’Antona, N.; Renis, M. Sugar and mineral enriched fraction from olive mill wastewater for promising cosmeceutical application: Characterization, in vitro and in vivo studies. Food Funct. 2017, 8, 4713–4722. [Google Scholar] [CrossRef]
- Malfa, G.A.; Tomasello, B.; Acquaviva, R.; Genovese, C.; La Mantia, A.; Cammarata, F.P.; Ragusa, M.; Renis, M.; Di Giacomo, C. Betula etnensis Raf. (Betulaceae) Extract Induced HO-1 Expression and Ferroptosis Cell Death in Human Colon Cancer Cells. Int. J. Mol. Sci. 2019, 20, 2723. [Google Scholar] [CrossRef]
- Malfa, G.A.; Tomasello, B.; Sinatra, F.; Villaggio, G.; Amenta, F.; Avola, R.; Renis, M. Reactive response evaluation of primary human astrocytes after methylmercury exposure. J. Neurosci. Res. 2014, 92, 95–103. [Google Scholar] [CrossRef]
- Tomasello, B.; Malfa, G.A.; Strazzanti, A.; Gangi, S.; Di Giacomo, C.; Basile, F.; Renis, M. Effects of physical activity on systemic oxidative/DNA status in breast cancer survivors. Oncol. Lett. 2017, 13, 441–448. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Ferrito, V.; Scifo, C.; Renis, M.; Tomasello, B. Effectiveness of Natural Compounds on DNA Damage in Coris julis (Linneaus 1758) from a Polluted Marine Area. Water Air Soil Pollut. 2017, 228, 228. [Google Scholar] [CrossRef]
- Acquaviva, R.; Sorrenti, V.; Santangelo, R.; Cardile, V.; Tomasello, B.; Malfa, G.; Vanella, L.; Amodeo, A.; Mastrojeni, S.; Pugliese, M.; et al. Effects of extract of Celtis aetnensis (Tornab.) Strobl twigs in human colon cancer cell cultures. Oncol. Rep. 2016, 36, 2298–2304. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tomasello, B.; Grasso, S.; Malfa, G.; Stella, S.; Favetta, M.; Renis, M. Double-Face Activity of Resveratrol in Voluntary Runners: Assessment of DNA Damage by Comet Assay. J. Med. Food 2012, 15, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Anfuso, C.D.; Olivieri, M.; Fidilio, A.; Lupo, G.; Rusciano, D.; Pezzino, S.; Gagliano, C.; Drago, F.; Bucolo, C. Gabapentin Attenuates Ocular Inflammation: In vitro and In vivo Studies. Front. Pharmacol. 2017, 8, 173. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pereira, C.; Pimentel, F.B.; Alves, R.C.; Ferreira, M.; Sarmento, B.; Amaral, M.H.; Oliveira, M.B.P.P. Are coffee silverskin extracts safe for topical use? An in vitro and in vivo approach. Ind Crop. Prod. 2015, 63, 167–174. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press: New York, NY, USA, 2005. [Google Scholar]
- Freundlich, H.M. Over the Adsorption in Solution. J. Phys. Chem. A 1906, 57, 385–470. [Google Scholar]
- Calisto, V.; Ferreira, C.I.; Oliveira, J.A.; Otero, M.; Esteves, V.I. Adsorptive removal of pharmaceuticals from water by commercial and waste-based carbons. J. Environ. Manag. 2015, 152, 83–90. [Google Scholar] [CrossRef]
- McCabe, W.L.; Smith, J.C.; Harriot, P. Unit Operations of Chemical Engineering, 7th ed.; Chemical Engineering Series; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Wilson, S.L.; Ahearne, M.; Hopkinson, A. An overview of current techniques for ocular toxicity testing. Toxicology 2015, 327, 32–46. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, M.; Cristaldi, M.; Pezzino, S.; Rusciano, D.; Tomasello, B.; Anfuso, C.D.; Lupo, G. Phenotypic characterization of the SIRC (Statens Seruminstitut Rabbit Cornea) cell line reveals a mixed epithelial and fibroblastic nature. Exp. Eye Res. 2018, 172, 123–127. [Google Scholar] [CrossRef]
- Cristaldi, M.; Olivieri, M.; Lupo, G.; Anfuso, C.D.; Pezzino, S.; Rusciano, D. N-hydroxymethylglycinate with EDTA is an efficient eye drop preservative with very low toxicity: An in vitro comparative study. Cutan Ocul. Toxicol. 2018, 37, 71–76. [Google Scholar] [CrossRef]
- Wroblewska, K.; Kucinska, M.; Murias, M.; Lulek, J. Characterization of new eye drops with choline salicylate and assessment of their irritancy by in vitro short time exposure tests. Saudi Pharm J. 2015, 23, 407–412. [Google Scholar] [CrossRef]
- Tomasello, B.; Malfa, G.; Galvano, F.; Renis, M. DNA damage in normal-weight obese syndrome measured by Comet assay. Mediterr. J. Nutr. Metab. 2011, 4, 99–104. [Google Scholar] [CrossRef]
- Pu, X.; Wang, Z.; Klaunig, J.E. Alkaline Comet Assay for Assessing DNA Damage in Individual Cells. Curr. Protoc. Toxicol. 2015, 65, 1–11. [Google Scholar]
- Rapisarda, V.; Loreto, C.; Ledda, C.; Musumeci, G.; Bracci, M.; Santarelli, L.; Renis, M.; Ferrante, M.; Cardile, V. Cytotoxicity, oxidative stress and genotoxicity induced by glass fibers on human alveolar epithelial cell line A549. Toxicol. In Vitro 2015, 29, 551–557. [Google Scholar] [CrossRef]
- Muscoli, C.; Fresta, M.; Cardile, V.; Palumbo, M.; Renis, M.; Puglisi, G.; Paolino, D.; Nisticò, S.; Rotiroti, D.; Mollace, V. Ethanol-induced injury in rat primary cortical astrocytes involves oxidative stress: Effect of idebenone. Neurosci. Lett. 2002, 329, 21–24. [Google Scholar] [CrossRef]
- Schlupp, P.; Schmidts, T.M.; Pössl, A.; Wildenhain, S.; Lo Franco, G.; Lo Franco, A.; Lo Franco, B. Effects of a Phenol-Enriched Purified Extract from Olive Mill Wastewater on Skin Cells. Cosmetics 2019, 6, 30. [Google Scholar] [CrossRef]
- Schaffer, S.; Podstawa, M.; Visioli, F.; Bogani, P.; Müller, W.E.; Eckert, G.P. Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. J. Agric. Food Chem. 2007, 55, 5043–5049. [Google Scholar] [CrossRef]
- Baci, D.; Gallazzi, M.; Cascini, C.; Tramacere, M.; De Stefano, D.; Bruno, A.; Noonan, D.M.; Albini, A. Downregulation of Pro-Inflammatory and Pro-Angiogenic Pathways in Prostate Cancer Cells by a Polyphenol-Rich Extract from Olive Mill Wastewater. Int. J. Mol. Sci. 2019, 20, 307. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Almeida, H.; Amaral, M.H.; Lobão, P.; Sousa Lobo, J.M. In situ gelling systems: A strategy to improve the bioavailability of ophthalmic pharmaceutical formulations. Drug Discov. Today 2014, 19, 400–412. [Google Scholar] [CrossRef]
- Zaki, R.; Hosny, K.M.; Khames, A.; Abd-elbary, A. Ketorolac tromethamine in-situ ocular hydrogel: Preparation, characterization and in vivo evaluation. Int. J. Drug Del. 2011, 3, 535–545. [Google Scholar]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]











| Ingredients | Composition (%) |
|---|---|
| PAD428-FR2 | 0.01 |
| Carbopol®980 | 0.20 |
| Pemulen™RT1-NF | 0.01 |
| EDTA | 0.02 |
| Glycerol | 1.15 |
| Na2HPO4·12 H2O | 0.25 |
| NaOH | 0.07 |
| Purified water | Up to 100 |
| Characterization | OMWW | PAD428-FR1 | PAD900-FR1 | PAD428-FR2 | PAD900-FR2 |
|---|---|---|---|---|---|
| Total Nitrogen (mg/L) | 350.0 ± 3.4 | 318.0 ± 3.1 | 320.0 ± 3.2 | 1.0 ± 0.2 | 1.0 ± 0.2 |
| Total Phosphorous (mg/L) | 186.0 ± 2.1 | 144.0 ± 2.3 | 149.0 ± 2.1 | 0.5 ± 0.01 | 0.5 ± 0.01 |
| COD (g/L) | 73.65 ± 1.43 | 44.80 ± 1.34 | 44.02 ± 1.55 | 20.42 ± 1.25 | 21.07 ± 1.29 |
| BOD5 (g/L) | 38.44 ± 2.12 | 26.42 ± 2.05 | 26.40 ± 2.19 | 15.60 ± 1.99 | 15.33 ± 1.92 |
| Total Polyphenols (g/L) | 5.20 ± 0.14 | 0.40 ± 0.04 | 0.38 ± 0.04 | 4.12 ± 0.11 | 3.94 ± 0.12 |
| Hydroxytyrosol (g/L) | 1.10 ± 0.08 | - | - | 0.90 ± 0.06 | 0.85 ± 0.06 |
| Tyrosol (g/L) | 0.14 ± 0.02 | - | - | 0.10 ± 0.02 | 0.08 ± 0.01 |
| Total Sugar (g/L) | 34.00 ± 2.18 | 24.22 ± 1.14 | 23.75 ± 2.09 | 4.70 ± 0.59 | 4.82 ± 0.32 |
| Freundlich Isotherm | Langmuir Isotherm | ||||
|---|---|---|---|---|---|
| R2 | N | KF | R2 | Q0 | KL |
| ((mg/g)(L/mg) 1/n) | L/mg | ||||
| 0.962 | 2.0 | 1.0 | 0.993 | 47.7 | 0.02 |
| Months | ||||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Hydroxytyrosol (%) | 100 | 99 | 98 | 98 |
| pH | 6.7 ± 0.1 | 6.7 ± 0.1 | 6.6 ± 0.1 | 6.7 ± 0.1 |
| Osmolality | 150.0 ± 1.0 | 153.0 ± 1.0 | 155.0 ± 1.0 | 155.0 ± 1.0 |
| Appearance | transparent | transparent | transparent | transparent |
| Characterization | OMWW | PAD428-FR1 | PAD428-FR2 |
|---|---|---|---|
| Total Nitrogen (mg/L) | 350.0 ± 3.4 | 204 ± 1.6 | 1.0 ± 0.2 |
| Total Phosphorous (mg/L) | 186.0 ± 2.1 | 97 ± 1.4 | 0.5 ± 0.1 |
| COD (g/L) | 73.65 ± 1.43 | 50.60 ± 1.93 | 20.42 ± 1.64 |
| BOD5 (g/L) | 38.44 ± 2.12 | 19.82 ± 2.24 | 15.60 ± 1.45 |
| Total Polyphenols (g/L) | 5.20 ± 0.14 | 2.10 ± 0.09 | 1.12 ± 0.08 |
| Hydroxytyrosol (g/L) | 1.10 ± 0.08 | not detected | 0.45 ± 0.07 |
| Tyrosol (g/L) | 0.14 ± 0.02 | not detected | 0.06 ± 0.01 |
| Total Sugar (g/L) | 34.00 ± 2.18 | 23.50 ± 3.11 | 3.55 ± 3.38 |
| Cost Item | Quantity (Units) | Unit Cost (€) | Total Item Cost (€) |
|---|---|---|---|
| Adsorbent (kg) | 32 | 38.00 | 1216.00 |
| Ethanol (L) | 500 | 1.80 | 900.00 |
| Manpower (hours) | 2 | 25.00 | 50.00 |
| Wastewater treatment (m3) | 2 | 0.80 | 1.60 |
| Energy (kWh) | 15 | 0.10 | 1.50 |
| Mains water (m3) | 2 | 0.30 | 0.60 |
| Total 10-cycles cost | 2169.70 | ||
| Average polyphenolic extract production for 10 cycles (kg) | 2 | ||
| Estimated cost per kg of extract (€/kg) | 1084.85 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mauro, M.D.; Fava, G.; Spampinato, M.; Aleo, D.; Melilli, B.; Saita, M.G.; Centonze, G.; Maggiore, R.; D’Antona, N. Polyphenolic Fraction from Olive Mill Wastewater: Scale-Up and in Vitro Studies for Ophthalmic Nutraceutical Applications. Antioxidants 2019, 8, 462. https://doi.org/10.3390/antiox8100462
Di Mauro MD, Fava G, Spampinato M, Aleo D, Melilli B, Saita MG, Centonze G, Maggiore R, D’Antona N. Polyphenolic Fraction from Olive Mill Wastewater: Scale-Up and in Vitro Studies for Ophthalmic Nutraceutical Applications. Antioxidants. 2019; 8(10):462. https://doi.org/10.3390/antiox8100462
Chicago/Turabian StyleDi Mauro, Maria Domenica, Giovanni Fava, Marcella Spampinato, Danilo Aleo, Barbara Melilli, Maria Grazia Saita, Giovanni Centonze, Riccardo Maggiore, and Nicola D’Antona. 2019. "Polyphenolic Fraction from Olive Mill Wastewater: Scale-Up and in Vitro Studies for Ophthalmic Nutraceutical Applications" Antioxidants 8, no. 10: 462. https://doi.org/10.3390/antiox8100462
APA StyleDi Mauro, M. D., Fava, G., Spampinato, M., Aleo, D., Melilli, B., Saita, M. G., Centonze, G., Maggiore, R., & D’Antona, N. (2019). Polyphenolic Fraction from Olive Mill Wastewater: Scale-Up and in Vitro Studies for Ophthalmic Nutraceutical Applications. Antioxidants, 8(10), 462. https://doi.org/10.3390/antiox8100462





