Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain
Abstract
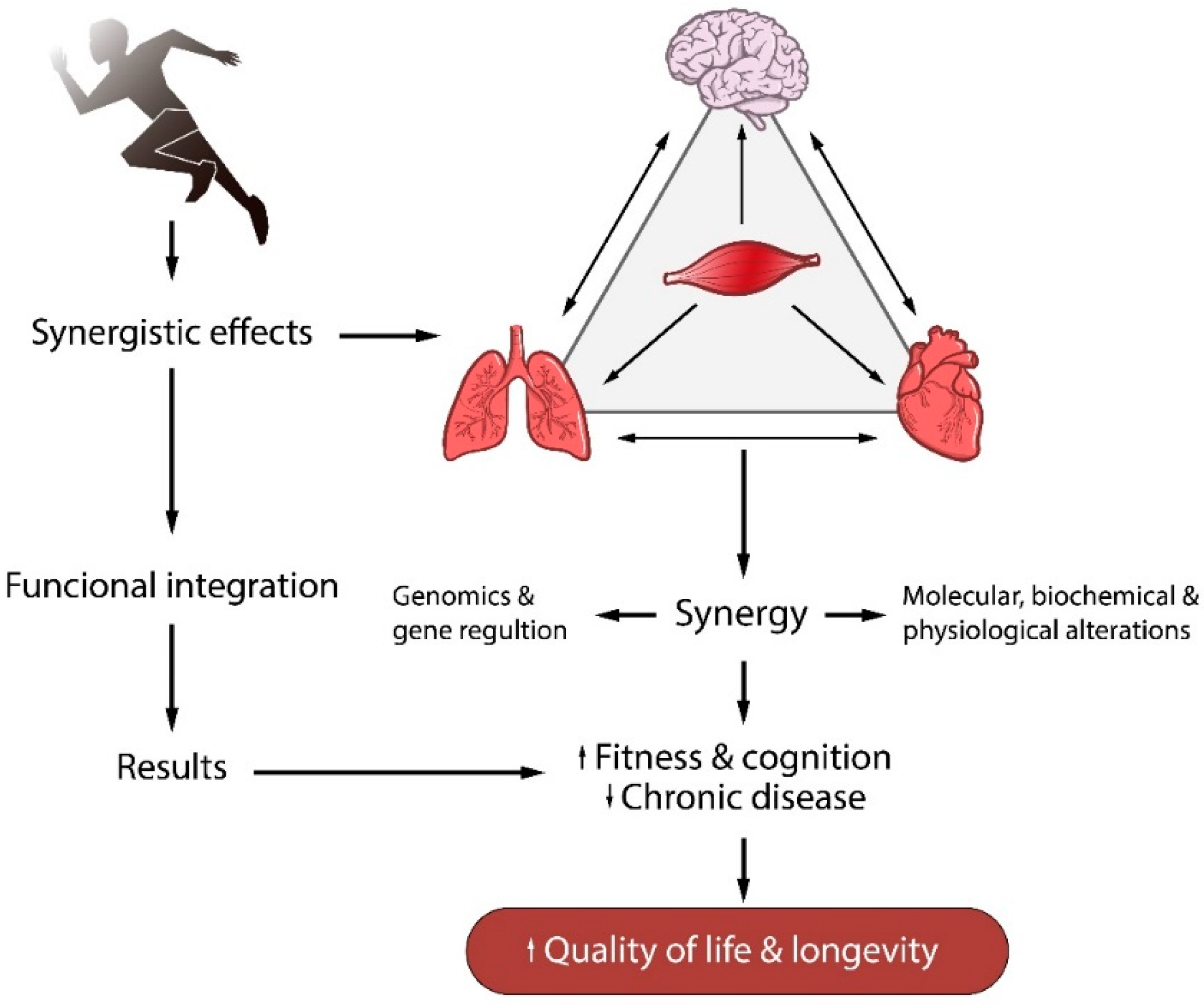
:1. Introduction
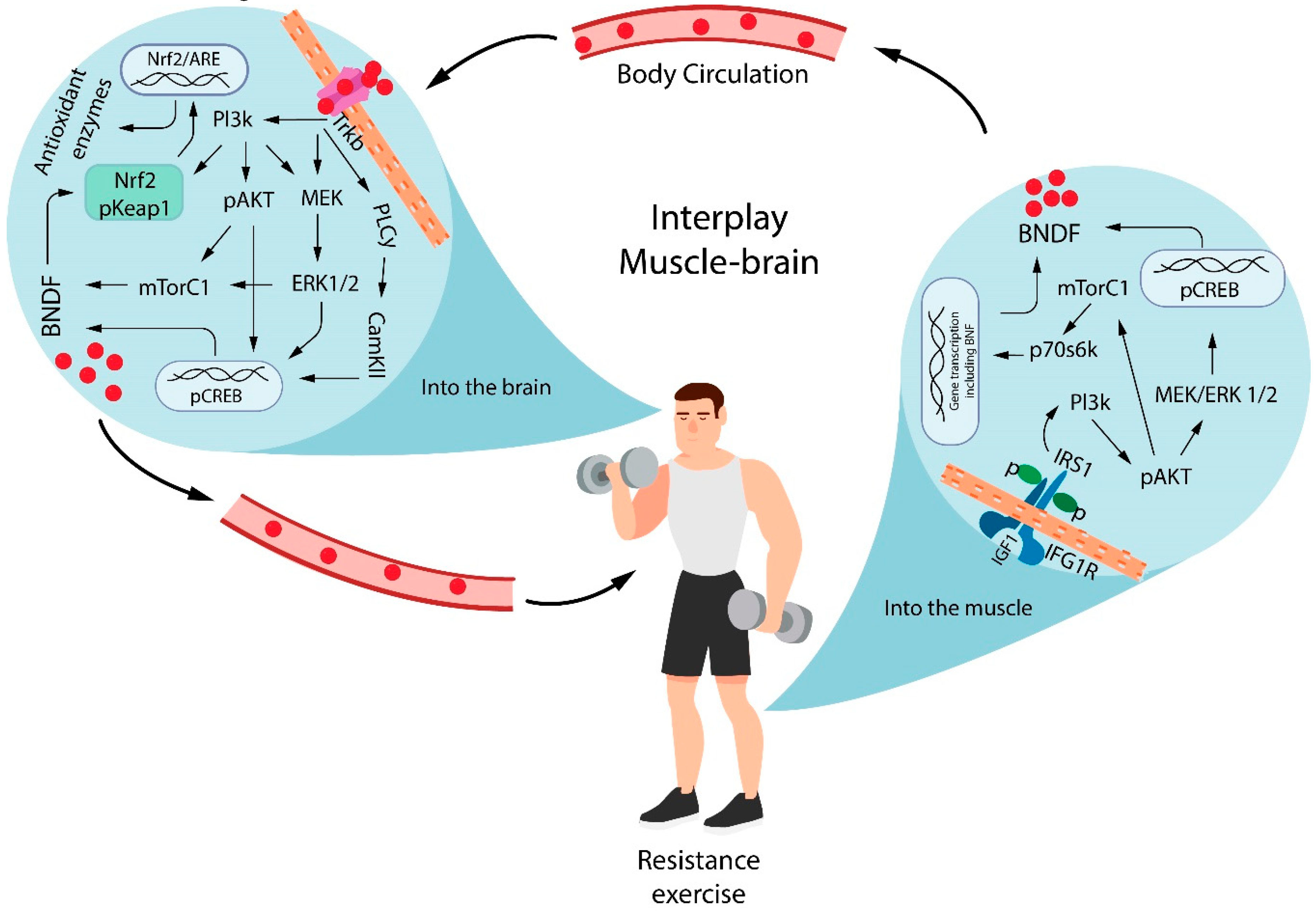
2. Mechanism of Resistance Exercise-Induced Neuroprotection: The Role of BDNF
3. Oxidative Stress in the Brain and Resistance Exercise
4. Resistance Exercise and Cognition
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantoani, L.C.; Dell’Era, S.; MacNee, W.; Rabinovich, R.A. Physical activity in patients with COPD: The impact of comorbidities. Expert Rev. Respir. Med. 2017, 11, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Febbraio, M.A. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014, 35, 262–269. [Google Scholar] [CrossRef]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2009, 39, 3–11. [Google Scholar] [CrossRef]
- Radak, Z.; Suzuki, K.; Higuchi, M.; Balogh, L.; Boldogh, I.; Koltai, E. Physical exercise, reactive oxygen species and neuroprotection. Free Radic. Biol. Med. 2016, 98, 187–196. [Google Scholar] [CrossRef]
- Souza, P.S.; Gonçalves, E.D.; Pedroso, G.S.; Farias, H.R.; Junqueira, S.C.; Marcon, R.; Tuon, T.; Cola, M.; Silveira, P.C.L.; Santos, A.R.; et al. Physical Exercise Attenuates Experimental Autoimmune Encephalomyelitis by Inhibiting Peripheral Immune Response and Blood-Brain Barrier Disruption. Mol. Neurobiol. 2017, 54, 4723–4737. [Google Scholar] [CrossRef]
- Tuon, T.; Souza, P.S.; Santos, M.F.; Pereira, F.T.; Pedroso, G.S.; Luciano, T.F.; De Souza, C.T.; Dutra, R.C.; Silveira, P.C.L.; Pinho, R.A. Physical Training Regulates Mitochondrial Parameters and Neuroinflammatory Mechanisms in an Experimental Model of Parkinson’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 261809. [Google Scholar] [CrossRef]
- Tuon, T.; Valvassori, S.S.; Dal Pont, G.C.; Paganini, C.S.; Pozzi, B.G.; Luciano, T.F.; Souza, P.S.; Quevedo, J.; Souza, C.T.; Pinho, R.A. Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res. Bull. 2014, 108, 106–112. [Google Scholar] [CrossRef]
- Jensen, C.S.; Bahl, J.M.; Østergaard, L.B.; Høgh, P.; Wermuth, L.; Heslegrave, A.; Zetterberg, H.; Heegaard, N.H.H.; Hasselbalch, S.G.; Simonsen, A.H. Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma. Exp. Gerontol. 2019, 121, 91–98. [Google Scholar] [CrossRef]
- Radak, Z.; Ihasz, F.; Koltai, E.; Goto, S.; Taylor, A.W.; Boldogh, I. The redox-associated adaptive response of brain to physical exercise. Free Radic. Res. 2014, 48, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [PubMed]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Secher, N.H.; Seifert, T.; Van Lieshout, J.J. Cerebral blood flow and metabolism during exercise: Implications for fatigue. J. Appl. Physiol. 2008, 104, 306–314. [Google Scholar] [CrossRef]
- Varendi, K.; Mätlik, K.; Andressoo, J.-O. From microRNA target validation to therapy: Lessons learned from studies on BDNF. Cell. Mol. Life Sci. 2015, 72, 1779–1794. [Google Scholar] [CrossRef]
- Mandel, A.L.; Ozdener, H.; Utermohlen, V. Identification of pro- and mature brain-derived neurotrophic factor in human saliva. Arch. Oral Biol. 2009, 54, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Halievski, K.; Nath, S.; Katsuno, M.; Adachi, H.; Sobue, G.; Breedlove, S.; Lieberman, A.; Jordan, C. Disease Affects Bdnf Expression in Synaptic and Extrasynaptic Regions of Skeletal Muscle of Three SBMA Mouse Models. Int. J. Mol. Sci. 2019, 20, 1314. [Google Scholar] [CrossRef]
- Omura, T.; Sano, M.; Omura, K.; Hasegawa, T.; Doi, M.; Sawada, T.; Nagano, A. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J. Peripher. Nerv. Syst. 2005, 10, 293–300. [Google Scholar] [CrossRef]
- Clow, C.; Jasmin, B.J. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol. Biol. Cell 2010, 21, 2182–2190. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.B.; Soderstrom, K.; Bakay, R.A.E.; Kordower, J.H. Neurotrophic factor therapy for Parkinson’s disease. Prog. Brain Res. 2010, 184, 237–264. [Google Scholar] [PubMed]
- Wang, Z.-H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.-Z.; Ye, K. Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates δ-Secretase by Upregulating C/EBPβ in Alzheimer’s Disease. Cell Rep. 2019, 28, 655–669.e5. [Google Scholar] [CrossRef] [PubMed]
- Marais, L.; Stein, D.J.; Daniels, W.M.U. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab. Brain Dis. 2009, 24, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Tripanichkul, W.; Gerdprasert, O.; Jaroensuppaperch, E. Estrogen reduces BDNF level, but maintains dopaminergic cell density in the striatum of MPTP mouse model. Int. J. Neurosci. 2010, 120, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Lesemann, A.; Reinel, C.; Hühnchen, P.; Pilhatsch, M.; Hellweg, R.; Klaissle, P.; Winter, C.; Steiner, B. MPTP-induced hippocampal effects on serotonin, dopamine, neurotrophins, adult neurogenesis and depression-like behavior are partially influenced by fluoxetine in adult mice. Brain Res. 2012, 1457, 51–69. [Google Scholar] [CrossRef]
- Tuon, T.; Valvassori, S.S.; Lopes-Borges, J.; Luciano, T.; Trom, C.B.; Silva, L.A.; Quevedo, J.; Souza, C.T.; Lira, F.S.; Pinho, R.A. Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience 2012, 227, 305–312. [Google Scholar] [CrossRef]
- Ortiz-López, L.; Vega-Rivera, N.M.; Babu, H.; Ramírez-Rodríguez, G.B. Brain-Derived Neurotrophic Factor Induces Cell Survival and the Migration of Murine Adult Hippocampal Precursor Cells During Differentiation In Vitro. Neurotox. Res. 2017, 31, 122–135. [Google Scholar] [CrossRef]
- Burkhalter, J.; Fiumelli, H.; Allaman, I.; Chatton, J.-Y.; Martin, J.-L. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J. Neurosci. 2003, 23, 8212–8220. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef]
- Ames, A. CNS energy metabolism as related to function. Brain Res. Brain Res. Rev. 2000, 34, 42–68. [Google Scholar] [CrossRef]
- Attwell, D.; Laughlin, S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001, 21, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Sasi, M.; Vignoli, B.; Canossa, M.; Blum, R. Neurobiology of local and intercellular BDNF signaling. Pflugers Arch. 2017, 469, 593–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balkowiec, A.; Katz, D.M. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J. Neurosci. 2002, 22, 10399–10407. [Google Scholar] [CrossRef] [PubMed]
- Goekint, M.; De Pauw, K.; Roelands, B.; Njemini, R.; Bautmans, I.; Mets, T.; Meeusen, R. Strength training does not influence serum brain-derived neurotrophic factor. Eur. J. Appl. Physiol. 2010, 110, 285–293. [Google Scholar] [CrossRef]
- Forti, L.N.; Njemini, R.; Beyer, I.; Eelbode, E.; Meeusen, R.; Mets, T.; Bautmans, I. Strength training reduces circulating interleukin-6 but not brain-derived neurotrophic factor in community-dwelling elderly individuals. Age 2014, 36, 9704. [Google Scholar] [CrossRef]
- Hvid, L.G.; Nielsen, M.K.F.; Simonsen, C.; Andersen, M.; Caserotti, P. Brain-derived neurotrophic factor (BDNF) serum basal levels is not affected by power training in mobility-limited older adults—A randomized controlled trial. Exp. Gerontol. 2017, 93, 29–35. [Google Scholar] [CrossRef]
- Yarrow, J.F.; White, L.J.; McCoy, S.C.; Borst, S.E. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci. Lett. 2010, 479, 161–165. [Google Scholar] [CrossRef]
- Coelho, F.M.; Pereira, D.S.; Lustosa, L.P.; Silva, J.P.; Dias, J.M.D.; Dias, R.C.D.; Queiroz, B.Z.; Teixeira, A.L.; Teixeira, M.M.; Pereira, L.S.M. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch. Gerontol. Geriatr. 2012, 54, 415–420. [Google Scholar] [CrossRef]
- Kim, H.; Song, B.; So, B.; Lee, O.; Song, W.; Kim, Y. Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: A pilot study. Psychiatry Res. 2014, 220, 792–796. [Google Scholar] [CrossRef]
- Walsh, J.J.; Scribbans, T.D.; Bentley, R.F.; Kellawan, J.M.; Gurd, B.; Tschakovsky, M.E. Neurotrophic growth factor responses to lower body resistance training in older adults. Appl. Physiol. Nutr. Metab. 2016, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hoffman, J.R.; Mangine, G.T.; Jajtner, A.R.; Townsend, J.R.; Beyer, K.S.; Wang, R.; La Monica, M.B.; Fukuda, D.H.; Stout, J.R. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. 2016, 121, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marston, K.J.; Newton, M.J.; Brown, B.M.; Rainey-Smith, S.R.; Bird, S.; Martins, R.N.; Peiffer, J.J. Intense resistance exercise increases peripheral brain-derived neurotrophic factor. J. Sci. Med. Sport 2017, 20, 899–903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forti, L.N.; Van Roie, E.; Njemini, R.; Coudyzer, W.; Beyer, I.; Delecluse, C.; Bautmans, I. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Exp. Gerontol. 2015, 70, 144–149. [Google Scholar] [CrossRef]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Seifert, T.; Brassard, P.; Wissenberg, M.; Rasmussen, P.; Nordby, P.; Stallknecht, B.; Adser, H.; Jakobsen, A.H.; Pilegaard, H.; Nielsen, H.B.; et al. Endurance training enhances BDNF release from the human brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R372–R377. [Google Scholar] [CrossRef] [Green Version]
- Novaes Gomes, F.G.; Fernandes, J.; Vannucci Campos, D.; Cassilhas, R.C.; Viana, G.M.; D’Almeida, V.; de Moraes Rêgo, M.K.; Buainain, P.I.; Cavalheiro, E.A.; Arida, R.M. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology 2014, 50, 106–117. [Google Scholar] [CrossRef]
- Vilela, T.C.; Muller, A.P.; Damiani, A.P.; Macan, T.P.; da Silva, S.; Canteiro, P.B.; de Sena Casagrande, A.; Pedroso, G.D.S.; Nesi, R.T.; de Andrade, V.M.; et al. Strength and Aerobic Exercises Improve Spatial Memory in Aging Rats Through Stimulating Distinct Neuroplasticity Mechanisms. Mol. Neurobiol. 2017, 54, 7928–7937. [Google Scholar] [CrossRef]
- Hwang, O. Role of Oxidative Stress in Parkinson’s Disease. Exp. Neurobiol. 2013, 22, 11. [Google Scholar] [CrossRef]
- Crichton, R.R.; Dexter, D.T.; Ward, R.J. Brain iron metabolism and its perturbation in neurological diseases. J. Neural Transm. 2011, 118, 301–314. [Google Scholar] [CrossRef]
- Lan, A.P.; Chen, J.; Chai, Z.F.; Hu, Y. The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. BioMetals 2016, 29, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Sian-Hülsmann, J.; Mandel, S.; Youdim, M.B.H.; Riederer, P. The relevance of iron in the pathogenesis of Parkinson’s disease. J. Neurochem. 2011, 118, 939–957. [Google Scholar] [CrossRef] [PubMed]
- Siamilis, S.; Jakus, J.; Nyakas, C.; Costa, A.; Mihalik, B.; Falus, A.; Radak, Z. The effect of exercise and oxidant–antioxidant intervention on the levels of neurotrophins and free radicals in spinal cord of rats. Spinal Cord 2009, 47, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Rasmussen, P.; Evans, K.A.; Bohm, A.M.; Zaar, M.; Nielsen, H.B.; Brassard, P.; Nordsborg, N.B.; Homann, P.H.; Raven, P.B.; et al. Hypoxia compounds exercise-induced free radical formation in humans; partitioning contributions from the cerebral and femoral circulation. Free Radic. Biol. Med. 2018, 124, 104–113. [Google Scholar] [CrossRef]
- Iofrida, C.; Daniele, S.; Pietrobono, D.; Fusi, J.; Galetta, F.; Trincavelli, M.L.; Bonuccelli, U.; Franzoni, F.; Martini, C. Influence of physical exercise on β-amyloid, α-synuclein and tau accumulation: an in vitro model of oxidative stress in human red blood cells. Arch. Ital. Biol. 2017, 155, 33–42. [Google Scholar]
- Roh, H.-T.; Cho, S.-Y.; Yoon, H.-G.; So, W.-Y. Effect of Exercise Intensity on Neurotrophic Factors and Blood-Brain Barrier Permeability Induced by Oxidative-Nitrosative Stress in Male College Students. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 239–246. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Q.; Jiang, H.; Du, J.; Zhou, C.; Yu, S.; Hashimoto, K.; Zhao, M. Impact of aerobic exercise on cognitive impairment and oxidative stress markers in methamphetamine-dependent patients. Psychiatry Res. 2018, 266, 328–333. [Google Scholar] [CrossRef]
- Um, H.S.; Kang, E.B.; Leem, Y.H.; Cho, I.H.; Yang, C.H.; Chae, K.R.; Hwang, D.Y.; Cho, J.Y. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int. J. Mol. Med. 2008, 22, 529–539. [Google Scholar]
- Speck, A.E.; Tromm, C.B.; Pozzi, B.G.; Paganini, C.S.; Tuon, T.; Silveira, P.C.L.; Aguiar, A.S.; Pinho, R.A. The dose-dependent antioxidant effects of physical exercise in the hippocampus of mice. Neurochem. Res. 2014, 39, 1496–1501. [Google Scholar] [CrossRef]
- Tuon, T.; Valvassori, S.S.; Lopes-Borges, J.; Fries, G.R.; Silva, L.A.; Kapczinski, F.; Quevedo, J.; Pinho, R.A. Effects of moderate exercise on cigarette smoke exposure-induced hippocampal oxidative stress values and neurological behaviors in mice. Neurosci. Lett. 2010, 475, 16–19. [Google Scholar] [CrossRef]
- Aguiar, A.S.; Tuon, T.; Pinho, C.A.; Silva, L.A.; Andreazza, A.C.; Kapczinski, F.; Quevedo, J.; Streck, E.L.; Pinho, R.A. Mitochondrial IV complex and brain neurothrophic derived factor responses of mice brain cortex after downhill training. Neurosci. Lett. 2007, 426, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.O.; Gadelha-Filho, C.V.J.; da Costa, A.E.M.; Feitosa, M.L.; de Araújo, D.P.; de Lucena, J.D.; de Aquino, P.E.A.; Lima, F.A.V.; Neves, K.R.T.; de Barros Viana, G.S. The Treadmill Exercise Protects against Dopaminergic Neuron Loss and Brain Oxidative Stress in Parkinsonian Rats. Oxid. Med. Cell. Longev. 2017, 2017, 2138169. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Z.; Ying, Z.; Radak, Z.; Gomez-Pinilla, F. Voluntary exercise may engage proteasome function to benefit the brain after trauma. Brain Res. 2010, 1341, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marosi, K.; Bori, Z.; Hart, N.; Sárga, L.; Koltai, E.; Radák, Z.; Nyakas, C. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience 2012, 226, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Radák, Z.; Silye, G.; Bartha, C.; Jakus, J.; Stefanovits-Bányai, E.; Atalay, M.; Marton, O.; Koltai, E. The effects of cocoa supplementation, caloric restriction, and regular exercise, on oxidative stress markers of brain and memory in the rat model. Food Chem. Toxicol. 2013, 61, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiar, A.S.; Duzzioni, M.; Remor, A.P.; Tristão, F.S.M.; Matheus, F.C.; Raisman-Vozari, R.; Latini, A.; Prediger, R.D. Moderate-Intensity Physical Exercise Protects Against Experimental 6-Hydroxydopamine-Induced Hemiparkinsonism Through Nrf2-Antioxidant Response Element Pathway. Neurochem. Res. 2016, 41, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P.C.O.; Quines, C.B.; Jardim, N.S.; Leite, M.R.; Nogueira, C.W. Resistance exercise reduces memory impairment induced by monosodium glutamate in male and female rats. Exp. Physiol. 2017, 102, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Özbeyli, D.; Sarı, G.; Özkan, N.; Karademir, B.; Yüksel, M.; Çilingir Kaya, Ö.T.; Kasımay Çakır, Ö. Protective effects of different exercise modalities in an Alzheimer’s disease-like model. Behav. Brain Res. 2017, 328, 159–177. [Google Scholar] [CrossRef]
- De Almeida, A.A.; Gomes da Silva, S.; Lopim, G.M.; Vannucci Campos, D.; Fernandes, J.; Cabral, F.R.; Arida, R.M. Resistance Exercise Reduces Seizure Occurrence, Attenuates Memory Deficits and Restores BDNF Signaling in Rats with Chronic Epilepsy. Neurochem. Res. 2017, 42, 1230–1239. [Google Scholar] [CrossRef]
- Henrique, J.S.; França, E.F.; Cardoso, F.D.S.; Serra, F.T.; de Almeida, A.A.; Fernandes, J.; Arida, R.M.; Gomes da Silva, S. Cortical and hippocampal expression of inflammatory and intracellular signaling proteins in aged rats submitted to aerobic and resistance physical training. Exp. Gerontol. 2018, 110, 284–290. [Google Scholar] [CrossRef]
- Park, S.H.; Yoon, J.H.; Seo, D.Y.; Kim, T.N.; Ko, J.R.; Han, J. Resistance Exercise Training Attenuates the Loss of Endogenous GLP-1 Receptor in the Hypothalamus of Type 2 Diabetic Rats. Int. J. Environ. Res. Public Health 2019, 16, 830. [Google Scholar] [CrossRef] [PubMed]
- Farzi, M.A.; Sadigh-Eteghad, S.; Ebrahimi, K.; Talebi, M. Exercise Improves Recognition Memory and Acetylcholinesterase Activity in the Beta Amyloid-Induced Rat Model of Alzheimer’s Disease. Ann. Neurosci. 2019, 25, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Shin, S.K.; Hong, S.B.; Kim, H.J. The effects of strength exercise on hippocampus volume and functional fitness of older women. Exp. Gerontol. 2017, 97, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.S.; Conrado de Freitas, M.; Gerosa-Neto, J.; Cholewa, J.M.; Rossi, F.E. Comparison Between Full-Body vs. Split-Body Resistance Exercise on the Brain-Derived Neurotrophic Factor and Immunometabolic Response. J. Strength Cond. Res. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Sanchéz, M.A.; Bustos-Cruz, R.H.; Velasco-Orjuela, G.P.; Quintero, A.P.; Tordecilla-Sanders, A.; Correa-Bautista, J.E.; Triana-Reina, H.R.; García-Hermoso, A.; González-Ruíz, K.; Peña-Guzmán, C.A.; et al. Acute Effects of High Intensity, Resistance, or Combined Protocol on the Increase of Level of Neurotrophic Factors in Physically Inactive Overweight Adults: The BrainFit Study. Front. Physiol. 2018, 9, 741. [Google Scholar] [CrossRef] [Green Version]
- Goldfield, G.S.; Kenny, G.P.; Prud’homme, D.; Holcik, M.; Alberga, A.S.; Fahnestock, M.; Cameron, J.D.; Doucette, S.; Hadjiyannakis, S.; Tulloch, H.; et al. Effects of aerobic training, resistance training, or both on brain-derived neurotrophic factor in adolescents with obesity: The hearty randomized controlled trial. Physiol. Behav. 2018, 191, 138–145. [Google Scholar] [CrossRef]
- Lan, Y.; Huang, Z.; Jiang, Y.; Zhou, X.; Zhang, J.; Zhang, D.; Wang, B.; Hou, G. Strength exercise weakens aerobic exercise-induced cognitive improvements in rats. PLoS ONE 2018, 13, e0205562. [Google Scholar] [CrossRef]
- LiCausi, F.; Hartman, N. Role of mTOR Complexes in Neurogenesis. Int. J. Mol. Sci. 2018, 19, 1544. [Google Scholar] [CrossRef]
- Sakamoto, K.; Karelina, K.; Obrietan, K. CREB: A multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011, 116, 1–9. [Google Scholar] [CrossRef]
- Ying, Z.; Roy, R.R.; Zhong, H.; Zdunowski, S.; Edgerton, V.R.; Gomez-Pinilla, F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience 2008, 155, 1070–1078. [Google Scholar] [CrossRef]
- Seki, K.; Yoshida, S.; Jaiswal, M.K. Molecular mechanism of noradrenaline during the stress-induced major depressive disorder. Neural Regen. Res. 2018, 13, 1159–1169. [Google Scholar] [PubMed]
- Browne, C.A.; Lucki, I. Antidepressant effects of ketamine: Mechanisms underlying fast-acting novel antidepressants. Front. Pharmacol. 2013, 4, 161. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, B.A.; Hake, H.S.; Ishiwata, T.; Farmer, C.E.; Loetz, E.C.; Fleshner, M.; Bland, S.T.; Greenwood, B.N. Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior. Behav. Brain Res. 2017, 323, 56–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taheri, P.; Keshavarzi, S.; Ebadi, M.; Motaghinejad, M.; Motevalian, M. Neuroprotective Effects of Forced Exercise and Bupropion on Chronic Methamphetamine-induced Cognitive Impairment via Modulation of cAMP Response Element-binding Protein/Brain-derived Neurotrophic Factor Signaling Pathway, Oxidative Stress, and Inflammatory Biomarkers in Rats. Adv. Biomed. Res. 2018, 7, 151. [Google Scholar] [PubMed]
- Ishii, T.; Mann, G. When and how does brain-derived neurotrophic factor activate Nrf2 in astrocytes and neurons? Neural Regen. Res. 2018, 13, 803. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Hur, W.; Gray, N.S. Small molecule modulators of antioxidant response pathway. Curr. Opin. Chem. Biol. 2011, 15, 162–173. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Lachman, M.E.; Neupert, S.D.; Bertrand, R.; Jette, A.M. The effects of strength training on memory in older adults. J. Aging Phys. Act. 2006, 14, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Gates, N.J.; Valenzuela, M.; Sachdev, P.S.; Singh, N.A.; Baune, B.T.; Brodaty, H.; Suo, C.; Jain, N.; Wilson, G.C.; Wang, Y.; et al. Study of Mental Activity and Regular Training (SMART) in at risk individuals: A randomised double blind, sham controlled, longitudinal trial. BMC Geriatr. 2011, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Mavros, Y.; Gates, N.; Wilson, G.C.; Jain, N.; Meiklejohn, J.; Brodaty, H.; Wen, W.; Singh, N.; Baune, B.T.; Suo, C.; et al. Mediation of Cognitive Function Improvements by Strength Gains After Resistance Training in Older Adults with Mild Cognitive Impairment: Outcomes of the Study of Mental and Resistance Training. J. Am. Geriatr. Soc. 2017, 65, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Liu-Ambrose, T.; Donaldson, M.G.; Ahamed, Y.; Graf, P.; Cook, W.L.; Close, J.; Lord, S.R.; Khan, K.M. Otago home-based strength and balance retraining improves executive functioning in older fallers: A randomized controlled trial. J. Am. Geriatr. Soc. 2008, 56, 1821–1830. [Google Scholar] [CrossRef]
- Best, J.R.; Chiu, B.K.; Liang Hsu, C.; Nagamatsu, L.S.; Liu-Ambrose, T. Long-Term Effects of Resistance Exercise Training on Cognition and Brain Volume in Older Women: Results from a Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2015, 21, 745–756. [Google Scholar] [CrossRef]
- Yoon, D.H.; Lee, J.-Y.; Song, W. Effects of Resistance Exercise Training on Cognitive Function and Physical Performance in Cognitive Frailty: A Randomized Controlled Trial. J. Nutr. Health Aging 2018, 22, 944–951. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Lee, K.S.; Fernandes, J.; Oliveira, M.G.M.; Tufik, S.; Meeusen, R.; de Mello, M.T. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 2012, 202, 309–317. [Google Scholar] [CrossRef]
- Kelley, B.J.; Petersen, R.C. Alzheimer’s disease and mild cognitive impairment. Neurol. Clin. 2007, 25, 577–609. [Google Scholar] [CrossRef]
- Yavas, E.; Gonzalez, S.; Fanselow, M.S. Interactions between the hippocampus, prefrontal cortex, and amygdala support complex learning and memory. F1000Research 2019, 8, 1292. [Google Scholar] [CrossRef] [Green Version]
- Cuttler, C.; Connolly, C.P.; LaFrance, E.M.; Lowry, T.M. Resist forgetting: Effects of aerobic and resistance exercise on prospective and retrospective memory. Sport. Exerc. Perform. Psychol. 2018, 7, 205–217. [Google Scholar] [CrossRef]
- Gardner, M.M.; Buchner, D.M.; Robertson, M.C.; Campbell, A.J. Practical implementation of an exercise-based falls prevention programme. Age Ageing 2001, 30, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Park, D.C.; Reuter-Lorenz, P. The adaptive brain: Aging and neurocognitive scaffolding. Annu. Rev. Psychol. 2009, 60, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. Aging and measures of processing speed. Biol. Psychol. 2000, 54, 35–54. [Google Scholar] [CrossRef]

| Part A-Pre-Clinical Studies | ||||
| Aim | Species | Results | Year of Publication and Reference | |
| 1 | Investigate the influence of aerobic and resistance training on the Central Nervous System in an experimental animal model of multiple sclerosis. | Mouse | Although aerobic exercise showed more prominent effects, strength exercise also contributed to neuroprotective mechanisms by modulating inflammatory parameters and oxidative stress. | 2017, [7] |
| 2 | Investigate the effects of strength and aerobic training on mitochondrial and inflammatory parameters in an experimental animal model of Parkinson’s disease. | Mouse | Both training protocols induced neuroprotection by modulating mitochondrial function and cerebral inflammation parameters. | 2015, [8] |
| 3 | Investigate the effects of two types of physical training on depressive-like behavior, and levels of proBDNF, brain-derived neurotrophic factor (BDNF), TrkB, in a mouse model of Parkinson’s disease. | Rat | Both types of physical exercise prevented depressive-like behavior and restored levels of proBDNF, BDNF, and TrkB in the striatum and hippocampus. | 2014, [9] |
| 4 | Investigate the effects of the nandrolone decanoate during a strength exercise program on cell proliferation, apoptotic status, and BDNF expression in the rat hippocampus. | Rat | The increase in the immunoreactivity of anti-apoptotic protein Bcl-2 (DG and CA3) induced by strength exercise was diminished by nandrolone decanoate. | 2014, [47] |
| 5 | Investigate the effect of aerobic and resistance training on spatial memory and hippocampal plasticity in aging rats. | Rat | Both aerobic and strength training improved spatial memory by distinct molecular neuroplastic mechanisms. | 2017, [48] |
| 6 | Verify the effects of resistance exercise on memory and motor co-ordination in male and female rats treated with monosodium glutamate. | Rat | Resistance exercise reduced memory and motor co-ordination impairment caused by monosodium glutamate. | 2017, [67] |
| 7 | Investigate the effects of aerobic, resistance, and combined exercise on Alzheimer’s disease animal model. | Rat | All training models reduced disease oxidative stress scores, increased antioxidant activity, and improved brain plasticity. | 2017, [68] |
| 8 | Investigate the effects of resistance exercise on the number of seizures, long-term memory, and expression of signaling proteins in rats with epilepsy. | Rat | Resistance exercise reduced memory deficits in rats with epilepsy and increased Insulin-like growth factor 1 and BDNF levels, as well as signaling protein activation. | 2017, [69] |
| 9 | Investigate the expression of inflammatory cytokines and chemokines and signaling proteins in aged rats undertaking aerobic and resistance exercise. | Rat | No significant difference in cytokines or signaling proteins in the cortex and hippocampus of old rats in response to resistance training was seen. | 2018, [70] |
| 10 | Verify the effects of resistance exercise training on hypothalamic glucagon-like peptide 1 receptor (GLP-1R) levels and its related signaling mechanisms in type II diabetes (T2DM). | Rat | Resistance training increased GLP-1R mRNA, protein kinase A, glucose transporter 2, and AKT and significantly decreased PKC-iota). Antioxidant enzymes and apoptotic factors were significantly improved in the hypothalamus. | 2019, [71] |
| 11 | Investigate the effects of aerobic and resistance exercise on the recognition memory and acetylcholinesterase (AChE) activity in a beta-amyloid (Aβ) model of AD in rats. | Rat | Both aerobic and strength training improved the exploration index. AChE activity increased in the Aβ-injected sedentary group but declined in the aerobic and resistance exercise groups. | 2019, [72] |
| Part B-Clinical Studies | ||||
| 1 | Investigate the effects of acute resistance exercise to-fatigue on serum BDNF levels in adult men (serum). | Human | Resistance exercise provided the necessary stimulus to increase peripheral serum BDNF. | 2017, [43] |
| 2 | Identify the effects of strength training on hippocampus volume in older women. | Human | Hippocampus volume was significantly increased after strength exercise. | 2017, [73] |
| 3 | Compare full-body versus split-body resistance training on BDNF levels in adult men. | Human | Resistance exercise increased BDNF levels in the serum of adult men. | 2018, [74] |
| 4 | Compare the response of neurotrophic factors NT3, NT4, and BDNF following one session of high-intensity exercise, resistance training, or both, in physically inactive overweight adult men. | Human | Acute resistance training and combined exercise increased neurotrophic factors in physically inactive overweight adults. | 2018, [75] |
| 5 | Investigate the effects of aerobic, resistance, and combined training on resting serum BDNF levels in adolescents with overweight and obesity. | Human | All training models increased BDNF levels. | 2018, [76] |
| 6 | Verify the effects of exercise combined with low- and high-intensity strength exercise in the brain. | Human | Strength exercise weakened aerobic exercise-induced cognitive improvements and hippocampal neurogenesis. | 2018, [77] |
| Program | RCT | Outcome | Resistance Training (RT) | Year of Publication and Reference | |||
|---|---|---|---|---|---|---|---|
| Duration | Volume | Overload | Supervision | ||||
| Otago exercise program | Yes | Prevent fall | 6 mo 3 times/wk | 2 × 10 repetitions | Ankle cuffs | No | 2008, [95] |
| Strong for Life | Yes | Muscle strength | 6 mo 3 times/wk 25 min | Uninformed | Elastic bands | No | 2006, [92] |
| Muscle strengthening | Yes | Muscle strength | 52 wk 1–2 times/wk 40 min | 2 × 6–8 repetitions 7RM method | Pneumatic Free weights (dumbbells) | Yes | 2015, [96] |
| Study of Mental and Resistance Training (SMART) | Yes | Cognition | 6 mo 2 times/wk | 3 × 8 repetitions 80% 1RM 15–18 Borg scale | Pneumatic Free weights (dumbbells) | Yes | 2011, 2017, [93,94] |
| Muscle strengthening | No | Cognition | 16 wk 3 times/week 40 min | 2–3 × 12–15 repetitions | Elastic bands High speed | Yes | 2018, [97] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinho, R.A.; Aguiar, A.S., Jr.; Radák, Z. Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain. Antioxidants 2019, 8, 529. https://doi.org/10.3390/antiox8110529
Pinho RA, Aguiar AS Jr., Radák Z. Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain. Antioxidants. 2019; 8(11):529. https://doi.org/10.3390/antiox8110529
Chicago/Turabian StylePinho, Ricardo A., Aderbal S. Aguiar, Jr., and Zsolt Radák. 2019. "Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain" Antioxidants 8, no. 11: 529. https://doi.org/10.3390/antiox8110529
APA StylePinho, R. A., Aguiar, A. S., Jr., & Radák, Z. (2019). Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain. Antioxidants, 8(11), 529. https://doi.org/10.3390/antiox8110529








