Maltol Improves APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Inflammation Response via NF-κB and PI3K/Akt Signal Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Experiments
2.3. Analysis of ALT and AST Biochemical Markers
2.4. Analysis of GSH, SOD, and MDA Oxidative Markers
2.5. Analysis of TNF-α and IL-1β Inflammation Markers
2.6. Histopathological Examination
2.7. Hoechst 33258 Staining
2.8. Immunohistochemistry and Immunofluorescence Staining
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
3.1. Maltol Ameliorated APAP-Induced Hepatic Dysfunction
3.2. Maltol Mitigated APAP-Induced Oxidative Stress Injury
3.3. Maltol Mitigated APAP-Induced Liver Histopathological Changes
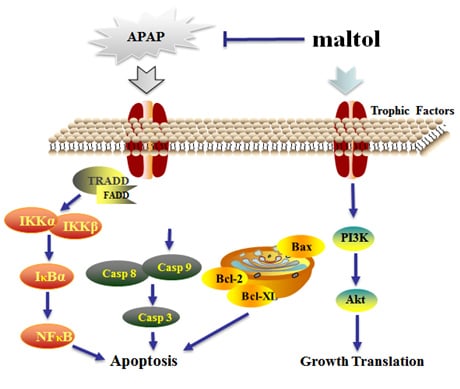
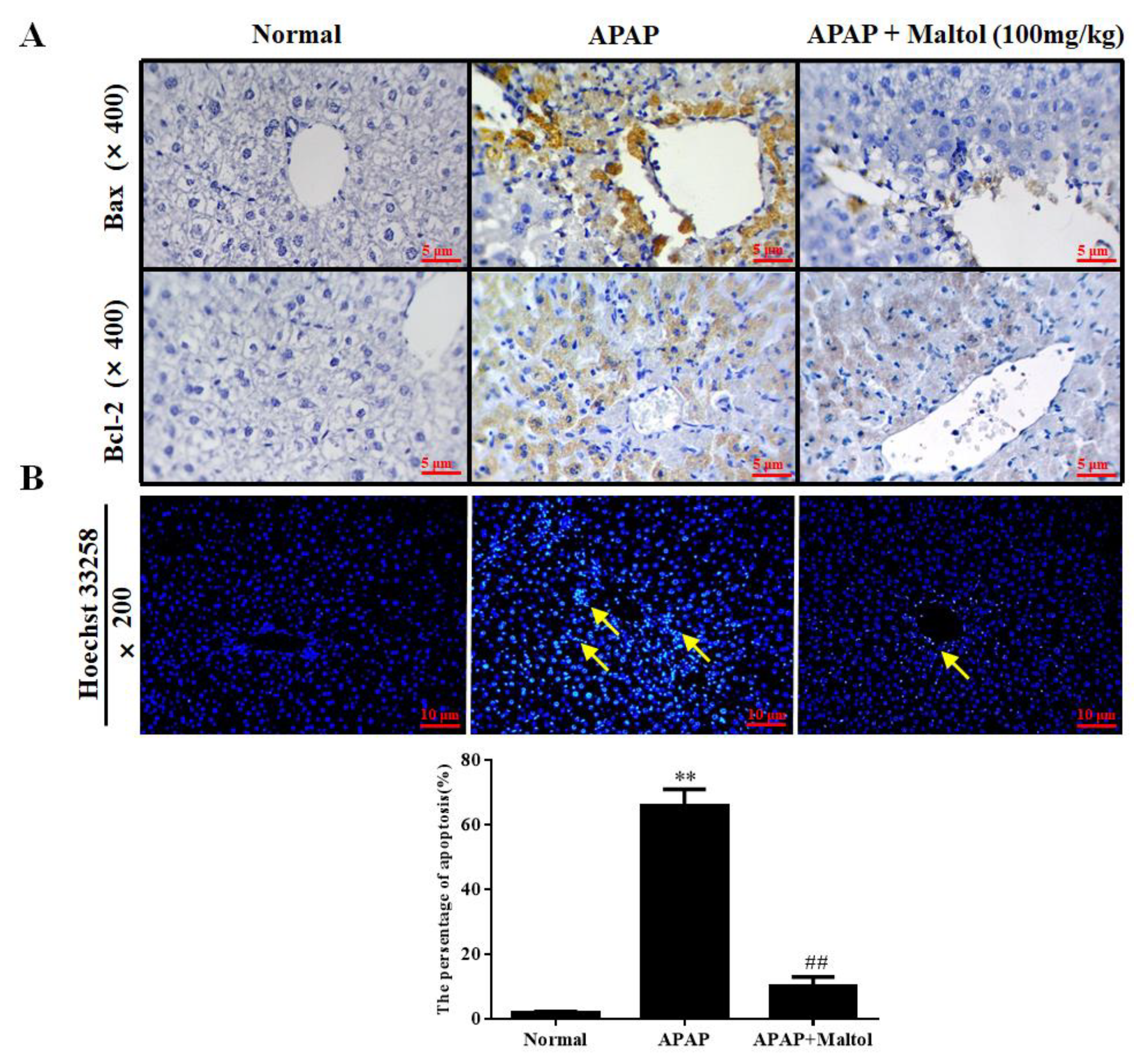
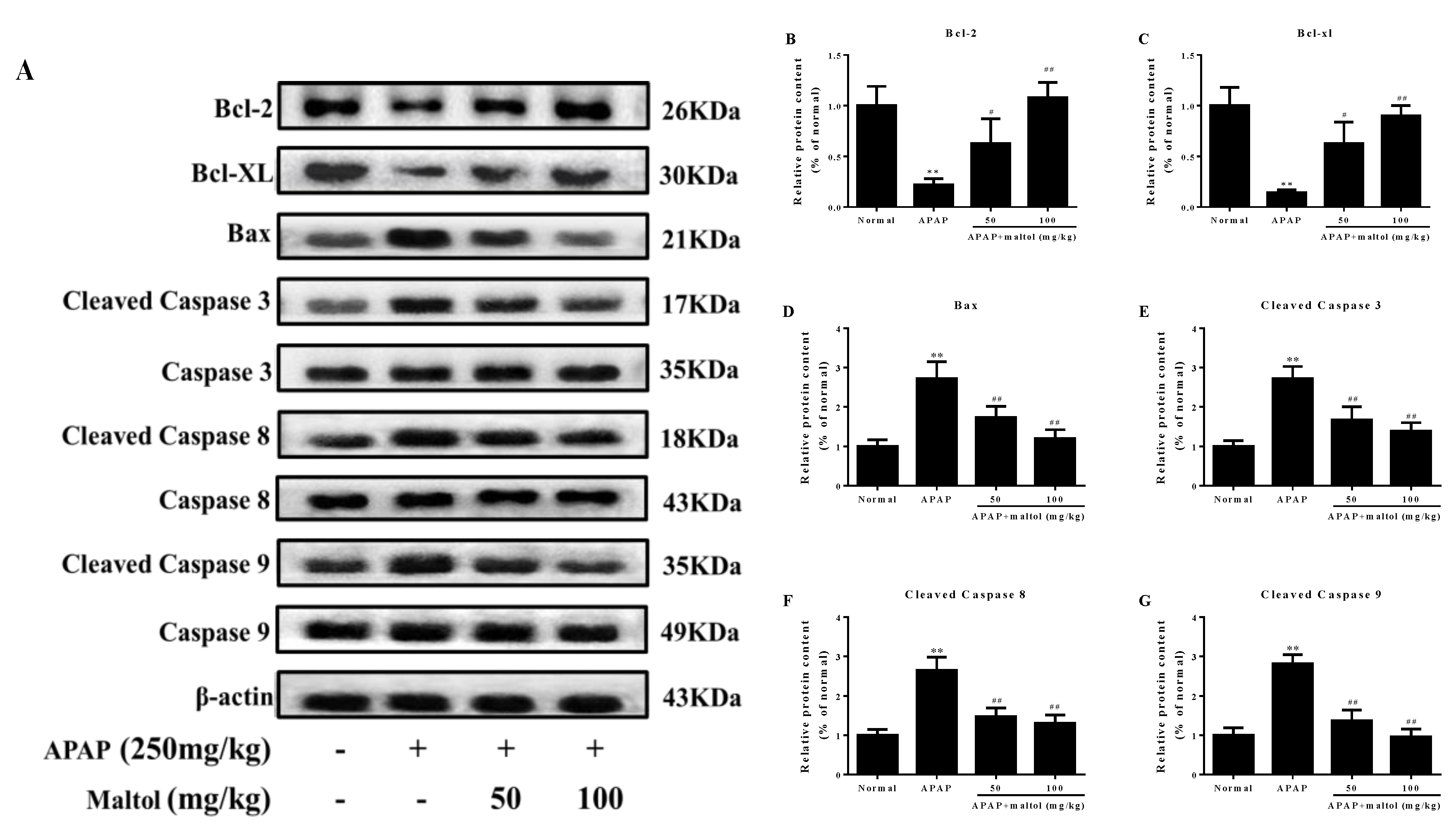
3.4. Maltol Mitigated APAP-Induced Apoptosis
3.5. Maltol Mitigated APAP-Induced Inflammatory Responses
3.6. Maltol Regulated the PI3K/Akt Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APAP | Acetaminophen |
| ALF | Acute Liver Failure |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| ROS | Reactive Oxygen Species |
| CYP2E1 | Cytochrome P450 E1 |
| 4-HNE | 4-hydroxynonenal |
| NAPQI | N-acetyl-P-aminophenol |
| H&E | Hematoxylin and Eosin |
| NF-κB | Nuclear factor-kappa B |
| Akt | Protein kinase B |
| PI3K | Phosphatidylinositol 3-kinase |
| IKKα | Inhibitor kappa B kinase α |
| IKKβ | Inhibitor kappa B kinase β |
References
- Jaeschke, H. Acetaminophen: Dose-dependent drug hepatotoxicity and acute liver failure in patients. Dig. Dis. 2015, 33, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Gandillet, A.; Vidal, I.; Alexandre, E.; Audet, M.; Chenard-Neu, M.P.; Stutzmann, J.; Heyd, B.; Jaeck, D.; Richert, L. Experimental models of acute and chronic liver failure in nude mice to study hepatocyte transplantation. Cell Transpl. 2005, 14, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.; Babar, A.; Choudhary, M.; Kutner, M.; Pyrsopoulos, N. Acetaminophen-induced hepatotoxicity: A comprehensive update. J. Clin. Transl. Hepatol. 2016, 4, 131–142. [Google Scholar] [PubMed]
- Hinson, J.A.; Reid, A.B.; McCullough, S.S.; James, L.P. Acetaminophen-induced hepatotoxicity: Role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab. Rev. 2004, 36, 805–822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cong, X.; Zheng, L.; Xu, L.; Yin, L.; Peng, J. Dioscin, a natural steroid saponin, shows remarkable protective effect against acetaminophen-induced liver damage in vitro and in vivo. Toxicol. Lett. 2012, 214, 69–80. [Google Scholar] [CrossRef]
- Brown, J.M.; Kuhlman, C.; Terneus, M.V.; Labenski, M.T.; Lamyaithong, A.B.; Ball, J.G.; Lau, S.S.; Valentovic, M.A. S-adenosyl-l-methionine protection of acetaminophen mediated oxidative stress and identification of hepatic 4-hydroxynonenal protein adducts by mass spectrometry. Toxicol. Appl. Pharmacol. 2014, 281, 174–184. [Google Scholar] [CrossRef] [Green Version]
- Jayasooriya, R.G.; Moon, D.O.; Yun, S.G.; Choi, Y.H.; Asami, Y.; Kim, M.O.; Jang, J.H.; Kim, B.Y.; Ahn, J.S.; Kim, G.Y. Verrucarin A enhances TRAIL-induced apoptosis via NF-kappaB-mediated Fas overexpression. Food Chem. Toxicol. 2013, 55, 1–7. [Google Scholar] [CrossRef]
- Leng, J.; Wang, Z.; Fu, C.L. NF-κB and AMPK/PI3K/Akt signaling pathways are involved in the protective effects of Platycodon grandiflorum saponins against acetaminophen-induced acute hepatotoxicity in mice. Phytother. Res. 2018, 32, 1–12. [Google Scholar] [CrossRef]
- Li, W.; Su, X.; Han, Y.; Xu, Q.; Zhang, J.; Wang, Z.; Wang, Y. Maltol, a Maillard reaction product, exerts anti-tumor efficacy in H22 tumor-bearing mice via improving immune function and inducing apoptosis. Rsc. Adv. 2015, 5, 101850–101859. [Google Scholar] [CrossRef]
- Sha, J.Y.; Zhou, Y.Y.; Yang, J.Y.; Leng, J.; Li, J.H.; Hu, J.N.; Liu, W.; Jiang, S.; Wang, Y.P.; Chen, C.; et al. Maltol (3-hydroxy-2-methyl-4-pyrone) slows D-galactose-induced brain aging process by damping the Nrf2/HO-1-mediated oxidative stress in mice. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef]
- Anwar-Mohamed, A.; El-Kadi, A.O. Induction of cytochrome P450 1a1 by the food flavoring agent, maltol. Toxicol In Vitro 2007, 21, 685–690. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Barathi, D.; Mathammal, R.; Balamani, J.; Jayamani, N. Spectroscopic properties, NLO, HOMO-LUMO and NBO of maltol. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 121, 245–253. [Google Scholar] [CrossRef]
- Kim, Y.B.; Oh, S.H.; Sok, D.E.; Kim, M.R. Neuroprotective effect of maltol against oxidative stress in brain of mice challenged with kainic acid. Nutr. Neurosci. 2004, 7, 33–39. [Google Scholar]
- Wei, A.; Mura, K.; Shibamoto, T. Antioxidative activity of volatile chemicals extracted from beer. J. Agric. Food Chem. 2001, 49, 4097–4101. [Google Scholar] [CrossRef]
- Mi, X.J.; Hou, J.G.; Jiang, S.; Liu, Z.; Tang, S.; Liu, X.X.; Wang, Y.P.; Chen, C.; Wang, Z.; Li, W. Maltol mitigates thioacetamide-induced liver fibrosis through TGF-beta1-mediated activation of PI3K/Akt signaling pathway. J. Agric. Food Chem. 2019, 67, 1392–1401. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Hou, J.G.; Zhou, Y.D.; He, Y.F.; Jiang, S.; Wang, Y.P.; Ren, S.; Li, W. The liver protection effects of maltol, a flavoring agent, on carbon tetrachloride-induced acute liver injury in mice via inhibiting apoptosis and inflammatory response. Molecules 2018, 23, 2120. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Q.; Hu, J.N.; Han, X.Y.; Li, W.; Zhao, L.C. Maltol, a food flavoring agent, attenuates acute alcohol-induced oxidative damage in mice. Nutrients 2015, 7, 682–696. [Google Scholar] [CrossRef]
- Li, W.; Yan, M.H.; Liu, Y.; Liu, Z.; Wang, Z.; Chen, C.; Zhang, J.; Sun, Y.S. Ginsenoside Rg5 ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of inflammation, oxidative stress, and apoptosis. Nutrients 2016, 8, 566. [Google Scholar] [CrossRef]
- Li, R.Y.; Zhang, W.Z.; Yan, X.T.; Hou, J.G.; Wang, Z.; Ding, C.B.; Liu, W.C.; Zheng, Y.N.; Chen, C.; Li, Y.R.; et al. Arginyl-fructosyl-glucose, a major maillard reaction product of red ginseng, attenuates cisplatin-induced acute kidney injury by regulating nuclear factor kappaB and phosphatidylinositol 3-kinase/protein kinase B signaling pathways. J. Agric. Food Chem. 2019, 67, 5754–5763. [Google Scholar] [CrossRef]
- Xu, X.Y.; Hu, J.N.; Liu, Z.; Zhang, R.; He, Y.F.; Hou, W.; Wang, Z.Q.; Yang, G.; Li, W. Saponins (Ginsenosides) from the leaves of panax quinquefolius ameliorated acetaminophen-induced hepatotoxicity in mice. J. Agric. Food Chem. 2017, 65, 3684–3692. [Google Scholar] [CrossRef]
- Yan, X.T.; Sun, Y.S.; Ren, S.; Zhao, L.C.; Liu, W.C.; Chen, C.; Wang, Z.; Li, W. Dietary alpha-mangostin provides protective effects against acetaminophen-induced hepatotoxicity in mice via Akt/mTOR-mediated inhibition of autophagy and apoptosis. Int. J. Mol. Sci. 2018, 19, 1335. [Google Scholar] [CrossRef]
- Michael Brown, J.; Ball, J.G.; Wright, M.S.; Van Meter, S.; Valentovic, M.A. Novel protective mechanisms for S-adenosyl-L-methionine against acetaminophen hepatotoxicity: Improvement of key antioxidant enzymatic function. Toxicol. Lett. 2012, 212, 320–328. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, F.; Lin, S.; Pan, X.; Jin, L.; Lu, T.; Shi, L.; Wang, Y.; Xu, A.; Li, X. Adiponectin protects against acetaminophen-induced mitochondrial dysfunction and acute liver injury by promoting autophagy in mice. J. Hepatol. 2014, 61, 825–831. [Google Scholar] [CrossRef]
- Song, Y.; Hong, S.; Iizuka, Y.; Kim, C.Y.; Seong, G.J. The neuroprotective effect of maltol against oxidative stress on rat retinal neuronal cells. Korean J. Ophthalmol. 2015, 29, 58–65. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.N.; Yan, M.H.; Xing, J.J.; Liu, W.C.; Li, W. Caspase-mediated anti-apoptotic effect of ginsenoside Rg5, a main rare ginsenoside, on acetaminophen-induced hepatotoxicity in mice. J. Agric. Food Chem. 2017, 65, 9226–9236. [Google Scholar] [CrossRef]
- Hu, J.N.; Xu, X.Y.; Li, W.; Wang, Y.M.; Liu, Y.; Wang, Z.; Wang, Y.P. Ginsenoside Rk1 ameliorates paracetamol-induced hepatotoxicity in mice through inhibition of inflammation, oxidative stress, nitrative stress and apoptosis. J. Ginseng Res. 2019, 43, 10–19. [Google Scholar] [CrossRef]
- McGill, M.R.; Jaeschke, H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013, 30, 2174–2187. [Google Scholar] [CrossRef]
- Hau, D.K.; Gambari, R.; Wong, R.S.; Yuen, M.C.; Cheng, G.Y.; Tong, C.S.; Zhu, G.Y.; Leung, A.K.; Lai, P.B.; Lau, F.Y.; et al. Phyllanthus urinaria extract attenuates acetaminophen induced hepatotoxicity: Involvement of cytochrome P450 CYP2E1. Phytomedicine 2009, 16, 751–760. [Google Scholar] [CrossRef]
- Shou, Y.; Li, N.; Li, L.; Borowitz, J.L.; Isom, G.E. NF-kappaB-mediated up-regulation of Bcl-X(S) and Bax contributes to cytochrome c release in cyanide-induced apoptosis. J. Neurochem. 2002, 81, 842–852. [Google Scholar] [CrossRef]
- McGill, M.R.; Sharpe, M.R.; Williams, C.D.; Taha, M.; Curry, S.C.; Jaeschke, H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 2012, 122, 1574–1583. [Google Scholar] [CrossRef] [Green Version]
- Jaeschke, H.; Duan, L.; Akakpo, J.Y.; Farhood, A.; Ramachandran, A. The role of apoptosis in acetaminophen hepatotoxicity. Food Chem. Toxicol. 2018, 118, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. BCL-2 protein family. Essential regulators of cell death. Preface. Adv. Exp. Med. Biol. 2010, 687, vii–viii. [Google Scholar] [PubMed]
- Moshari, S.; Nejati, V.; Najafi, G.; Razi, M. Nanomicelle curcumin-induced DNA fragmentation in testicular tissue; Correlation between mitochondria dependent apoptosis and failed PCNA-related hemostasis. Acta Histochem. 2017, 119, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.J.; Bentley, I.S.; Tanner, A.R.; Saunders, P.K.; Millward-Sadler, G.H.; Wright, R. Oxygen-derived free radicals promote hepatic injury in the rat. Gastroenterology 1985, 89, 1114–1122. [Google Scholar] [CrossRef]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.L.; Kamata, H.; Karin, M. IKK/NF-kappaB signaling: Balancing life and death—A new approach to cancer therapy. J. Clin. Invest. 2005, 115, 2625–2632. [Google Scholar] [CrossRef]
- Robinson, S.M.; Mann, D.A. Role of nuclear factor kappaB in liver health and disease. Clin. Sci. 2010, 118, 691–705. [Google Scholar] [CrossRef]
- Bieghs, V.; Trautwein, C. The innate immune response during liver inflammation and metabolic disease. Trends Immunol. 2013, 34, 446–452. [Google Scholar] [CrossRef]
- Yu, L.; She, T.; Li, M.; Shi, C.; Han, L.; Cheng, M. Tetramethylpyrazine inhibits angiotensin II-induced cardiomyocyte hypertrophy and tumor necrosis factor-alpha secretion through an NF-kappaB-dependent mechanism. Int. J. Mol. Med. 2013, 32, 717–722. [Google Scholar] [CrossRef]
- Rasul, A.; Ding, C.; Li, X.; Khan, M.; Yi, F.; Ali, M.; Ma, T. Dracorhodin perchlorate inhibits PI3K/Akt and NF-kappaB activation, up-regulates the expression of p53, and enhances apoptosis. Apoptosis 2012, 17, 1104–1119. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Cheng, H.; Lv, H.; Cheng, G.; Ci, X. Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3β pathway. Free Radic. Biol. Med. 2016, 101, 401–412. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Hao, W.; Hu, J.; Mi, X.; Han, Y.; Ren, S.; Jiang, S.; Wang, Y.; Li, X.; Li, W. Maltol Improves APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Inflammation Response via NF-κB and PI3K/Akt Signal Pathways. Antioxidants 2019, 8, 395. https://doi.org/10.3390/antiox8090395
Wang Z, Hao W, Hu J, Mi X, Han Y, Ren S, Jiang S, Wang Y, Li X, Li W. Maltol Improves APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Inflammation Response via NF-κB and PI3K/Akt Signal Pathways. Antioxidants. 2019; 8(9):395. https://doi.org/10.3390/antiox8090395
Chicago/Turabian StyleWang, Zi, Weinan Hao, Junnan Hu, Xiaojie Mi, Ye Han, Shen Ren, Shuang Jiang, Yingping Wang, Xindian Li, and Wei Li. 2019. "Maltol Improves APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Inflammation Response via NF-κB and PI3K/Akt Signal Pathways" Antioxidants 8, no. 9: 395. https://doi.org/10.3390/antiox8090395
APA StyleWang, Z., Hao, W., Hu, J., Mi, X., Han, Y., Ren, S., Jiang, S., Wang, Y., Li, X., & Li, W. (2019). Maltol Improves APAP-Induced Hepatotoxicity by Inhibiting Oxidative Stress and Inflammation Response via NF-κB and PI3K/Akt Signal Pathways. Antioxidants, 8(9), 395. https://doi.org/10.3390/antiox8090395