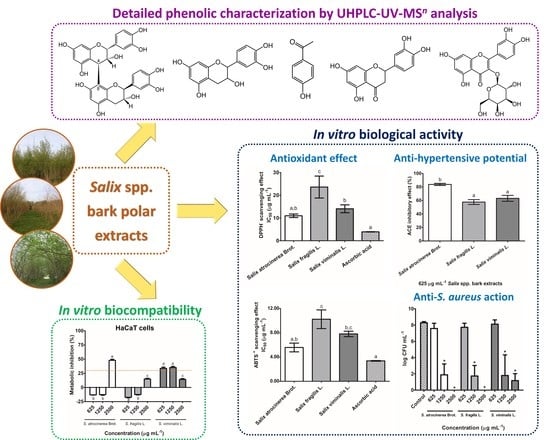
The Health-Promoting Potential of Salix spp. Bark Polar Extracts: Key Insights on Phenolic Composition and In Vitro Bioactivity and Biocompatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sampling of Salix spp. Barks
2.3. Extraction of Phenolic Compounds
2.4. Total Phenolic Content
2.5. Identification of Phenolic Compounds by UHPLC-DAD-MSn Analysis
2.6. Quantification of Phenolic Compounds by UHPLC-UV Analysis
2.7. Antioxidant Activity
2.7.1. DPPH Free Radical Scavenging Effect
2.7.2. ABTS Radical Cation Scavenging Effect
2.8. Angiotensin-I Converting Enzyme Inhibitory Activity
2.9. Inhibitory Effect Against Staphylococcus aureus Growth
2.10. In Vitro Biocompatibility
2.10.1. Mammalian Cell Lines
2.10.2. Metabolic Inhibition via XTT Assay
2.11. Statistical Analysis
3. Results
3.1. Extractive Yield and Total Phenolic Content
3.2. Phenolic Composition
3.2.1. Identification of Phenolic Compounds
Flavan-3-ols
Acetophenones
Hydroxybenzoic Acids
Flavanones
Flavonols
3.2.2. Quantification of Identified Phenolic Compounds by UHPLC-UV Analysis
3.3. In Vitro Bioactivity of Salix spp. Bark Polar Extracts
3.3.1. Antioxidant Activity
3.3.2. Angiotensin-I Converting Enzyme Inhibitory Activity
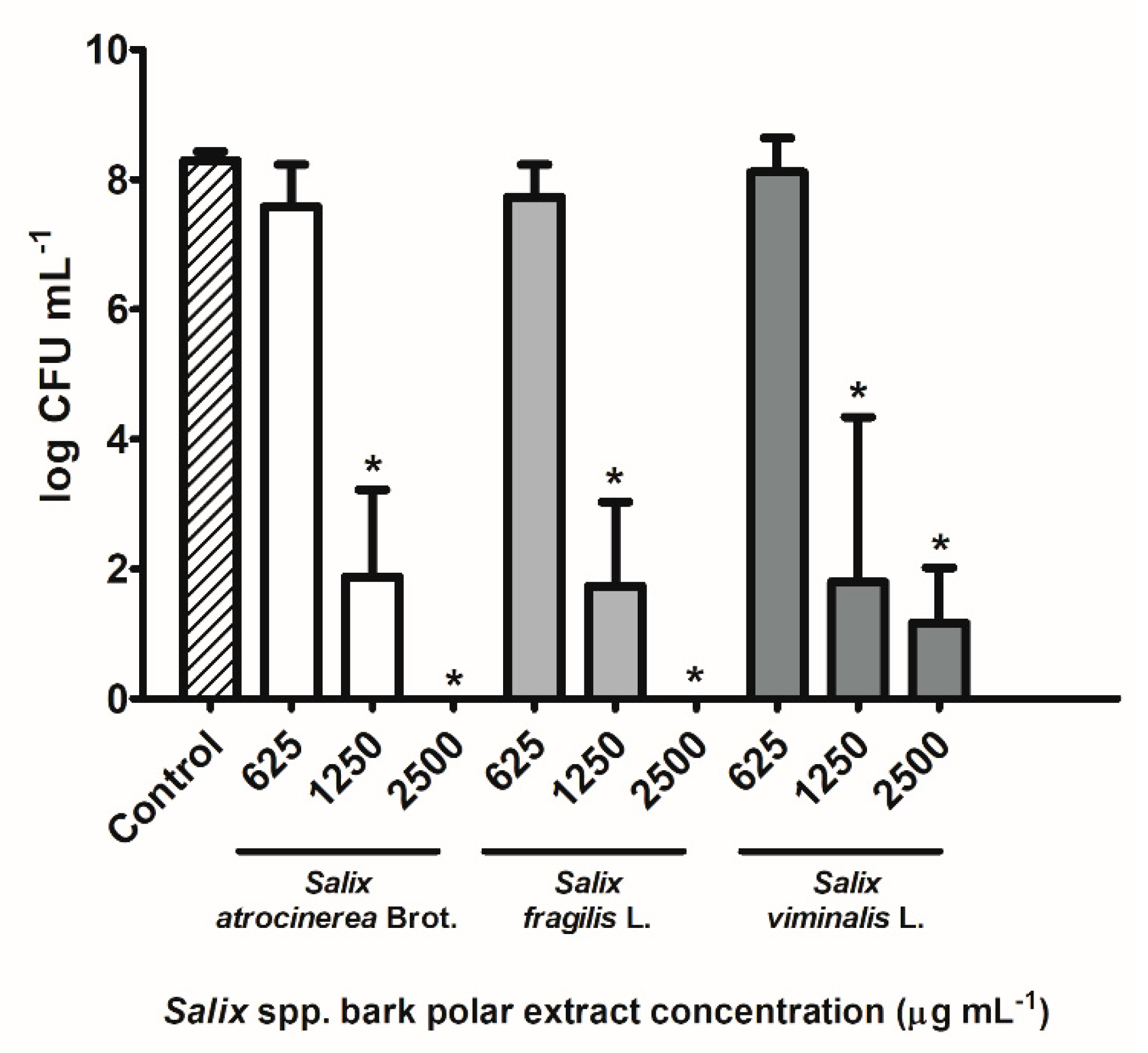
3.3.3. Inhibitory Effect against S. aureus Growth
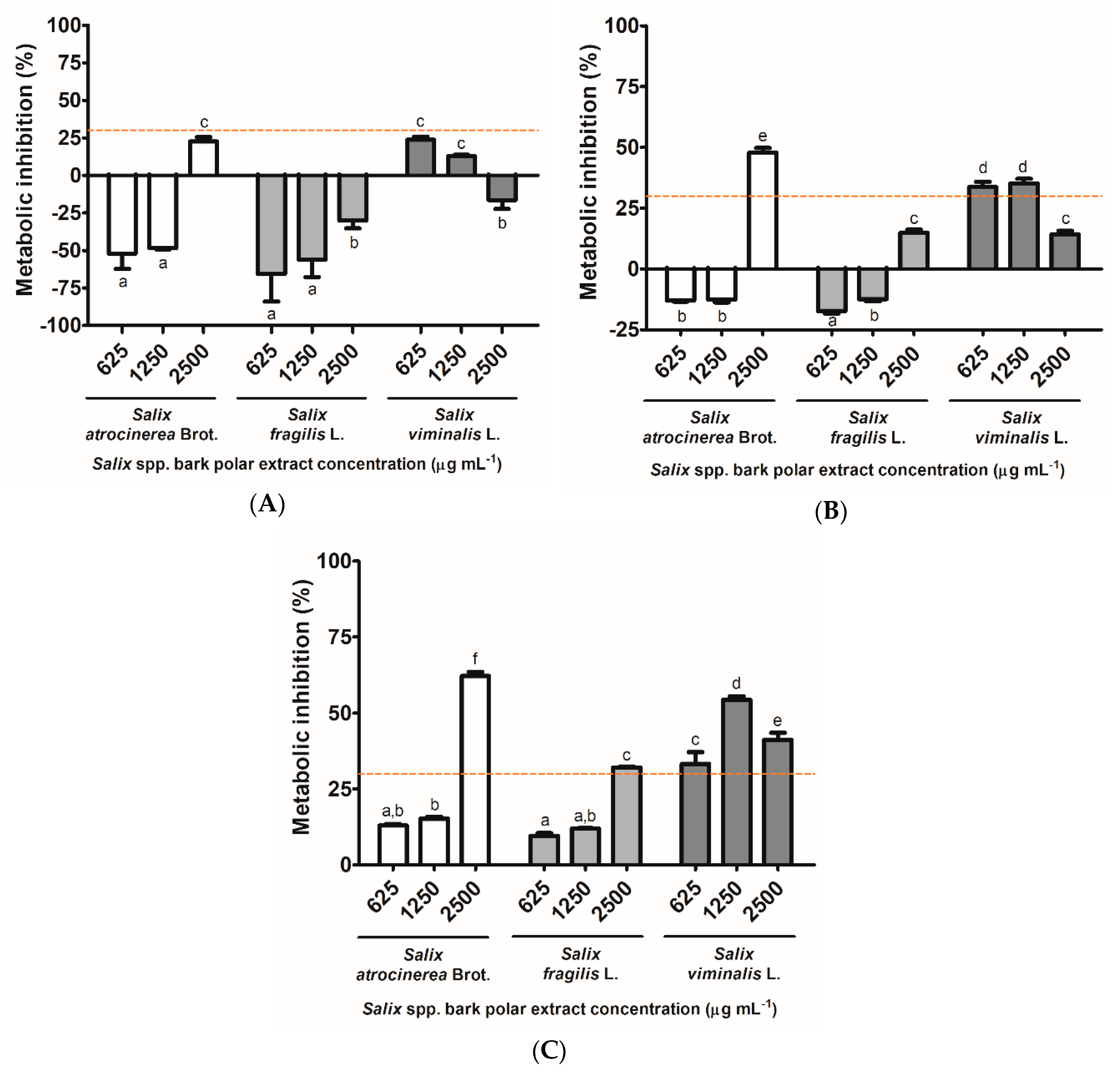
3.4. In Vitro Biocompatibility of Salix spp. Bark Polar Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EC A Sustainable Bioeconomy for Europe. Strengthening the Connection between Economy, Society and the Environment—Updated Bioeconomy Strategy; Publications Office of the European Union: Brussels, Belgium, 2018; pp. 4–16. [Google Scholar]
- Parajuli, R.; Knudsen, M.T.; Dalgaard, T. Multi-criteria assessment of yellow, green, and woody biomasses: Pre-screening of potential biomasses as feedstocks for biorefineries. Biofuels Bioprod. Biorefining 2015, 9, 545–566. [Google Scholar] [CrossRef]
- Wickham, J.; Rice, B.; Finnan, J.; McConnon, R. A Review of Past and Current Research on Short Rotation Coppice in Ireland and Abroad: Report prepared for COFORD and Sustainable Energy Authority of Ireland; COFORD, National Council for Forest Research and Development: Dublin, Ireland, 2010; pp. 1–36. [Google Scholar]
- Djomo, S.N.; Kasmioui, O.E.L.; Ceulemans, R. Energy and greenhouse gas balance of bioenergy production from poplar and willow: A review. Glob. Chang. Biol. Bioenergy 2011, 3, 181–197. [Google Scholar] [CrossRef]
- Setty, A.R.; Sigal, L.H. Herbal medications commonly used in the practice of rheumatology: Mechanisms of action, efficacy, and side effects. Semin. Arthritis Rheum. 2005, 34, 773–784. [Google Scholar] [CrossRef]
- EMA European Union herbal monograph on Salix (various species including S. purpurea L., S. daphnoides Vill., S. fragilis L.), cortex; London, 2017. Available online: https://www.ema.europa.eu/documents/herbal-monograph/final-european-union-herbal-monograph-salix-various-species-including-s-purpurea-l-s-daphnoides-vill_en.pdf (accessed on 5 July 2019).
- Bonaterra, G.A.; Heinrich, E.U.; Kelber, O.; Weiser, D.; Metz, J.; Kinscherf, R. Anti-inflammatory effects of the willow bark extract STW 33-I (Proaktiv®) in LPS-activated human monocytes and differentiated macrophages. Phytomedicine 2010, 17, 1106–1113. [Google Scholar] [CrossRef]
- Bonaterra, G.A.; Kelber, O.; Weiser, D.; Metz, J.; Kinscherf, R. In vitro anti-proliferative effects of the willow bark extract STW 33-I. Arzneimittelforschung 2010, 60, 330–335. [Google Scholar] [CrossRef]
- Agnolet, S.; Wiese, S.; Verpoorte, R.; Staerk, D. Comprehensive analysis of commercial willow bark extracts by new technology platform: Combined use of metabolomics, high-performance liquid chromatography–solid-phase extraction–nuclear magnetic resonance spectroscopy and high-resolution radical scavenging assay. J. Chromatogr. A 2012, 1262, 130–137. [Google Scholar]
- Dou, J.; Xu, W.; Koivisto, J.J.; Mobley, J.K.; Padmakshan, D.; Kögler, M.; Xu, C.; Willför, S.; Ralph, J.; Vuorinen, T. Characteristics of hot water extracts from the bark of cultivated willow (Salix sp.). ACS Sustain. Chem. Eng. 2018, 6, 5566–5573. [Google Scholar] [CrossRef]
- Kammerer, B.; Kahlich, R.; Biegert, C.; Gleiter, C.H.; Heide, L. HPLC-MS/MS analysis of willow bark extracts contained in pharmaceutical preparations. Phytochem. Anal. 2005, 16, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Poblocka-Olech, L.; Glod, D.; Wiwart, M.; Zielinski, J.; Migas, P. HPLC of flavanones and chalcones in different species and clones of Salix. Acta Pol. Pharm. 2013, 70, 27–34. [Google Scholar] [PubMed]
- Sulima, P.; Krauze-Baranowska, M.; Przyborowski, J.A. Variations in the chemical composition and content of salicylic glycosides in the bark of Salix purpurea from natural locations and their significance for breeding. Fitoterapia 2017, 118, 118–125. [Google Scholar] [CrossRef]
- Actis-Goretta, L.; Ottaviani, J.I.; Keen, C.L.; Fraga, C.G. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett. 2003, 555, 597–600. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Shimada, A.; Miyano, A.; Nishida, T.; Mitsunaga, T. In vitro screening of angiotensin I-converting enzyme inhibitors from Japanese cedar (Cryptomeria japonica). J. Wood Sci. 1998, 44, 463–468. [Google Scholar] [CrossRef]
- Han, X.; Pan, J.; Ren, D.; Cheng, Y.; Fan, P.; Lou, H. Naringenin-7-O-glucoside protects against doxorubicin-induced toxicity in H9c2 cardiomyocytes by induction of endogenous antioxidant enzymes. Food Chem. Toxicol. 2008, 46, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, S.M.; Graikou, K.; Sotiroudis, G.; Glowniak, K.; Chinou, I. Two new iridoids from selected Penstemon species-antimicrobial activity. Nat. Prod. Res. 2013, 27, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit. Rev. Food Sci. Nutr. 2018, 1–14. [Google Scholar] [CrossRef]
- Kusumawati, I.; Indrayanto, G. Chapter 15—Natural Antioxidants in Cosmetics. In Studies in Natural Products Chemistry, 1st ed.; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, the Netherlands, 2013; Volume 40, pp. 485–505. [Google Scholar]
- Kilfoyle, B.E.; Kaushik, D.; Terebetski, J.L.; Bose, S.; Michniak-Kohn, B.B. The use of quercetin and curcumin in skin care consumer products. In Formulating, Packaging, and Marketing of Natural Cosmetic Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 259–286. [Google Scholar]
- Coscueta, E.R.; Campos, D.A.; Osório, H.; Nerli, B.B.; Pintado, M. Enzymatic soy protein hydrolysis: A tool for biofunctional food ingredient production. Food Chem. X 2019, 1, 100006. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Parreira, P.; Soares, B.I.G.; Freire, C.S.R.; Silvestre, A.J.D.; Reis, C.A.; Martins, M.C.L.; Duarte, M.F. Eucalyptus spp. outer bark extracts inhibit Helicobacter pylori Growth: In vitro studies. Ind. Crop. Prod. 2017, 105, 207–214. [Google Scholar] [CrossRef]
- WHO Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 5 July 2019).
- Puchalska, P.; Alegre, M.L.M.; López, M.C.G. Isolation and characterization of peptides with antihypertensive activity in foodstuffs. Crit. Rev. Food Sci. Nutr. 2015, 55, 521–551. [Google Scholar] [CrossRef]
- ECDC Surveillance of antimicrobial resistance in Europe 2017. In Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); ECDC: Stockholm, Sweden, 2018; pp. 54–56.
- CDC Staphylococcal (Staph) Food Poisoning. Available online: https://www.cdc.gov/foodsafety/diseases/staphylococcal.html (accessed on 5 July 2019).
- Masika, P.J.; Sultana, N.; Afolayan, A.J.; Houghton, P.J. Isolation of two antibacterial compounds from the bark of Salix capensis. S. Afr. J. Bot. 2005, 71, 441–443. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Krauze-Baranowska, M.; Głód, D.; Kawiak, A.; Łojkowska, E. Chromatographic analysis of simple phenols in some species from the genus Salix. Phytochem. Anal. 2010, 21, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Pobłocka-Olech, L.; van Nederkassel, A.-M.; Vander Heyden, Y.; Krauze-Baranowska, M.; Glód, D.; Baczek, T. Chromatographic analysis of salicylic compounds in different species of the genus Salix. J. Sep. Sci. 2007, 30, 2958–2966. [Google Scholar] [CrossRef] [PubMed]
- Pobłocka-Olech, L.; Krauze-Baranowska, M. SPE-HPTLC of procyanidins from the barks of different species and clones of Salix. J. Pharm. Biomed. Anal. 2008, 48, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.A.B.; Moreirinha, C.; Santos, S.A.O.; Almeida, A.; Freire, C.S.R.; Silva, A.M.S.; Silvestre, A.J.D. Valorisation of bark lipophilic fractions from three Portuguese Salix species: A systematic study of the chemical composition and inhibitory activity on Escherichia coli. Ind. Crop. Prod. 2019, 132, 245–252. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Santos, S.A.O.; Guerra, Â.R.; Guerreiro, O.; Freire, C.S.R.; Rocha, S.M.; Duarte, M.F.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of different morphological parts of Cynara cardunculus L. var. altilis (DC). Ind. Crop. Prod. 2014, 61, 460–471. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Domingues, R.M.A.; Oliveira, C.S.D.; Villaverde, J.J.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Secondary metabolites from Eucalyptus grandis wood cultivated in Portugal, Brazil and South Africa. Ind. Crop. Prod. 2017, 95, 357–364. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2, 2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sentandreu, M.Á.; Toldrá, F. A rapid, simple and sensitive fluorescence method for the assay of angiotensin-I converting enzyme. Food Chem. 2006, 97, 546–554. [Google Scholar] [CrossRef]
- Belley, A.; Neesham-Grenon, E.; Arhin, F.F.; McKay, G.A.; Parr, T.R.; Moeck, G. Assessment by time-kill methodology of the synergistic effects of oritavancin in combination with other antimicrobial agents against Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 3820–3822. [Google Scholar] [CrossRef]
- Friedrich, W.; Eberhardt, A.; Galensa, R. Investigation of proanthocyanidins by HPLC with electrospray ionization mass spectrometry. Eur. Food Res. Technol. 2000, 211, 56–64. [Google Scholar] [CrossRef]
- Teixeira, N.; Azevedo, J.; Mateus, N.; de Freitas, V. Proanthocyanidin screening by LC-ESI-MS of Portuguese red wines made with teinturier grapes. Food Chem. 2016, 190, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.O.; Vilela, C.; Camacho, J.F.; Cordeiro, N.; Gouveia, M.; Freire, C.S.R.; Silvestre, A.J.D. Profiling of lipophilic and phenolic phytochemicals of four cultivars from cherimoya (Annona cherimola Mill.). Food Chem. 2016, 211, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V. Flavonoids in wine. In Flavonoids: Chemistry, Biochemistry, and Applications; Andersen, Ø.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 263–318. [Google Scholar]
- Attygalle, A.B.; Ruzicka, J.; Varughese, D.; Bialecki, J.B.; Jafri, S. Low-energy collision-induced fragmentation of negative ions derived from ortho-, meta-, and para-hydroxyphenyl carbaldehydes, ketones, and related compounds. J. Mass Spectrom. 2007, 42, 1207–1217. [Google Scholar] [CrossRef]
- Mageroy, M.H.; Parent, G.; Germanos, G.; Giguère, I.; Delvas, N.; Maaroufi, H.; Bauce, É.; Bohlmann, J.; Mackay, J.J. Expression of the β-glucosidase gene Pgβglu-1 underpins natural resistance of white spruce against spruce budworm. Plant J. 2015, 81, 68–80. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Vallverdú-Queralt, A.; Jáuregui, O.; Medina-Remón, A.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Improved characterization of tomato polyphenols using liquid chromatography/electrospray ionization linear ion trap quadrupole Orbitrap mass spectrometry and liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2986–2992. [Google Scholar] [CrossRef]
- Chang, Q.; Wong, Y.-S. Identification of flavonoids in Hakmeitau beans (Vigna sinensis) by high-performance liquid chromatography−electrospray mass spectrometry (LC-ESI/MS). J. Agric. Food Chem. 2004, 52, 6694–6699. [Google Scholar] [CrossRef]
- Kubo, S.; Hashida, K.; Makino, R.; Magara, K.; Kenzo, T.; Kato, A. Aorigele Chemical composition of desert willow (Salix psammophila) grown in the Kubuqi desert, inner Mongolia, China: Bark extracts associated with environmental adaptability. J. Agric. Food Chem. 2013, 61, 12226–12231. [Google Scholar] [CrossRef] [PubMed]
- Tawfeek, N.; Sobeh, M.; Hamdan, D.I.; Farrag, N.; Roxo, M.; El-Shazly, A.M.; Wink, M. Phenolic compounds from Populus alba L. and Salix subserrata Willd. (Salicaceae) counteract oxidative stress in Caenorhabditis elegans. Molecules 2019, 24, 1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enayat, S.; Banerjee, S. Comparative antioxidant activity of extracts from leaves, bark and catkins of Salix aegyptiaca sp. Food Chem. 2009, 116, 23–28. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Krauze-Baranowska, M.; Wiwart, M. HPTLC determination of catechins in different clones of the genus Salix. JPC-J. Planar Chromatogr.-Mod. TLC 2007, 20, 61–64. [Google Scholar]
- Gligorić, E.; Igić, R.; Suvajdžić, L.; Grujić-Letić, N. Species of the genus Salix L.: Biochemical screening and molecular docking approach to potential acetylcholinesterase inhibitors. Appl. Sci. 2019, 9, 1842. [Google Scholar]
- Freischmidt, A.; Jürgenliemk, G.; Kraus, B.; Okpanyi, S.N.; Müller, J.; Kelber, O.; Weiser, D.; Heilmann, J. Contribution of flavonoids and catechol to the reduction of ICAM-1 expression in endothelial cells by a standardised willow bark extract. Phytomedicine 2012, 19, 245–252. [Google Scholar] [CrossRef]
- Piazzini, V.; Bigagli, E.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Enhanced solubility and permeability of salicis cortex extract by formulating as a microemulsion. Planta Med. 2018, 84, 976–984. [Google Scholar] [CrossRef] [Green Version]
- Touati, R.; Santos, S.A.O.; Rocha, S.M.; Belhamel, K.; Silvestre, A.J.D. Phenolic composition and biological prospecting of grains and stems of Retama sphaerocarpa. Ind. Crop. Prod. 2017, 95, 244–255. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.L.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Tahvanainen, J. The effect of the sample preparation method of extractable phenolics of Salicaceae species. Planta Med. 1989, 55, 55–58. [Google Scholar] [CrossRef]
- Meier, B.; Julkunen-Tiitto, R.; Tahvanainen, J.; Sticher, O. Comparative high-performance liquid and gas-liquid chromatographic determination of phenolic glucosides in Salicaceae species. J. Chromatogr. A 1988, 442, 175–186. [Google Scholar] [CrossRef]
- Ishikado, A.; Sono, Y.; Matsumoto, M.; Robida-Stubbs, S.; Okuno, A.; Goto, M.; King, G.L.; Blackwell, T.K.; Makino, T. Willow bark extract increases antioxidant enzymes and reduces oxidative stress through activation of Nrf2 in vascular endothelial cells and Caenorhabditis elegans. Free Radic. Biol. Med. 2013, 65, 1506–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaiter, A.; Becker, L.; Petit, J.; Zimmer, D.; Karam, M.-C.; Baudelaire, É; Scher, J.; Dicko, A. Antioxidant and antiacetylcholinesterase activities of different granulometric classes of Salix alba (L.) bark powders. Powder Technol. 2016, 301, 649–656. [Google Scholar] [CrossRef]
- Durak, A.; Gawlik-Dziki, U.; Sugier, D. Coffee enriched with willow (Salix purpurea and Salix myrsinifolia) bark preparation—interactions of antioxidative phytochemicals in a model system. J. Funct. Foods 2015, 18, 1106–1116. [Google Scholar] [CrossRef]
- Janceva, S.; Lauberte, L.; Dizhbite, T.; Krasilnikova, J.; Telysheva, G.; Dzenis, M. Protective effects of proanthocyanidins extracts from the bark of deciduous trees in lipid systems. Holzforschung 2017, 71, 675–680. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.A.; Garbuz, S.A.; Malfanov, I.L.; Ptitsyn, L.R. Screening of Russian medicinal and edible plant extracts for angiotensin I-converting enzyme (ACE I) inhibitory activity. Russ. J. Bioorgan. Chem. 2013, 39, 743–749. [Google Scholar] [CrossRef]
- Eldeen, I.M.S.; Elgorashi, E.E.; van Staden, J. Antibacterial, anti-inflammatory, anti-cholinesterase and mutagenic effects of extracts obtained from some trees used in South African traditional medicine. J. Ethnopharmacol. 2005, 102, 457–464. [Google Scholar] [CrossRef]
- Masika, P.J.; Afolayan, A.J. Antimicrobial activity of some plants used for the treatment of livestock disease in the Eastern Cape, South Africa. J. Ethnopharmacol. 2002, 83, 129–134. [Google Scholar] [CrossRef]
- Jeon, S.H.; Chun, W.; Choi, Y.J.; Kwon, Y.S. Cytotoxic constituents from the bark of Salix hulteni. Arch. Pharm. Res. 2008, 31, 978–982. [Google Scholar] [CrossRef]



| Standard Compound | λ (nm) A | Concentration Range (µg mL−1) | Linear Regression Equation B | r2 | LOD (µg mL−1) | LOQ (µg mL−1) |
|---|---|---|---|---|---|---|
| Catechin | 280 | 0.10–30.29 | y = 93621x + 17212 | 0.9998 | 0.52 | 1.74 |
| m-Hydroxybenzoic acid | 235 | 0.51–30.89 | y = 245747x + 909936 | 0.9929 | 3.42 | 11.40 |
| Naringenin | 280 | 0.11–21.17 | y = 398130x + 61541 | 0.9990 | 0.87 | 2.89 |
| Piceol | 280 | 0.30–18.23 | y = 765733x + 59082 | 0.9992 | 0.68 | 2.25 |
| Quercetin | 370 | 0.10–19.21 | y = 320421x − 99949 | 0.9989 | 0.85 | 2.83 |
| Salix spp. | EY (% of Dry Bark, w/w) | TPC (g GAE kg−1 of Dry Bark) | TPC (mg GAE g−1 of Extract) |
|---|---|---|---|
| Salix atrocinerea Brot. | 15.1 ± 1.7 b | 44.47 ± 6.68 b | 293.36 ± 19.52 b |
| Salix fragilis L. | 9.7 ± 0.3 a | 17.47 ± 3.19 a | 179.06 ± 30.64 a |
| Salix viminalis L. | 10.1 ± 0.8 a | 24.76 ± 0.82 a | 246.44 ± 16.58 a,b |
| No. | RT (min) | Compound | λmax (nm) | [M−H]− (m/z) | MSn Product Ions (m/z) B | Id. |
|---|---|---|---|---|---|---|
| 1 | 3.79 | (Epi)gallocatechin-(epi)catechin dimer isomer | 233, 273 | 593 | MS2: 575, 525, 467, 441, 425, 423, 407, 303, 289, 245 MS3: 245 | [40,41] |
| 2 | 6.68 | B-type procyanidin dimer isomer 1 | 237, 277 | 577 | MS2: 559, 451, 425, 407, 289, 287, 245 MS3: 245, 229, 205 | [41] |
| 3 | 6.77 | Picein | 229, 264 | 343 A | MS2: 297, 135, 120 | [11] |
| 4 | 7.19 | Procyanidin B1 | 236, 278 | 577 | MS2: 559, 451, 425, 407, 289, 287, 245 MS3: 245 | Co |
| 5 | 7.37 | Catechin | 235, 278 | 289 | MS2: 271, 245, 205, 203, 179 | Co |
| 6 | 7.61 | B-type procyanidin dimer isomer 2 | 237, 278 | 577 | MS2: 559, 451, 425, 407, 289, 287, 245 MS3: 245, 205 | [41] |
| 7 | 10.10 | Piceol | 229, 274 | 135 | MS2: 93 | Co |
| 8 | 12.24 | B-type procyanidin dimer isomer 3 | 241, 279 | 577 | MS2: 559, 451, 425, 407, 289 MS3: 289, 245 | [41,42] |
| 9 | 12.27 | Salicylic acid | 241, 299 | 137 | MS2: 93 | [11] |
| 10 | 13.86 | Naringenin-O-hexoside isomer 1 | 241, 277 | 433 | MS2: 433, 416, 365, 313, 271, 151 MS3: 151 | [11] |
| 11 | 14.52 | Naringenin-O-hexoside isomer 2 | 241, 274 | 433 | MS2: 313, 271, 251, 151 MS3: 151, 107 | [11] |
| 12 | 14.88 | Quercetin 3-O-galactoside | 241, 268, 346 | 463 | MS2: 417, 395, 379, 343, 301, 300, 271, 179, 151 MS3: 179, 151 | Co |
| 13 | 18.09 | Eriodictyol-O-hexoside isomer | 238, 282, 330sh | 449 | MS2: 431, 413, 403, 381, 297; 287, 269, 175, 151, 135 MS3: 287, 269, 151, 135, 125, 107 | [33] |
| 14 | 19.88 | Eriodictyol | 238, 284, 330sh | 287 | MS2: 287, 151, 135, 125, 107 | Co |
| 15 | 23.23 | Naringenin | 237, 279 | 271 | MS2: 227, 177, 151, 119, 107 | Co |
| No. | Compound | λ (nm) | mg kg−1 of Dry Weight | mg g−1 of Extract | ||||
|---|---|---|---|---|---|---|---|---|
| Salix atrocinerea Brot. | Salix fragilis L. | Salix viminalis L. | Salix atrocinerea Brot. | Salix fragilis L. | Salix viminalis L. | |||
| 1 | (Epi)gallocatechin-(epi)catechin dimer isomer A | 280 | 213 | − | − | 1.40 | − | − |
| 2 | B-type procyanidin dimer isomer 1 A | 280 | − | − | 19 | − | − | 0.19 |
| 4 | Procyanidin B1A | 280 | 404 F(4+5) | − | 159 F(4+5+6) | 2.70 F(4+5) | − | 1.55 F(4+5+6) |
| 5 | Catechin A | 280 | F(4+5) | 146 | F(4+5+6) | F(4+5) | 1.51 | F(4+5+6) |
| 6 | B-type procyanidin dimer isomer 2 A | 280 | − | − | F(4+5+6) | − | − | F(4+5+6) |
| 8 | B-type procyanidin dimer isomer 3 B | 235 | − | − | F(8+9), G | − | − | F(8+9), G |
| Σ Flavan-3-ols | 617 | 146 | 178 | 4.10 | 1.51 | 1.73 | ||
| 3 | Picein C | 280 | 797 | 27 | − | 5.32 | 0.28 | − |
| 7 | Piceol C | 280 | 1358 | 1537 | − | 9.10 | 15.87 | − |
| Σ Acetophenones | 2155 | 1564 | − | 14.42 | 16.15 | − | ||
| 9 | Salicylic acid B | 235 | traces | 58 | 200 F(8+9), G | traces | 0.59 | 2.00 F(8+9), G |
| Σ Hydroxybenzoic acids | traces | 58 | 200 | traces | 0.59 | 2.00 | ||
| 10 | Naringenin-O-hexoside isomer 1 D | 280 | 6 | − | − | 0.04 | − | − |
| 11 | Naringenin-O-hexoside isomer 2 D | 280 | 13 | − | − | 0.09 | − | − |
| 13 | Eriodictyol-O-hexoside isomer D | 280 | − | − | 51 | − | − | 0.50 |
| 14 | Eriodictyol D | 280 | − | − | 52 | − | − | 0.51 |
| 15 | Naringenin D | 280 | 44 | 5 | − | 0.30 | 0.05 | − |
| Σ Flavanones | 64 | 5 | 103 | 0.43 | 0.05 | 1.00 | ||
| 12 | Quercetin 3-O-galactoside E | 370 | 35 | 6 | 10 | 0.23 | 0.06 | 0.09 |
| Σ Flavonols | 35 | 6 | 10 | 0.23 | 0.06 | 0.09 | ||
| TOTAL | 2871 | 1779 | 490 | 19.18 | 18.37 | 4.83 | ||
| Salix spp. Bark Extract/Reference | DPPH• Scavenging Effect | ABTS•+ Scavenging Effect | ||
|---|---|---|---|---|
| IC50 (µg mL−1) | IC50 (mg AAE g−1 of Dry Bark) | AAI | IC50 (µg mL−1) | |
| Salix atrocinerea Brot. | 10.98 ± 0.77 a,b | 54.41 ± 8.22 b | 5.64 | 5.58 ± 0.72 a,b |
| Salix fragilis L. | 23.62 ± 4.82 c | 16.79 ± 3.54 a | 2.62 | 10.24 ± 1.54 c |
| Salix viminalis L. | 14.06 ± 1.73 b | 28.63 ± 4.34 a | 4.40 | 7.82 ± 0.45 b,c |
| Ascorbic acid | 3.92 ± 0.08 a | ˗ | - | 3.37 ± 0.06 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, P.A.B.; Moreirinha, C.; Silva, S.; Costa, E.M.; Veiga, M.; Coscueta, E.; Santos, S.A.O.; Almeida, A.; Pintado, M.M.; Freire, C.S.R.; et al. The Health-Promoting Potential of Salix spp. Bark Polar Extracts: Key Insights on Phenolic Composition and In Vitro Bioactivity and Biocompatibility. Antioxidants 2019, 8, 609. https://doi.org/10.3390/antiox8120609
Ramos PAB, Moreirinha C, Silva S, Costa EM, Veiga M, Coscueta E, Santos SAO, Almeida A, Pintado MM, Freire CSR, et al. The Health-Promoting Potential of Salix spp. Bark Polar Extracts: Key Insights on Phenolic Composition and In Vitro Bioactivity and Biocompatibility. Antioxidants. 2019; 8(12):609. https://doi.org/10.3390/antiox8120609
Chicago/Turabian StyleRamos, Patrícia A. B., Catarina Moreirinha, Sara Silva, Eduardo M. Costa, Mariana Veiga, Ezequiel Coscueta, Sónia A. O. Santos, Adelaide Almeida, M. Manuela Pintado, Carmen S. R. Freire, and et al. 2019. "The Health-Promoting Potential of Salix spp. Bark Polar Extracts: Key Insights on Phenolic Composition and In Vitro Bioactivity and Biocompatibility" Antioxidants 8, no. 12: 609. https://doi.org/10.3390/antiox8120609
APA StyleRamos, P. A. B., Moreirinha, C., Silva, S., Costa, E. M., Veiga, M., Coscueta, E., Santos, S. A. O., Almeida, A., Pintado, M. M., Freire, C. S. R., Silva, A. M. S., & Silvestre, A. J. D. (2019). The Health-Promoting Potential of Salix spp. Bark Polar Extracts: Key Insights on Phenolic Composition and In Vitro Bioactivity and Biocompatibility. Antioxidants, 8(12), 609. https://doi.org/10.3390/antiox8120609