Structure, Antioxidant, and Hypoglycemic Activities of Arabinoxylans Extracted by Multiple Methods from Triticale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AX Extraction
2.3. Monosaccharide Composition
2.4. MW Determination
2.5. Infrared Spectroscopic Analysis
2.6. Determination of Free or Esterified FA Content
2.7. Measurement of Hypoglycemic Activity of AXs
2.7.1. α-Amylase Inhibition Assay
2.7.2. Determination of Glucose Absorption Capacity
2.7.3. Glucose Dialysis Retardation Index (GDRI)
2.8. Estimation of Antioxidant Activity of AXs
2.8.1. Hydroxyl Radical-Scavenging Assay
2.8.2. 2,2-Diphenyl-1-picrylhydrazyl (DPPH)-Scavenging Assay
2.8.3. Reductive Ability
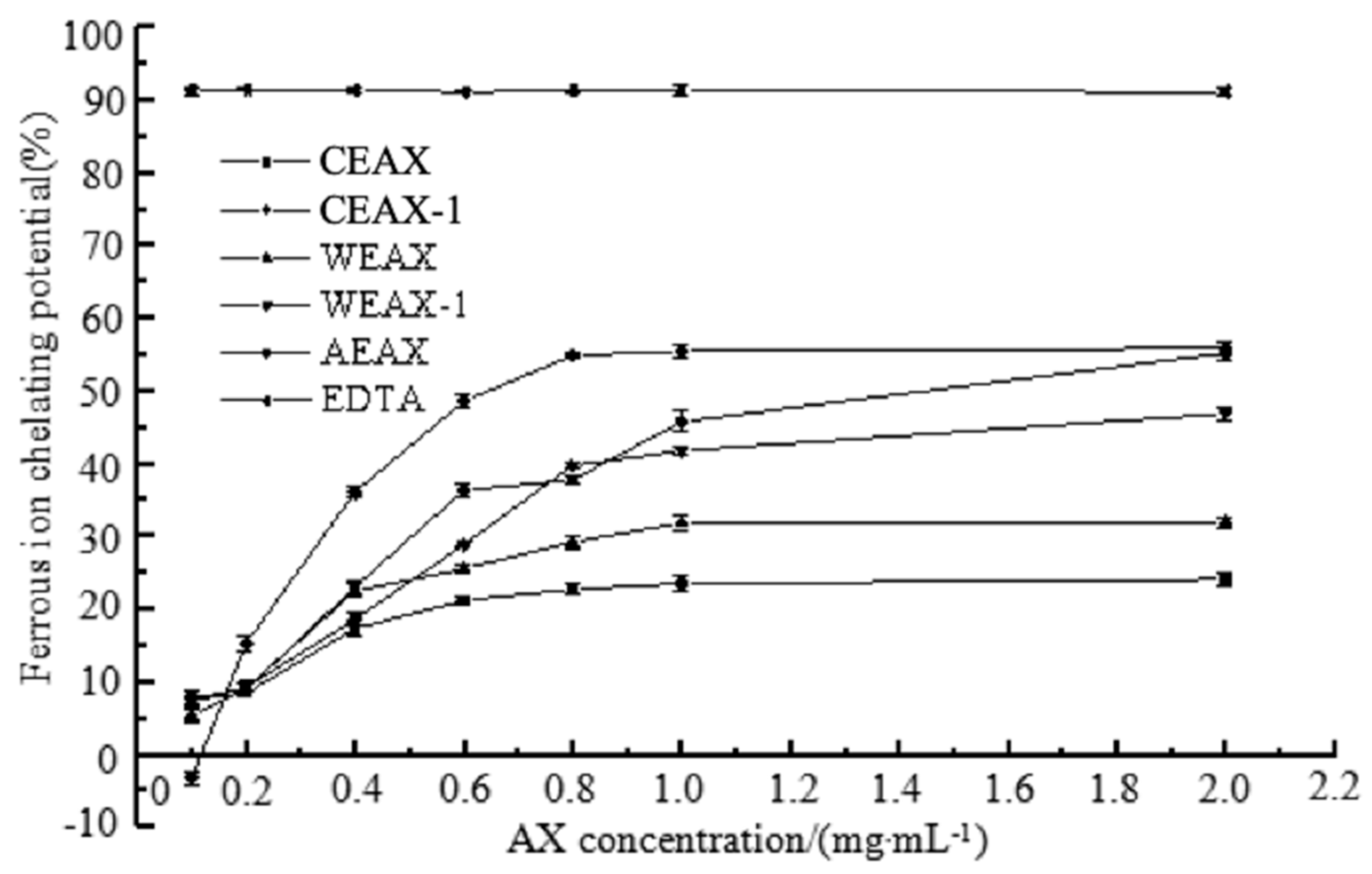
2.8.4. Metal-Chelating Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Characterization of AXs
3.2. Antioxidant Activity of AXs
3.3. Hypoglycemic Activity of AXs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Döring, C.; Jekle, M.; Becker, T. Technological and Analytical Methods for Arabinoxylan Quantification from Cereals. Crit. Rev. Food Technol. 2016, 56, 999–1011. [Google Scholar] [CrossRef]
- Snelders, J.; Dornez, E.; Delcour, J.A.; Courtin, C.M. Ferulic Acid Content and Appearance Determine the Antioxidant Capacity of Arabinoxylanoligosaccharides. J. Agric. Food Chem. 2013, 61, 10173–10182. [Google Scholar] [CrossRef]
- Malunga, L.N.; Beta, T. Antioxidant capacity of arabinoxylan oligosaccharide fractions prepared from wheat aleurone using Trichoderma viride or Neocallimastix patriciarum xylanase. Food Chem. 2015, 167, 311–319. [Google Scholar] [CrossRef]
- Malunga, L.N.; Izydorczyk, M.; Beta, T. Effect of water-extractable arabinoxylans from wheat aleurone and bran on lipid peroxidation and factors influencing their antioxidant capacity. Bioact. Carbohydr. Diet. Fibre 2017, 10, 20–26. [Google Scholar] [CrossRef]
- Yuwang, P.; Sulaeva, I.; Hell, J.; Henniges, U.; Bohmdorfer, S.; Rosenau, T.; Chitsomboon, B.; Tongta, S. Phenolic compounds and antioxidant properties of arabinoxylan hydrolysates from defatted rice bran. J. Sci. Food Agric. 2018, 98, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Bijalwan, V.; Ali, U.; Kesarwani, A.K.; Yadav, K.; Mazumder, K. Hydroxycinnamic acid bound arabinoxylans from millet brans-structural features and antioxidant activity. Int. J. Biol. Macromol. 2016, 88, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Gemen, R.; De Vries, J.F.; Slavin, J.L. Relationship between molecular structure of cereal dietary fiber and health effects: Focus on glucose/insulin response and gut health. Nutr. Rev. 2011, 69, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.L.; Hedemann, M.S.; Laerke, H.N.; Jorgensen, H.; Mutt, S.J.; Herzig, K.H.; Bach Knudsen, K.E. Concentrated arabinoxylan but not concentrated beta-glucan in wheat bread has similar effects on postprandial insulin as whole-grain rye in porto-arterial catheterized pigs. J. Agric. Food Chem. 2013, 61, 7760–7768. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D. α-Amylase Inhibitors: A Review of Raw Material and Isolated Compounds from Plant Source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef]
- Malunga, L.N.; Eck, P.; Beta, T. Inhibition of Intestinal alpha-Glucosidase and Glucose Absorption by Feruloylated Arabinoxylan Mono- and Oligosaccharides from Corn Bran and Wheat Aleurone. J. Nutr. Metab. 2016, 2016, 1932532. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, F.; Ni, H.; Mo, Z.; Wan, J.-B.; Hua, D.; Yan, C. Structural characterization, α-glucosidase inhibitory and DPPH scavenging activities of polysaccharides from guava. Carbohydr. Polym. 2016, 144, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Beta, T.; Sun, S.; Corke, H. Protein characteristics of Chinese black-grained wheat. Food Chem. 2006, 98, 463–472. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, S.W.; Gu, X.; Zhang, J. Isolation and structural characterization of water unextractable arabinoxylans from Chinese black-grained wheat bran. Carbohydr. Polym. 2011, 85, 615–621. [Google Scholar] [CrossRef]
- Fadel, A.; Mahmoud, A.M.; Ashworth, J.J.; Li, W.; Ng, Y.L.; Plunkett, A. Health-related effects and improving extractability of cereal arabinoxylans. Int. J. Biol. Macromol. 2018, 109, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, X.; Guo, Y.; Wang, Q.; Peng, D.; Cao, L. Comparison of the immunological activities of arabinoxylans from wheat bran with alkali and xylanase-aided extraction. Carbohydr. Polym. 2010, 81, 784–789. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.Y.; Wang, Y.X.; Nie, S.P.; Li, C. Analysis of the monosaccharide composition of purified polysaccharides in Ganoderma atrum by capillary gas chromatography. Phytochem. Anal. 2010, 20, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Adriana, M.O.; Elizabeth, C.M.; Yolanda, L.F.; Agustín, R.C.; Jaime, L.M.; Patricia, T.C.; Alma, C.M. Characterization of water extractable arabinoxylans from a spring wheat flour: Rheological properties and microstructure. Molecules 2013, 18, 8417–8428. [Google Scholar]
- Apostolidis, E.; Lee, C. In Vitro Potential of Ascophyllum nodosum Phenolic Antioxidant-Mediated α-Glucosidase and α-Amylase Inhibition. J. Food Sci. 2010, 75, H97–H102. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.C.; Li, Y.; Fu, L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J. Agric. Food Chem. 2001, 49, 1026–1029. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Karnjanapratum, S.; Benjakul, S. Antioxidative gelatin hydrolysate from unicorn leatherjacket skin as affected by prior autolysis. Int. Aquat. Res. 2015, 7, 101–114. [Google Scholar] [CrossRef]
- Aguedo, M.; Fougnies, C.; Dermience, M.; Richel, A. Extraction by three processes of arabinoxylans from wheat bran and characterization of the fractions obtained. Carbohydr. Polym. 2014, 105, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, J.; Lindhauer, M. Pilot-scale isolation of glucuronoarabinoxylans from wheat bran. Carbohydr. Polym. 2005, 59, 225–230. [Google Scholar] [CrossRef]
- Maes, C.; Delcour, J. Structural Characterisation of Water-extractable and Water-unextractable Arabinoxylans in Wheat Bran. J. Cereal Sci. 2002, 35, 315–326. [Google Scholar] [CrossRef]
- Collins, T.; Gerday, C.; Feller, G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol. Rev. 2010, 29, 3–23. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Abdelhamid, A.; Rihouey, C.; Le Cerf, D.; Bouraoui, A.; Majdoub, H. Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: Characterization, antioxidant, anti-inflammatory and analgesic activities. Carbohydr. Polym. 2018, 185, 127–137. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Jian, L.; Haifeng, Z.; Jian, C.; Wei, F.; Jianjun, D.; Weibao, K.; Junyong, S.; Yu, C.; Guolin, C. Evolution of phenolic compounds and antioxidant activity during malting. J. Agric. Food Chem. 2007, 55, 10994–11001. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; Conde, E.; Moure, A.; Domínguez, H.; Parajó, J.C. Characterization, refining and antioxidant activity of saccharides derived from hemicelluloses of wood and rice husks. Food Chem. 2013, 141, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Pristov, J.B.; Mitrović, A.; Spasojevic, I. A comparative study of antioxidative activities of cell-wall polysaccharides. Carbohydr. Res. 2011, 346, 2255–2259. [Google Scholar] [CrossRef]
- Izydorczyk, M.; Biliaderis, C.; Bushuk, W. Oxidative gelation studies of water-soluble pentosans from wheat. J. Cereal Sci. 1990, 11, 153–169. [Google Scholar] [CrossRef]
- Dervilly-Pinel, G.; Rimsten, L.; Saulnier, L.; Andersson, R.; Åman, P. Water-extractable Arabinoxylan from Pearled Flours of Wheat, Barley, Rye and Triticale. Evidence for the Presence of Ferulic Acid Dimers and their Involvement in Gel Formation. J. Cereal Sci. 2001, 34, 207–214. [Google Scholar] [CrossRef]
- Veenashri, B.; Muralikrishna, G. In vitro anti-oxidant activity of xylo-oligosaccharides derived from cereal and millet brans—A comparative study. Food Chem. 2011, 126, 1475–1481. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Cao, C.; Huang, Q.; Zhang, B.; Li, C.; Fu, X. Physicochemical characterization and in vitro hypoglycemic activities of polysaccharides from Sargassum pallidum by microwave-assisted aqueous two-phase extraction. Int. J. Biol. Macromol. 2018, 109, 357–368. [Google Scholar] [CrossRef]
- Vaugelade, P.; Hoebler, C.; Guillon, F.; Lahaye, M.; Duée, P.-H. Non-starch polysaccharides extracted from seaweed can modulate intestinal absorption of glucose and insulin response in the pig. Reprod. Nutr. Dev. 2000, 40, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Li, S.; Fu, Y.; Li, C.; Chen, D.; Chen, H. Arabinoxylan structural characteristics, interaction with gut microbiota and potential health functions. J. Funct. Foods 2019, 54, 536–551. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Muñoz, A.L.; Fernández-Bolaños, J. Pectin extracted from thermally treated olive oil by-products: Characterization, physico-chemical properties, in vitro bile acid and glucose binding. Food Hydrocoll. 2015, 43, 311–321. [Google Scholar] [CrossRef] [Green Version]







| Samples | Yield (%) |
|---|---|
| CEAX | 5.27 ± 0.09 |
| CEAX-1 | 14.95 ± 0.17 |
| WEAX | 1.19 ± 0.17 |
| WEAX-1 | 17.41 ± 0.13 |
| AEAX | 19.83 ± 0.16 |
| Samples | Arabinose (%) | Xylose (%) | Rhamnose (%) | Mannose (%) | Glucose (%) | Galactose (%) | Ara/Xyl |
|---|---|---|---|---|---|---|---|
| CEAX | 18.19 | 73.57 | n.d. | 1.78 | 3.44 | 3.01 | 0.25 |
| CEAX-1 | 55.90 | 36.83 | n.d. | 2.84 | n.d. | 4.43 | 1.52 |
| WEAX | 34.29 | 51.53 | n.d. | 2.30 | 7.29 | 4.59 | 0.67 |
| WEAX-1 | 53.37 | 41.25 | n.d. | n.d. | 2.17 | 3.21 | 1.29 |
| AEAX | 48.18 | 42.33 | n.d. | n.d. | 5.90 | 3.58 | 1.14 |
| Samples | Mw | Mn | Mw/Mn |
|---|---|---|---|
| CEAX | 2.8314 × 104 | 3744 | 7.56 |
| CEAX-1 | 1.76876 × 105 | 9259 | 19.10 |
| WEAX | 3.0730 × 104 | 3312 | 9.28 |
| WEAX-1 | 1.61989 × 105 | 9274 | 17.47 |
| AEAX | 2.10638 × 105 | 10,498 | 20.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Chen, Z.; Fu, Y.; Liu, J.; Lin, S.; Zhang, Q.; Liu, Y.; Wu, D.; Lin, D.; Han, G.; et al. Structure, Antioxidant, and Hypoglycemic Activities of Arabinoxylans Extracted by Multiple Methods from Triticale. Antioxidants 2019, 8, 584. https://doi.org/10.3390/antiox8120584
Chen H, Chen Z, Fu Y, Liu J, Lin S, Zhang Q, Liu Y, Wu D, Lin D, Han G, et al. Structure, Antioxidant, and Hypoglycemic Activities of Arabinoxylans Extracted by Multiple Methods from Triticale. Antioxidants. 2019; 8(12):584. https://doi.org/10.3390/antiox8120584
Chicago/Turabian StyleChen, Hong, Zhuoyun Chen, Yuanfang Fu, Jiao Liu, Siying Lin, Qing Zhang, Yuntao Liu, Dingtao Wu, Derong Lin, Guoquan Han, and et al. 2019. "Structure, Antioxidant, and Hypoglycemic Activities of Arabinoxylans Extracted by Multiple Methods from Triticale" Antioxidants 8, no. 12: 584. https://doi.org/10.3390/antiox8120584
APA StyleChen, H., Chen, Z., Fu, Y., Liu, J., Lin, S., Zhang, Q., Liu, Y., Wu, D., Lin, D., Han, G., Wang, L., & Qin, W. (2019). Structure, Antioxidant, and Hypoglycemic Activities of Arabinoxylans Extracted by Multiple Methods from Triticale. Antioxidants, 8(12), 584. https://doi.org/10.3390/antiox8120584








