PKR Promotes Oxidative Stress and Apoptosis of Human Articular Chondrocytes by Causing Mitochondrial Dysfunction through p38 MAPK Activation—PKR Activation Causes Apoptosis in Human Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Chondrocytes Isolation
2.3. Investigation of Proteins
2.4. Measurement of Mitochondria ROS Concentration
2.5. PKR Knockdown
2.6. Investigation of Mitochondrial Membrane Potential
2.7. Mitochondrial Biogenesis
2.8. Antioxidant Activity
2.9. Investigation of Apoptosis
2.10. Statistical Analyses
3. Results
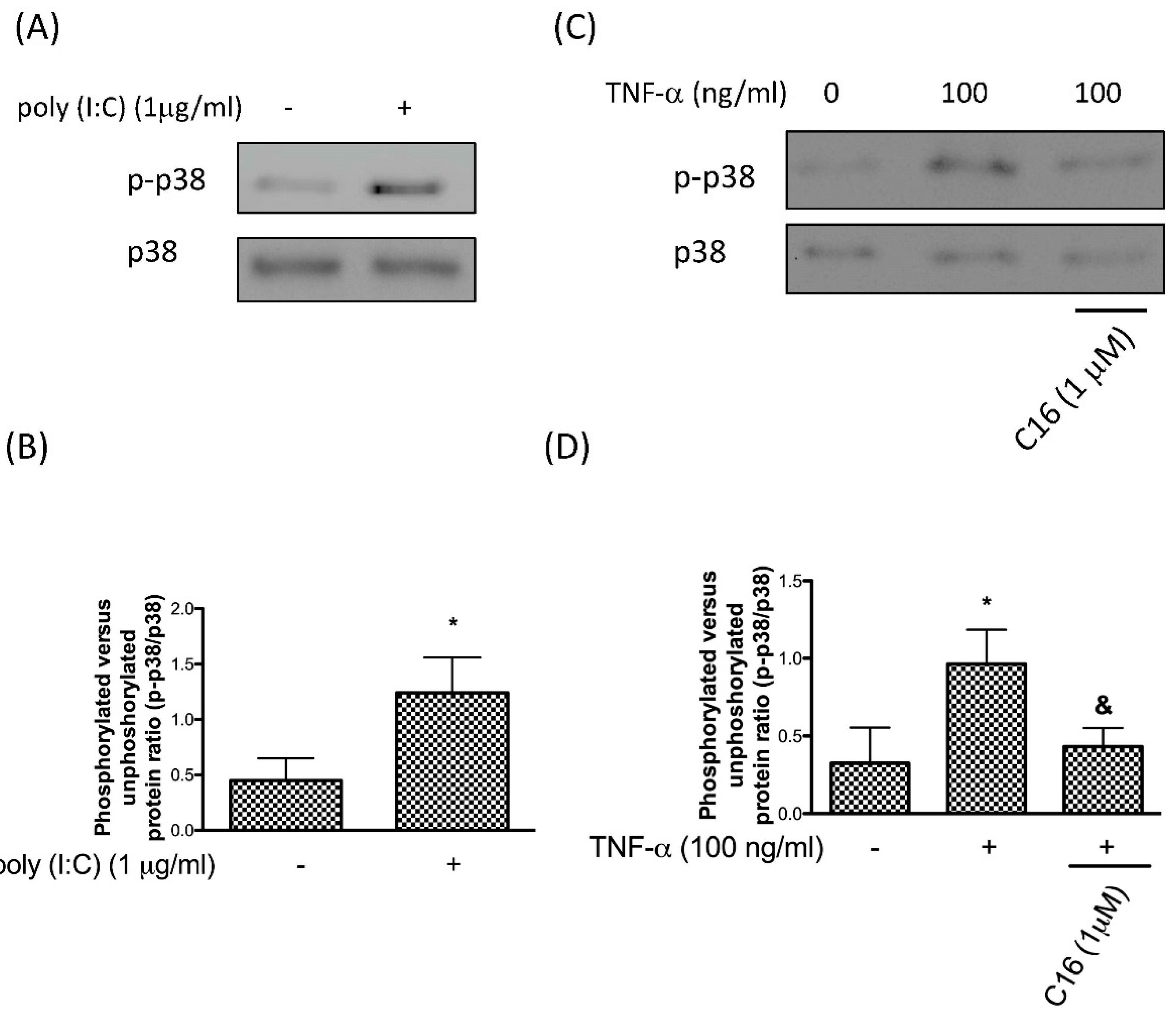
3.1. TNF-α Activates p38 MAPK via PKR in Chondrocytes
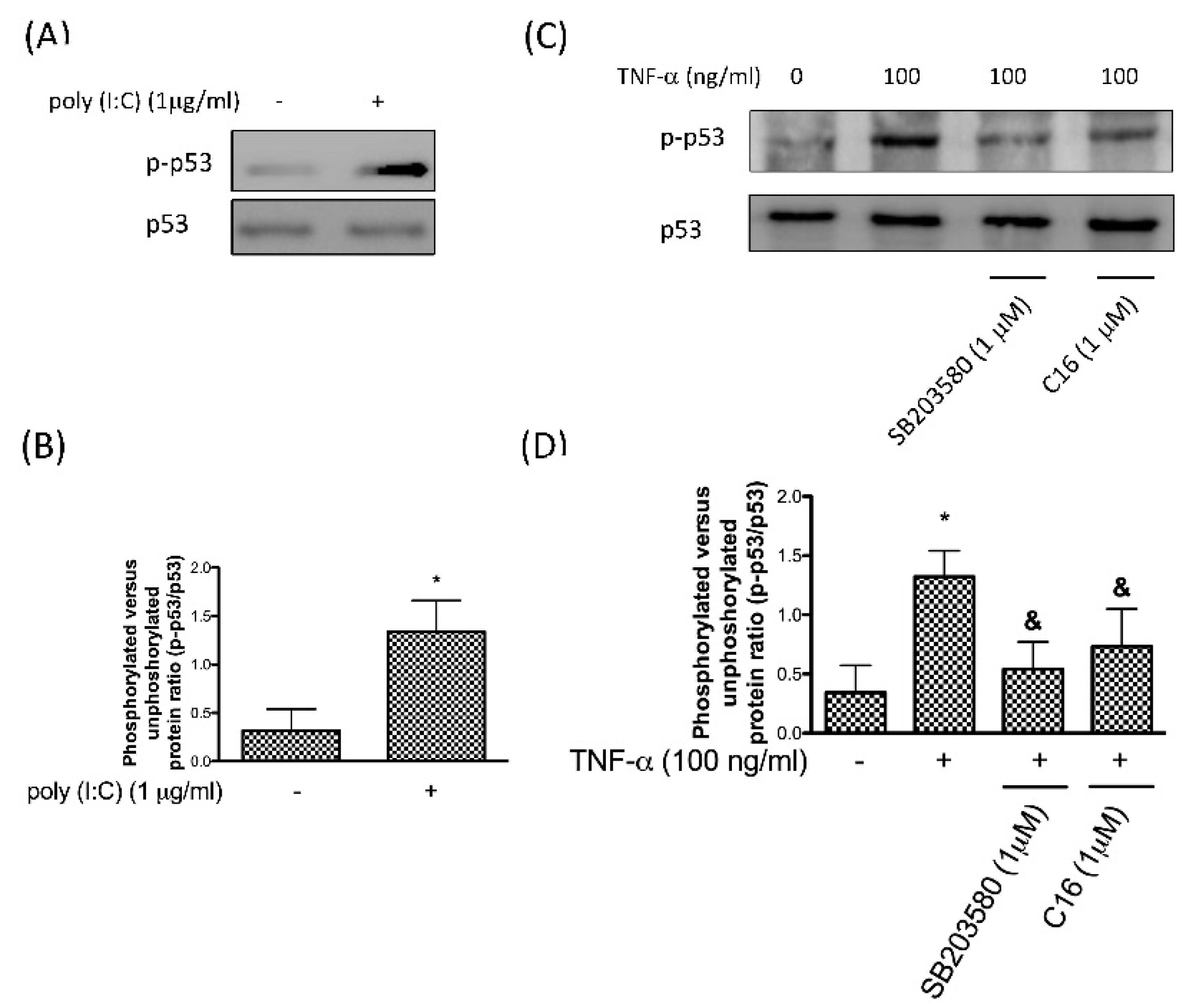
3.2. Phosphorylation of p53 after TNF-α-Induced p38 MAPK Activation Is Mediated by PKR
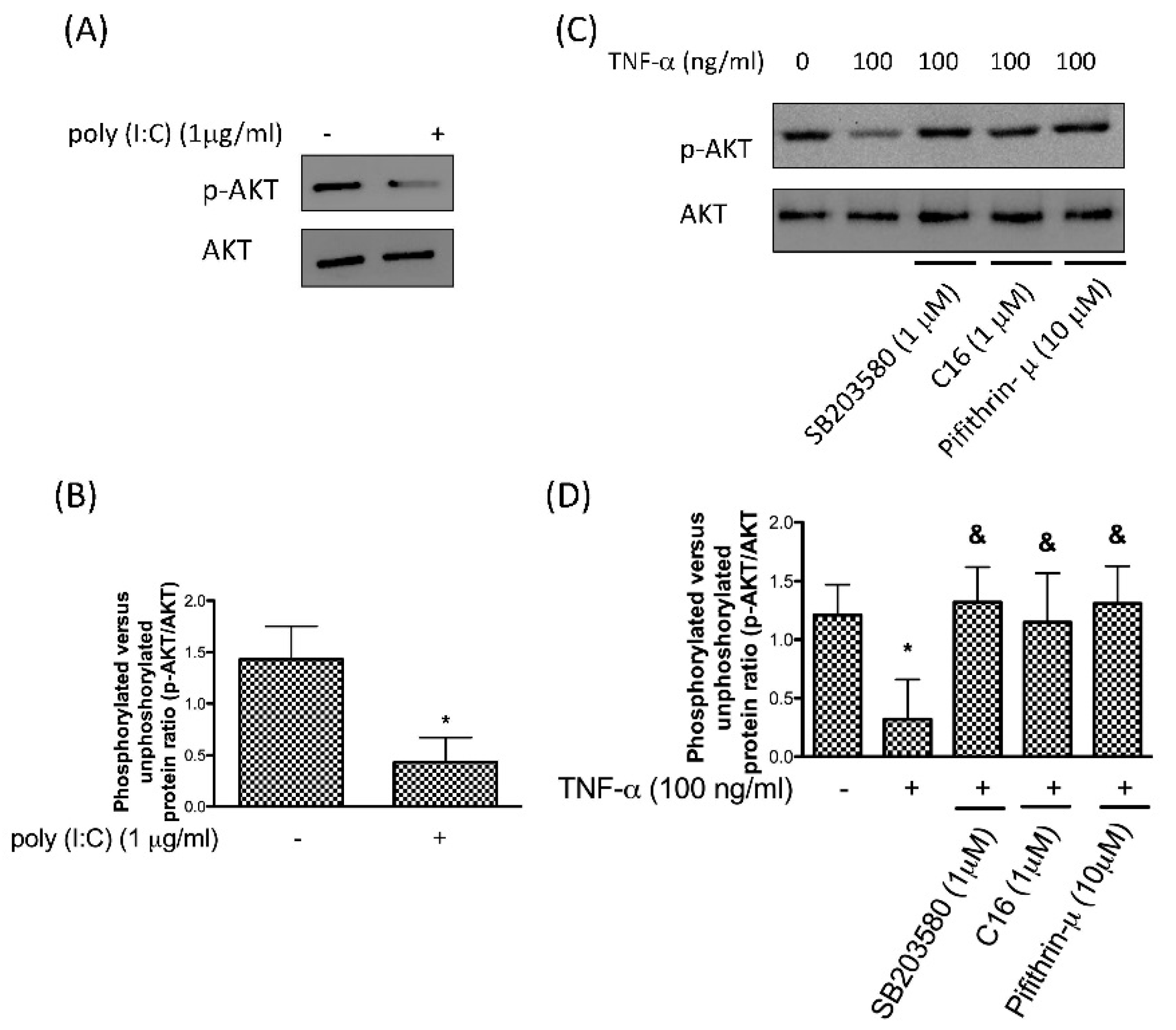
3.3. Downregulation of Phosphor-AKT by TNF-α Stimulation Is through the PKR/p38 MAPK/p53 Pathway
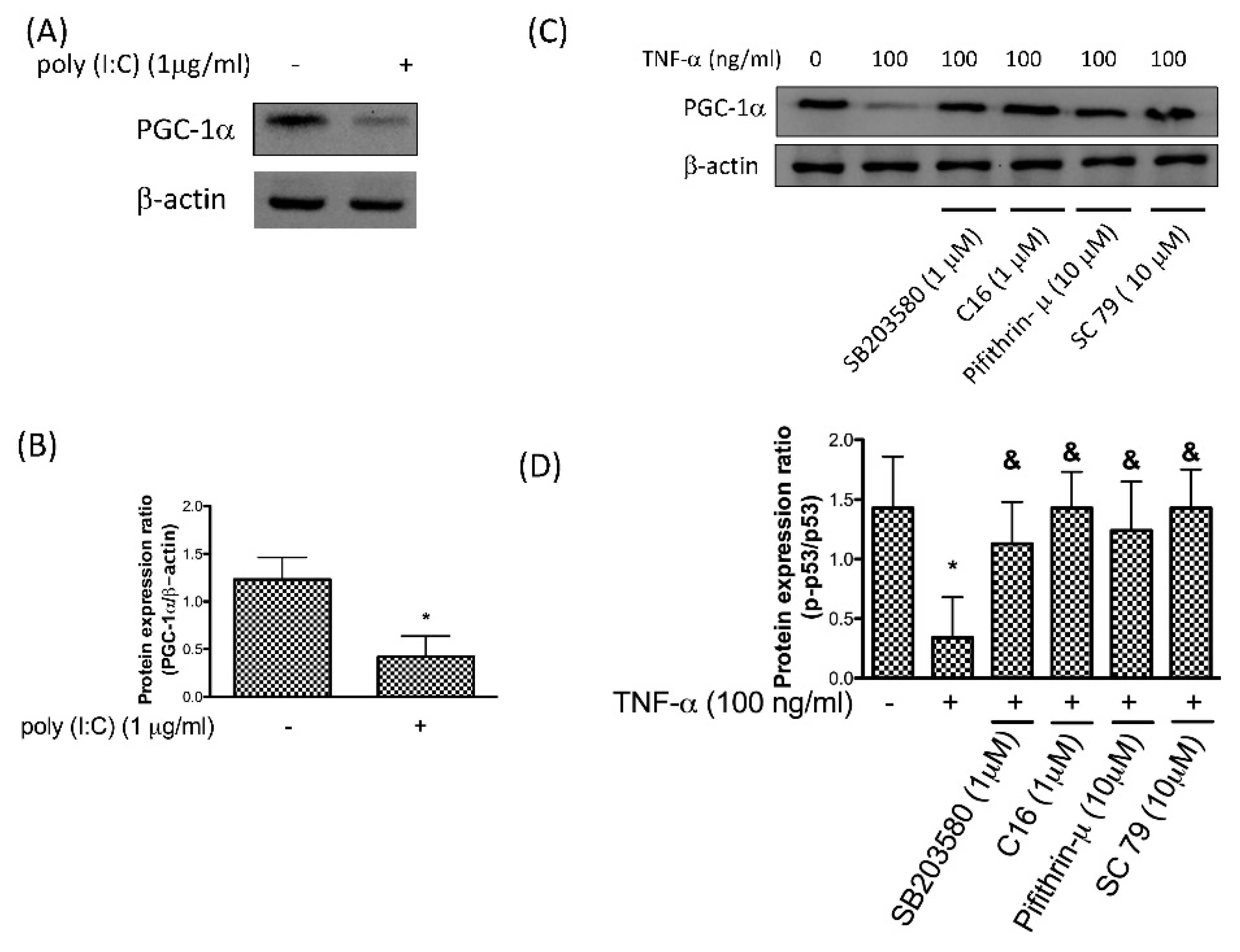
3.4. Reduction of PGC-1α by TNF-α Is via the PKR/p38 MAPK/p53/AKT Pathway
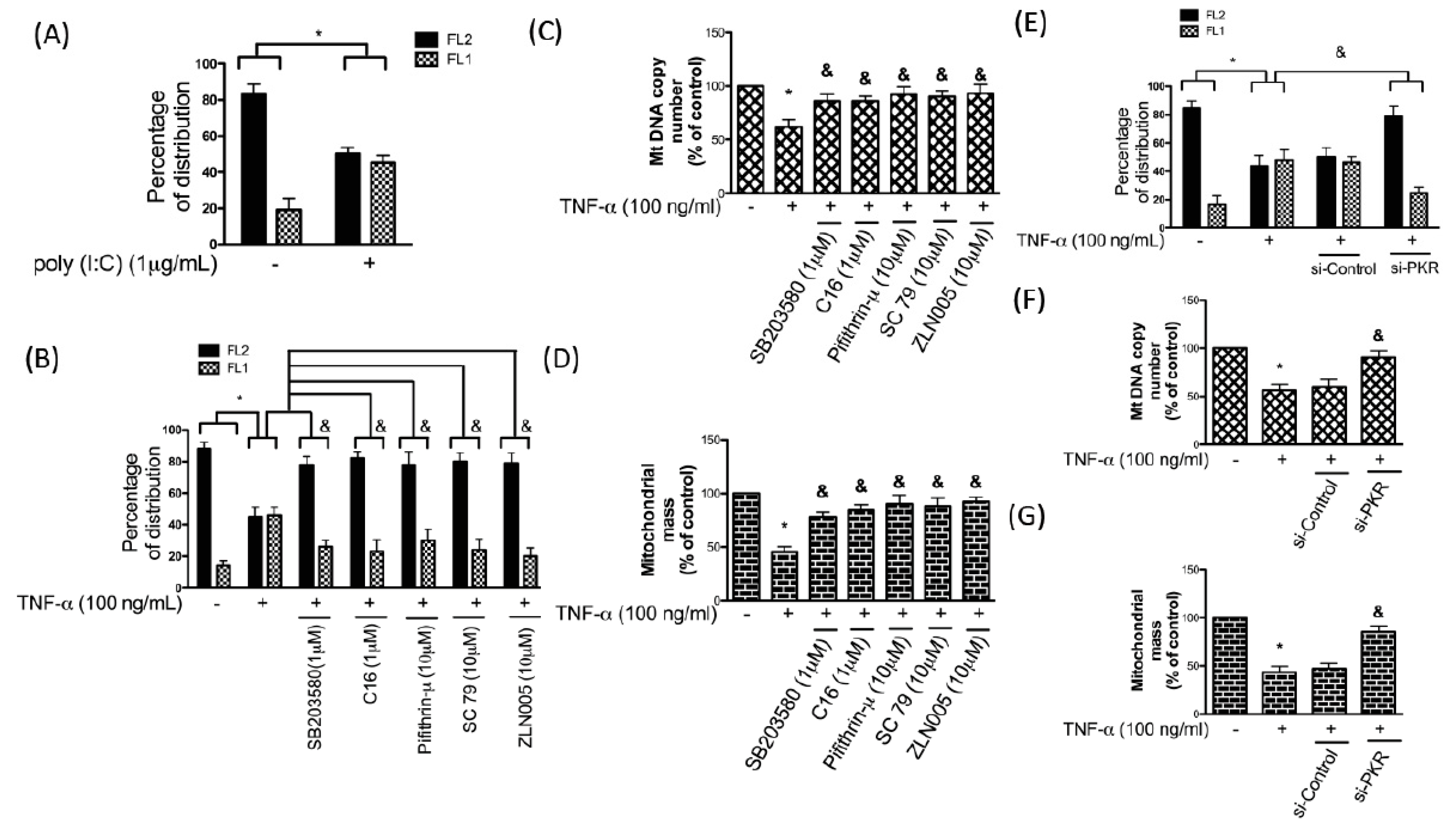
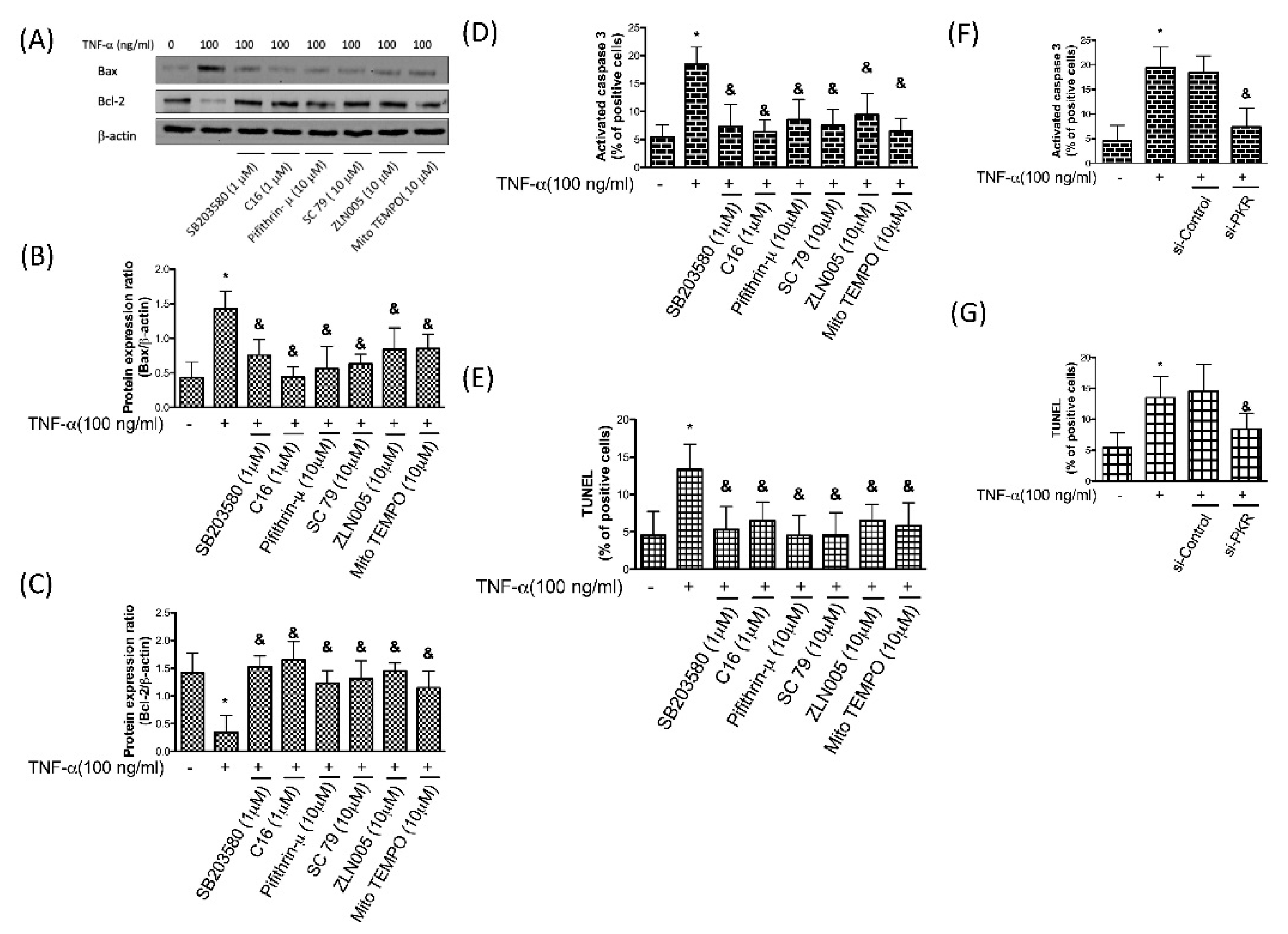
3.5. TNF-α-Induced Apoptosis in Chondrocytes Is Mediated by the PKR/p38 MAPK/p53/AKT/PGC-1α Pathway
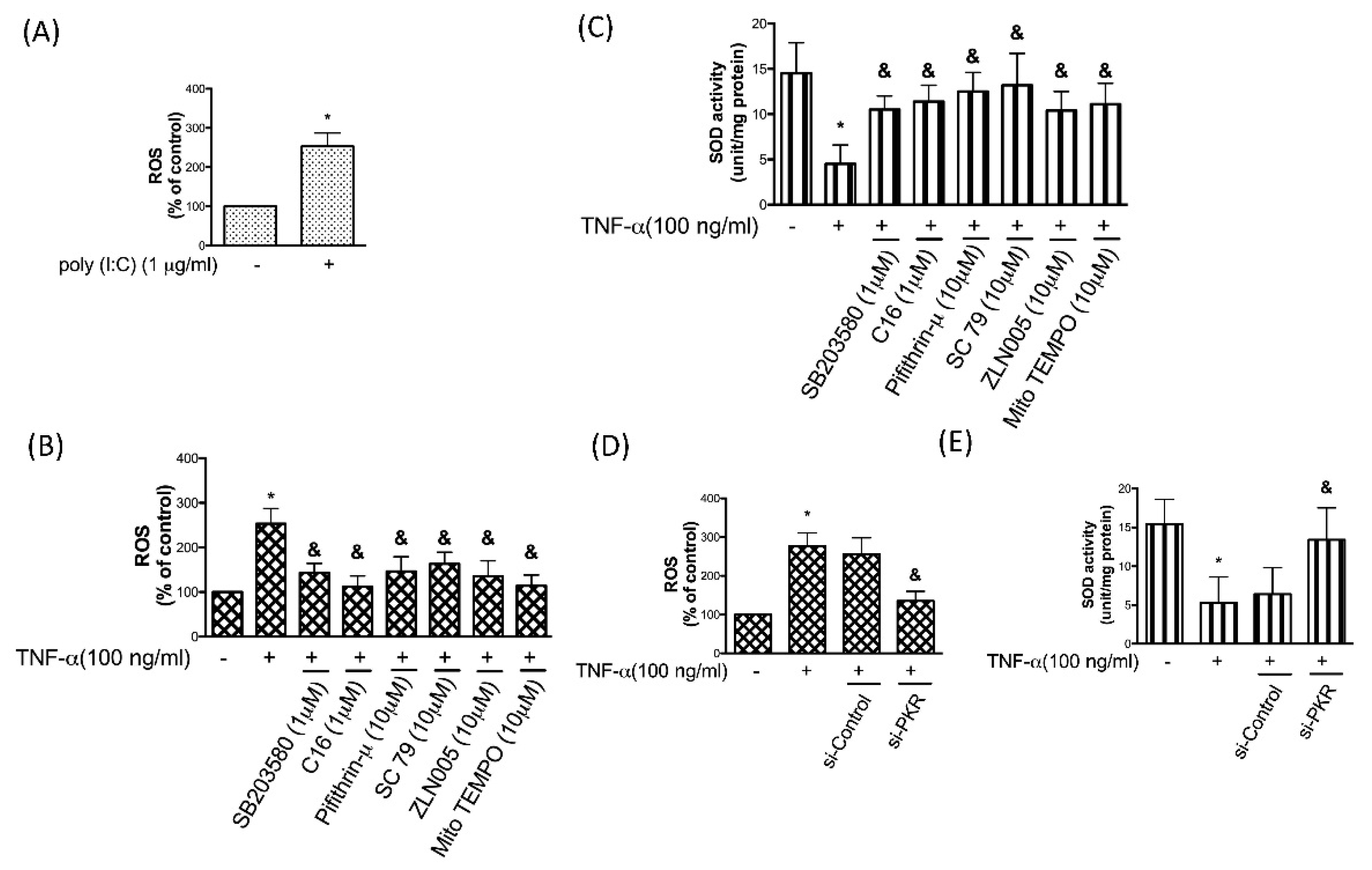
3.6. PKR is Associated with the TNF-α-Induced Oxidative Stress in Chondrocytes
3.7. TNF-α-Induced Apoptosis in Chondrocytes Is Due to the Accumulation of Oxidative Stress via PKR/p38 MAPK/p53/AKT/PGC-1α Signaling
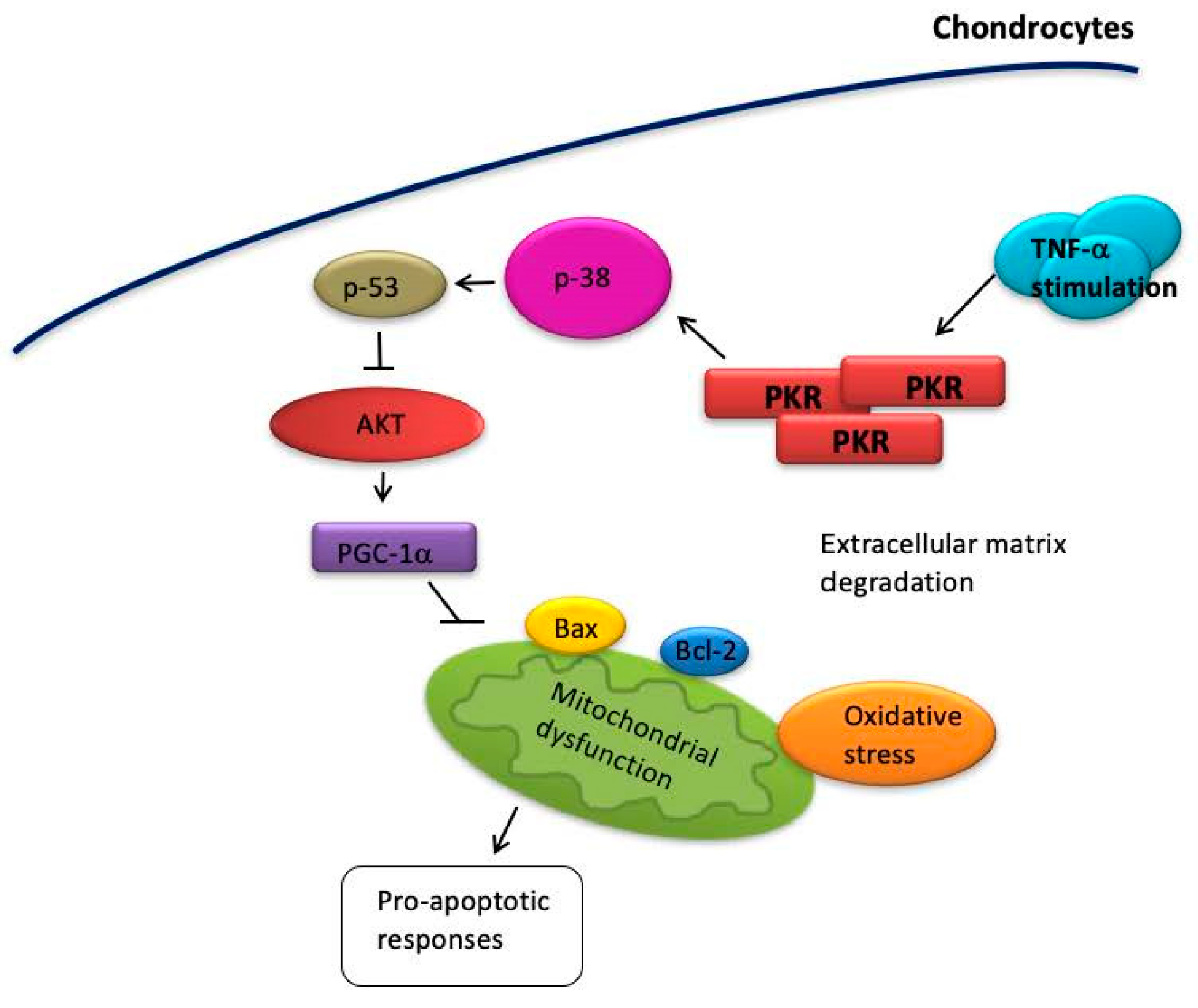
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthr. Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Jinks, C.; Jordan, K.; Croft, P. Osteoarthritis as a public health problem: The impact of developing knee pain on physical function in adults living in the community: (KNEST 3). Rheumatology 2007, 46, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Kong, X.; Fitzgerald, G.K. Psychological health impact on 2-year changes in pain and function in persons with knee pain: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2011, 19, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T. Osteoarthritis as a disease of mechanics. Osteoarthr. Cartil. 2013, 21, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, D.; Yuan, Y.; Min, J. New insights on the MMP-13 regulatory network in the pathogenesis of early osteoarthritis. Arthr. Res. Ther. 2017, 19, 248. [Google Scholar] [CrossRef] [PubMed]
- D’Lima, D.D.; Hashimoto, S.; Chen, P.C.; Colwell, C.W., Jr.; Lotz, M.K. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthr. Cartil. 2001, 9, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Guitian, R.; Vazquez-Martul, E.; de Toro, F.J.; Galdo, F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthr. Rheum. 1998, 41, 284–289. [Google Scholar] [CrossRef]
- Kim, H.T.; Lo, M.Y.; Pillarisetty, R. Chondrocyte apoptosis following intraarticular fracture in humans. Osteoarthr. Cartil. 2002, 10, 747–749. [Google Scholar] [CrossRef]
- Hashimoto, S.; Takahashi, K.; Amiel, D.; Coutts, R.D.; Lotz, M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthr. Rheum. 1998, 41, 1266–1274. [Google Scholar] [CrossRef]
- Blanco, F.J.; Ochs, R.L.; Schwarz, H.; Lotz, M. Chondrocyte apoptosis induced by nitric oxide. Am. J. Pathol. 1995, 146, 75–85. [Google Scholar] [PubMed]
- Aizawa, T.; Kon, T.; Einhorn, T.A.; Gerstenfeld, L.C. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J. Orthop. Res. 2001, 19, 785–796. [Google Scholar] [CrossRef]
- Lopez-Armada, M.J.; Carames, B.; Lires-Dean, M.; Cillero-Pastor, B.; Ruiz-Romero, C.; Galdo, F.; Blanco, F.J. Cytokines, tumor necrosis factor-α and interleukin-1β, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthr. Cartil. 2006, 14, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Carames, B.; Lopez-Armada, M.J.; Cillero-Pastor, B.; Lires-Dean, M.; Vaamonde, C.; Galdo, F.; Blanco, F.J. Differential effects of tumor necrosis factor-α and interleukin-1β on cell death in human articular chondrocytes. Osteoarthr. Cartil. 2008, 16, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.H.; Wu, C.H.; Jou, I.M.; Tu, Y.K.; Hung, C.H.; Hsieh, P.L.; Tsai, K.L. PKR activation causes inflammation and MMP-13 secretion in human degenerated articular chondrocytes. Redox Biol. 2018, 14, 72–81. [Google Scholar] [CrossRef]
- Tam, C.L.; Hofbauer, M.; Towle, C.A. Requirement for protein kinase R in interleukin-1α-stimulated effects in cartilage. Biochem. Pharmacol. 2007, 74, 1636–1641. [Google Scholar] [CrossRef]
- Gilbert, S.J.; Duance, V.C.; Mason, D.J. Does protein kinase R mediate TNF-α-and ceramide-induced increases in expression and activation of matrix metalloproteinases in articular cartilage by a novel mechanism? Arthr. Res. Ther. 2004, 6, R46–R55. [Google Scholar] [CrossRef]
- Yeung, M.C.; Liu, J.; Lau, A.S. An essential role for the interferon-inducible, double-stranded RNA-activated protein kinase PKR in the tumor necrosis factor-induced apoptosis in U937 cells. Proc. Natl. Acad. Sci. USA 1996, 93, 12451–12455. [Google Scholar] [CrossRef]
- Srivastava, S.P.; Kumar, K.U.; Kaufman, R.J. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J. Biol. Chem. 1998, 273, 2416–2423. [Google Scholar] [CrossRef]
- Lee, W.J.; Ou, H.C.; Hsu, W.C.; Chou, M.M.; Tseng, J.J.; Hsu, S.L.; Tsai, K.L.; Sheu, W.H. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J. Vasc. Surg. 2010, 52, 1290–1300. [Google Scholar] [CrossRef]
- Ou, H.C.; Chou, W.C.; Hung, C.H.; Chu, P.M.; Hsieh, P.L.; Chan, S.H.; Tsai, K.L. Galectin-3 aggravates ox-LDL-induced endothelial dysfunction through LOX-1 mediated signaling pathway. Environ. Toxicol. 2019, 34, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Zhou, H.Y.; Fu, Y.Y.; Li, Y.Y.; Wu, F.; Gu, M.; Wu, L.Y.; Xia, C.M.; Dong, T.C.; Li, J.Y.; et al. Novel small-molecule PGC-1α transcriptional regulator with beneficial effects on diabetic db/db mice. Diabetes 2013, 62, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Steinle, J.J. Epac1 inhibits PKR to reduce NLRP3 inflammasome proteins in retinal endothelial cells. J. Inflamm. Res. 2019, 12, 153–159. [Google Scholar] [CrossRef]
- Sekihara, K.; Harashima, N.; Tongu, M.; Tamaki, Y.; Uchida, N.; Inomata, T.; Harada, M. Pifithrin-mu, an inhibitor of heat-shock protein 70, can increase the antitumor effects of hyperthermia against human prostate cancer cells. PLoS ONE 2013, 8, e78772. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cai, F.; Chen, X.; Luo, M.; Hu, L.; Lu, Y. The role of mitochondria-derived reactive oxygen species in hyperthermia-induced platelet apoptosis. PLoS ONE 2013, 8, e75044. [Google Scholar] [CrossRef]
- Yui, N.; Yoshioka, H.; Fujiya, H.; Musha, H.; Beppu, M.; Karasawa, R.; Yudoh, K. The DNA repair enzyme apurinic/apyrimidinic endonuclease (Apex nuclease) 2 has the potential to protect against down-regulation of chondrocyte activity in osteoarthritis. Int. J. Mol. Sci. 2014, 15, 14921–14934. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.H.; Foster, B.K.; Zhou, X.F.; Cowin, A.J.; Xian, C.J. TNF-α mediates p38 MAP kinase activation and negatively regulates bone formation at the injured growth plate in rats. J. Bone Miner. Res. 2006, 21, 1075–1088. [Google Scholar] [CrossRef]
- Zwerina, J.; Hayer, S.; Redlich, K.; Bobacz, K.; Kollias, G.; Smolen, J.S.; Schett, G. Activation of p38 MAPK is a key step in tumor necrosis factor-mediated inflammatory bone destruction. Arthr. Rheum. 2006, 54, 463–472. [Google Scholar] [CrossRef]
- Goh, K.C.; deVeer, M.J.; Williams, B.R. The protein kinase PKR is required for p38 MAPK activation and the innate immune response to bacterial endotoxin. EMBO J. 2000, 19, 4292–4297. [Google Scholar] [CrossRef]
- Hashimoto, S.; Nishiyama, T.; Hayashi, S.; Fujishiro, T.; Takebe, K.; Kanzaki, N.; Kuroda, R.; Kurosaka, M. Role of p53 in human chondrocyte apoptosis in response to shear strain. Arthr. Rheum. 2009, 60, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Bulavin, D.V.; Saito, S.; Hollander, M.C.; Sakaguchi, K.; Anderson, C.W.; Appella, E.; Fornace, A.J., Jr. Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 1999, 18, 6845–6854. [Google Scholar] [CrossRef] [PubMed]
- Perfettini, J.L.; Castedo, M.; Nardacci, R.; Ciccosanti, F.; Boya, P.; Roumier, T.; Larochette, N.; Piacentini, M.; Kroemer, G. Essential role of p53 phosphorylation by p38 MAPK in apoptosis induction by the HIV-1 envelope. J. Exp. Med. 2005, 201, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, T.M.; Leal, J.F.; Seger, R.; Taya, Y.; Oren, M. Cross-talk between Akt, p53 and Mdm2: Possible implications for the regulation of apoptosis. Oncogene 2002, 21, 1299–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Lotz, M.; Terkeltaub, R.; Liu-Bryan, R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor γ coactivator 1α. Arthr. Rheumatol. 2015, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ahn, N.; Jung, S.; Park, S. Effects of intermittent ladder-climbing exercise training on mitochondrial biogenesis and endoplasmic reticulum stress of the cardiac muscle in obese middle-aged rats. Korean J. Physiol. Pharmacol. 2017, 21, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yin, P.H.; Lu, C.Y.; Chi, C.W.; Wei, Y.H. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem. J. 2000, 348, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Pawlowski, J.; Kraft, A.S. Bax-induced apoptotic cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 529–531. [Google Scholar] [CrossRef]
- Goldring, M.B. Osteoarthritis and cartilage: The role of cytokines. Curr. Rheumatol. Rep. 2000, 2, 459–465. [Google Scholar] [CrossRef]
- Shlopov, B.V.; Gumanovskaya, M.L.; Hasty, K.A. Autocrine regulation of collagenase 3 (matrix metalloproteinase 13) during osteoarthritis. Arthr. Rheum. 2000, 43, 195–205. [Google Scholar] [CrossRef]
- Tetlow, L.C.; Adlam, D.J.; Woolley, D.E. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: Associations with degenerative changes. Arthr. Rheum. 2001, 44, 585–594. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Feng, Z.; Wang, P.; Zhu, W.; Cui, S. Catalase enhances viability of human chondrocytes in culture by reducing reactive oxygen species and counteracting tumor necrosis factor-α-induced apoptosis. Cellular Physiol. Biochem. 2018, 49, 2427–2442. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Romero, C.; Calamia, V.; Mateos, J.; Carreira, V.; Martinez-Gomariz, M.; Fernandez, M.; Blanco, F.J. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: A decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol. Cell. Proteom. 2009, 8, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Gavriilidis, C.; Miwa, S.; von Zglinicki, T.; Taylor, R.W.; Young, D.A. Mitochondrial dysfunction in osteoarthritis is associated with down-regulation of superoxide dismutase 2. Arthr. Rheum. 2013, 65, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.J.; Blain, E.J.; Al-Sabah, A.; Zhang, Y.; Duance, V.C.; Mason, D.J. Protein kinase R plays a pivotal role in oncostatin M and interleukin-1 signalling in bovine articular cartilage chondrocytes. Eur. Cells Mater. 2012, 23, 41–57. [Google Scholar] [CrossRef]
- Der, S.D.; Yang, Y.L.; Weissmann, C.; Williams, B.R. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 3279–3283. [Google Scholar] [CrossRef]
- Lee, S.B.; Esteban, M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology 1994, 199, 491–496. [Google Scholar] [CrossRef]
- Hattori, Y.; Kojima, T.; Kato, D.; Matsubara, H.; Takigawa, M.; Ishiguro, N. A selective estrogen receptor modulator inhibits tumor necrosis factor-α-induced apoptosis through the ERK1/2 signaling pathway in human chondrocytes. Biochem. Biophys. Res. Commun. 2012, 421, 418–424. [Google Scholar] [CrossRef]
- Yeung, M.C.; Lau, A.S. Tumor suppressor p53 as a component of the tumor necrosis factor-induced, protein kinase PKR-mediated apoptotic pathway in human promonocytic U937 cells. J Biol. Chem. 1998, 273, 25198–25202. [Google Scholar] [CrossRef]
- Yoon, C.H.; Lee, E.S.; Lim, D.S.; Bae, Y.S. PKR, a p53 target gene, plays a crucial role in the tumor-suppressor function of p53. Proc. Natl. Acad. Sci. USA 2009, 106, 7852–7857. [Google Scholar] [CrossRef] [PubMed]
- Lomas, C.; Tang, X.D.; Chanalaris, A.; Saklatvala, J.; Vincent, T.L. Cyclic mechanical load causes global translational arrest in articular chondrocytes: A process which is partially dependent upon PKR phosphorylation. Eur. Cells Mater. 2011, 22, 178–189. [Google Scholar] [CrossRef]
- Morimoto, H.; Baba, R.; Haneji, T.; Doi, Y. Double-stranded RNA-dependent protein kinase regulates insulin-stimulated chondrogenesis in mouse clonal chondrogenic cells, ATDC-5. Cell Tissue Res. 2013, 351, 41–47. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.-H.; Wu, C.-H.; Jou, I.-M.; Tu, Y.-K.; Hung, C.-H.; Chou, W.-C.; Chang, Y.-C.; Hsieh, P.-L.; Tsai, K.-L. PKR Promotes Oxidative Stress and Apoptosis of Human Articular Chondrocytes by Causing Mitochondrial Dysfunction through p38 MAPK Activation—PKR Activation Causes Apoptosis in Human Chondrocytes. Antioxidants 2019, 8, 370. https://doi.org/10.3390/antiox8090370
Ma C-H, Wu C-H, Jou I-M, Tu Y-K, Hung C-H, Chou W-C, Chang Y-C, Hsieh P-L, Tsai K-L. PKR Promotes Oxidative Stress and Apoptosis of Human Articular Chondrocytes by Causing Mitochondrial Dysfunction through p38 MAPK Activation—PKR Activation Causes Apoptosis in Human Chondrocytes. Antioxidants. 2019; 8(9):370. https://doi.org/10.3390/antiox8090370
Chicago/Turabian StyleMa, Ching-Hou, Chin-Hsien Wu, I-Ming Jou, Yuan-Kun Tu, Ching-Hsia Hung, Wan-Ching Chou, Yun-Ching Chang, Pei-Ling Hsieh, and Kun-Ling Tsai. 2019. "PKR Promotes Oxidative Stress and Apoptosis of Human Articular Chondrocytes by Causing Mitochondrial Dysfunction through p38 MAPK Activation—PKR Activation Causes Apoptosis in Human Chondrocytes" Antioxidants 8, no. 9: 370. https://doi.org/10.3390/antiox8090370
APA StyleMa, C.-H., Wu, C.-H., Jou, I.-M., Tu, Y.-K., Hung, C.-H., Chou, W.-C., Chang, Y.-C., Hsieh, P.-L., & Tsai, K.-L. (2019). PKR Promotes Oxidative Stress and Apoptosis of Human Articular Chondrocytes by Causing Mitochondrial Dysfunction through p38 MAPK Activation—PKR Activation Causes Apoptosis in Human Chondrocytes. Antioxidants, 8(9), 370. https://doi.org/10.3390/antiox8090370





