Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Animals and Experimental Ethics Protocol
2.3. Experimental Design and Sampling
2.4. Hepatic Lead Concentration
2.5. Liver Function Parameters
2.6. Hepatic Inflammatory Biomarkers
2.7. Hepatic Apoptotic Biomarkers
2.8. Hepatic Oxidative Stress Markers
2.9. Liver Antioxidant Capacity
2.10. Gene Expression Analysis
2.11. Immunohistochemical Examination
2.12. Western Blotting Analyses
2.13. Histopathological Examination
2.14. Statistical Analysis
3. Results
3.1. Clinical Signs and Mortality
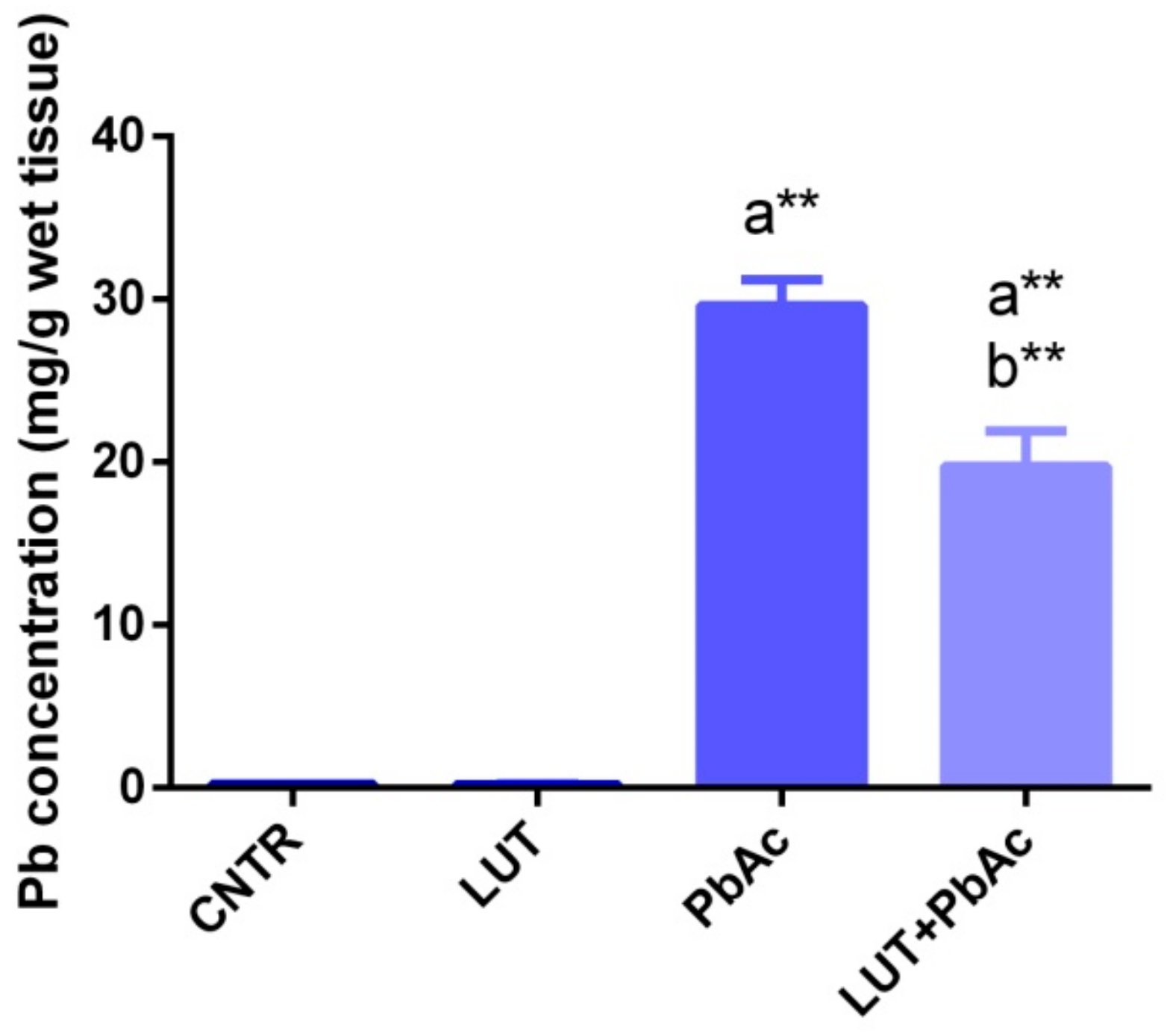
3.2. Lead Concentration in Liver Tissue
3.3. Liver Function Markers
3.4. Antioxidant/Oxidant Capacity
3.5. Inflammatory Markers
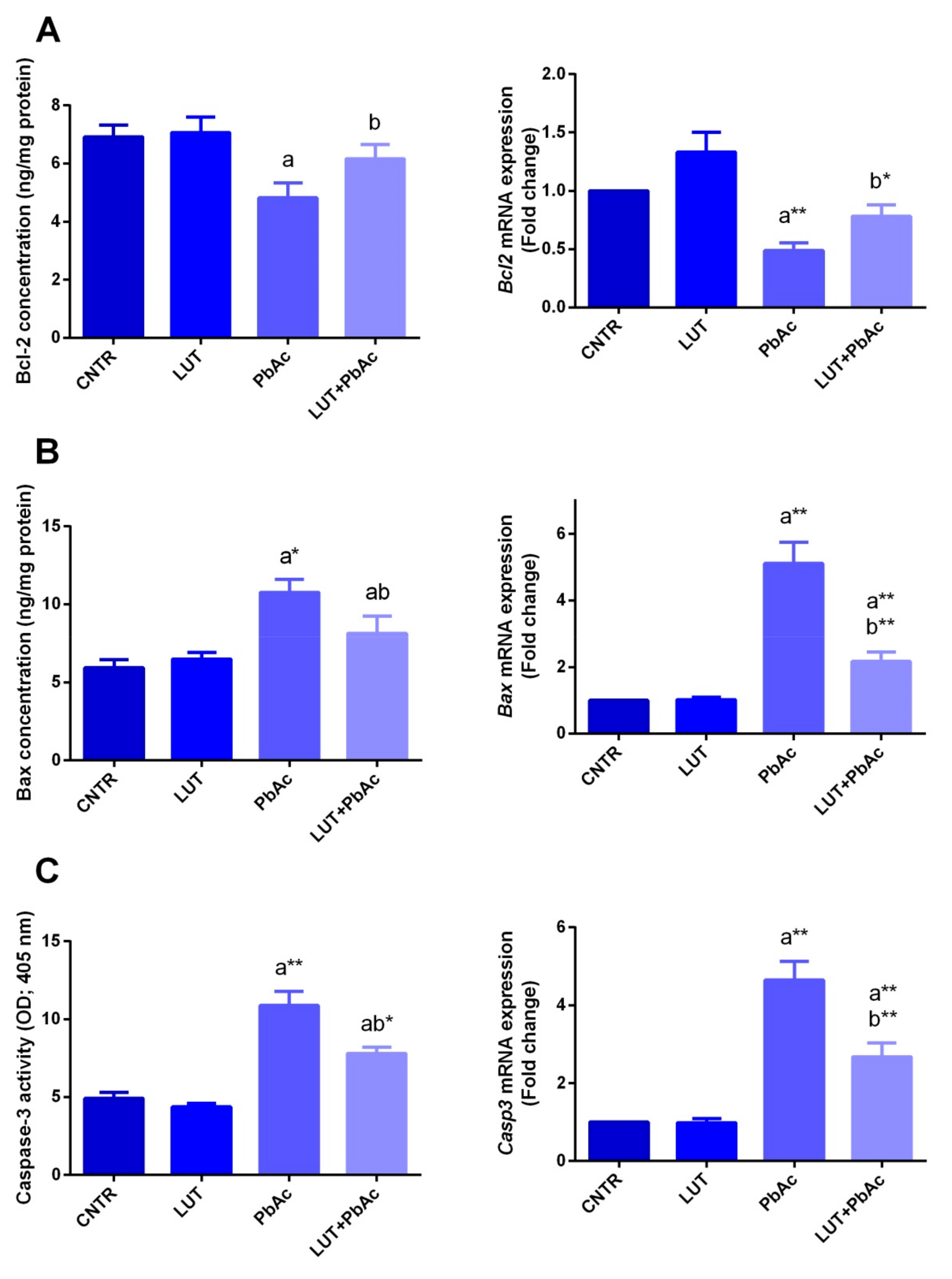
3.6. Hepatic Apoptotic Parameters
3.7. Alterations in Hepatic Tissue Architecture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bischoff, K.; Mukai, M.; Ramaiah, S.K. Liver toxicity. In Veterinary Toxicology; Academic Press Elsevier: Amsterdam, The Netherlands, 2018; pp. 239–257. [Google Scholar]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Mudipalli, A. Lead hepatotoxicity & potential health effects. Indian J. Med Res. 2007, 126, 518. [Google Scholar] [PubMed]
- Assi, M.A.; Hezmee, M.N.M.; Haron, A.W.; Sabri, M.Y.M.; Rajion, M.A. The detrimental effects of lead on human and animal health. Vet. World 2016, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- Basgen, J.M.; Sobin, C. Early chronic low-level lead exposure produces glomerular hypertrophy in young c57bl/6j mice. Toxicol. Lett. 2014, 225, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujaibel, L.M.; Kilarkaje, N. Mitogen-activated protein kinase signaling and its association with oxidative stress and apoptosis in lead-exposed hepatocytes. Environ. Toxicol. 2015, 30, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lewis, G. Environmental toxicity and poor cognitive outcomes in children and adults. J. Environ. Health 2014, 76, 130. [Google Scholar]
- Pant, N.; Kumar, G.; Upadhyay, A.; Patel, D.; Gupta, Y.; Chaturvedi, P. Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environ. Sci. Pollut. Res. 2014, 21, 11066–11074. [Google Scholar] [CrossRef]
- Moneim, A.E.A. Flaxseed oil as a neuroprotective agent on lead acetate-induced monoamineric alterations and neurotoxicity in rats. Biol. Trace Elem. Res. 2012, 148, 363–370. [Google Scholar] [CrossRef]
- Aziz, F.M. Protective effects of latex of ficus carica l. Against lead acetate-induced hepatotoxicity in rats. Jordan J. Biol. Sci. 2012, 147, 1–7. [Google Scholar]
- El-Boshy, M.E.; Refaat, B.; Qasem, A.H.; Khan, A.; Ghaith, M.; Almasmoum, H.; Mahbub, A.; Almaimani, R.A. The remedial effect of thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ. Sci. Pollut. Res. 2019, 26, 22736–22746. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: A review. Asian Pac. J. Cancer Prev. 2014, 15, 5501–5508. [Google Scholar] [CrossRef]
- Ziyan, L.; Yongmei, Z.; Nan, Z.; Ning, T.; Baolin, L. Evaluation of the anti-inflammatory activity of luteolin in experimental animal models. Planta Med. 2007, 73, 221–226. [Google Scholar] [CrossRef]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Luteolin as an anti-inflammatory and anti-allergic constituent of perilla frutescens. Biol. Pharm. Bull. 2002, 25, 1197–1202. [Google Scholar] [CrossRef] [Green Version]
- Ju, W.; Wang, X.; Shi, H.; Chen, W.; Belinsky, S.A.; Lin, Y. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor-κb pathway and sensitization of apoptosis in lung cancer cells. Mol. Pharmacol. 2007, 71, 1381–1388. [Google Scholar] [CrossRef]
- Coleta, M.; Campos, M.G.; Cotrim, M.D.; de Lima, T.C.M.; da Cunha, A.P. Assessment of luteolin (3′,4′,5,7-tetrahydroxyflavone) neuropharmacological activity. Behav. Brain Res. 2008, 189, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Woodman, O.L.; Chan, E. Vascular and anti-oxidant actions of flavonols and flavones. Clin. Exp. Pharm. Physiol. 2004, 31, 786–790. [Google Scholar] [CrossRef]
- Yang, D.; Tan, X.; Lv, Z.; Liu, B.; Baiyun, R.; Lu, J.; Zhang, Z. Regulation of Sirt1/Nrf2/TNF-alpha signaling pathway by luteolin is critical to attenuate acute mercuric chloride exposure induced hepatotoxicity. Sci. Rep. 2016, 6, 37157. [Google Scholar] [CrossRef] [PubMed]
- Tai, M.; Zhang, J.; Song, S.; Miao, R.; Liu, S.; Pang, Q.; Wu, Q.; Liu, C. Protective effects of luteolin against acetaminophen-induced acute liver failure in mouse. Int. Immunopharmacol. 2015, 27, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Domitrovic, R.; Jakovac, H.; Milin, C.; Radosevic-Stasic, B. Dose- and time-dependent effects of luteolin on carbon tetrachloride-induced hepatotoxicity in mice. Exp. Toxicol. Pathol. 2009, 61, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Cholbi, M.R.; Paya, M.; Alcaraz, M.J. Inhibitory effects of phenolic compounds on CCl4-induced microsomal lipid peroxidation. Experientia 1991, 47, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Kalbolandi, S.M.; Gorji, A.V.; Babaahmadi-Rezaei, H.; Mansouri, E. Luteolin confers renoprotection against ischemia–reperfusion injury via involving Nrf2 pathway and regulating miR320. Mol. Biol. Rep. 2019, 46, 4039–4047. [Google Scholar] [CrossRef]
- Szkoda, J.; Zmudzki, J. Determination of lead and cadmium in biological material by graphite furnace atomic absorption spectrometry method. Bull. Vet. Inst. Pulawy 2005, 49, 89–92. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15n]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzym. 1985, 113, 484–490. [Google Scholar]
- Almeer, R.S.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Moneim, A.E.A. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019, 9, 5825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Moneim, A.M.; El-Toweissy, M.Y.; Ali, A.M.; Allah, A.A.M.A.; Darwish, H.S.; Sadek, I.A. Curcumin ameliorates lead (Pb2+)-induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biol. Trace Elem. Res. 2015, 168, 206–220. [Google Scholar] [CrossRef] [PubMed]
- BaSalamah, M.A.; Abdelghany, A.H.; El-Boshy, M.; Ahmad, J.; Idris, S.; Refaat, B. Vitamin D alleviates lead induced renal and testicular injuries by immunomodulatory and antioxidant mechanisms in rats. Sci. Rep. 2018, 8, 4853. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, O.I.; El-Nahas, A.F.; El-Sayed, Y.S.; Ashry, K.M. Ginger extract modulates Pb-induced hepatic oxidative stress and expression of antioxidant gene transcripts in rat liver. Pharm. Biol. 2016, 54, 1164–1172. [Google Scholar] [CrossRef]
- Mabrouk, A.; Salah, I.B.H.; Chaieb, W.; Cheikh, H.B. Protective effect of thymoquinone against lead-induced hepatic toxicity in rats. Environ. Sci. Pollut. Res. 2016, 23, 12206–12215. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, L.; Zhao, H.; Liu, X.; Gao, L.; Cheng, N.; Cao, W. Complexation of luteolin with lead (II): Spectroscopy characterization and theoretical researches. J. Inorg. Biochem. 2019, 193, 25–30. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Regulska, E. Spectroscopic study of molecular structure, antioxidant activity and biological effects of metal hydroxyflavonol complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 757–771. [Google Scholar] [CrossRef]
- Adhikari, A.; Darbar, S.; Chatterjee, T.; Das, M.; Polley, N.; Bhattacharyya, M.; Bhattacharya, S.; Pal, D.; Pal, S.K. Spectroscopic studies on dual role of natural flavonoids in detoxification of lead poisoning: Bench-to-bedside preclinical trial. ACS Omega 2018, 3, 15975–15987. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Chen, F.-X.; Wang, L.; Wang, J.; Jin, S.; Ma, Y.-M. Protective effects of blueberries (Vaccinium corymbosum L.) extract against cadmium-induced hepatotoxicity in mice. Environ. Toxicol. Pharm. 2014, 37, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, B.; Qi, S.; He, J.; Zheng, H. Protective effects of salidroside on lead acetate-induced oxidative stress and hepatotoxicity in sprague-dawley rats. Biol. Trace Elem. Res. 2019, 191, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Offor, S.J.; Mbagwu, H.O.; Orisakwe, O.E. Lead induced hepato-renal damage in male albino rats and effects of activated charcoal. Front Pharm. 2017, 8, 107. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, X.; Yang, D.; Lu, J.; Liu, B.; Baiyun, R.; Zhang, Z. Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signaling pathway in rats. Oncotarget 2017, 8, 40982. [Google Scholar]
- Moneim, A.E.A. Indigofera oblongifolia prevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS ONE 2016, 11, e0158965. [Google Scholar]
- Liu, C.-M.; Ma, J.-Q.; Sun, Y.-Z. Puerarin protects the rat liver against oxidative stress-mediated DNA damage and apoptosis induced by lead. Exp. Toxicol. Pathol. 2012, 64, 575–582. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; Dkhil, M.A.; Al-Quraishy, S. The redox status in rats treated with flaxseed oil and lead-induced hepatotoxicity. Biol. Trace Elem. Res. 2011, 143, 457–467. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Abdel Moneim, A.E.; Bauomy, A.A.; Khalil, M.; Al-Shaebi, E.M.; Al-Quraishy, S. Chlorogenic acid prevents hepatotoxicity in arsenic-treated mice: Role of oxidative stress and apoptosis. Mol. Biol. Rep. 2019, 46. [Google Scholar] [CrossRef]
- Albasher, G.; Albrahim, T.; Alsultan, N.; Alfaraj, S.; Alharthi, M.S.; Kassab, R.B.; Abdel Moneim, A.E. Red beetroot extract mitigates chlorpyrifos-induced reprotoxicity associated with oxidative stress, inflammation, and apoptosis in rats. Environ. Sci. Pollut. Res. Int. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and Nf-kappaB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of cell protection by heme oxygenase-1. Annu. Rev. Pharm. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, F.; Li, X.; Li, L.; Yuan, J.; Chen, J. t-BHQ provides protection against lead neurotoxicity via Nrf2/HO-1 pathway. Oxidative Med. Cell. Longev. 2016, 2016, 2075915. [Google Scholar] [CrossRef]
- Mabrouk, A. Protective effect of thymoquinone against lead-induced antioxidant defense system alteration in rat liver. Acta Biol. Hung. 2017, 68, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Yang, J.; Wang, J. Modulatory effect of luteolin on redox homeostasis and inflammatory cytokines in a mouse model of liver cancer. Oncol. Lett. 2016, 12, 4767–4772. [Google Scholar] [CrossRef] [Green Version]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Cheng, I.F.; Breen, K. On the ability of four flavonoids, baicilein, luteolin, naringenin, and quercetin, to suppress the fenton reaction of the iron-ATP complex. Biometals: Int. J. Role Met. Ions Biol. Biochem. Med. 2000, 13, 77–83. [Google Scholar] [CrossRef]
- Sadik, C.D.; Sies, H.; Schewe, T. Inhibition of 15-lipoxygenases by flavonoids: Structure-activity relations and mode of action. Biochem. Pharmacol. 2003, 65, 773–781. [Google Scholar] [CrossRef]
- Wruck, C.J.; Claussen, M.; Fuhrmann, G.; Romer, L.; Schulz, A.; Pufe, T.; Waetzig, V.; Peipp, M.; Herdegen, T.; Gotz, M.E. Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent keap1-Nrf2-ARE pathway. J. Neural Transm. Suppl. 2007, 72, 57–67. [Google Scholar]
- Samy, R.P.; Gopalakrishnakone, P.; Ignacimuthu, S. Anti-tumor promoting potential of luteolin against 7,12-dimethylbenz (a) anthracene-induced mammary tumors in rats. Chem. Biol. Interact. 2006, 164, 1–14. [Google Scholar] [CrossRef] [PubMed]
- George, V.C.; Kumar, D.R.N.; Suresh, P.K.; Kumar, S.; Kumar, R.A. Comparative studies to evaluate relative in vitro potency of luteolin in inducing cell cycle arrest and apoptosis in HaCaT and A375 cells. Asian Pac. J. Cancer Prev. 2013, 14, 631–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Brakati, A.Y.; Kassab, R.B.; Lokman, M.S.; Elmahallawy, E.K.; Amin, H.K.; Moneim, A.E.A. Soursop fruit extract mitigates scopolamine-induced amnesia and oxidative stress via activating cholinergic and Nrf2/HO-1 pathways. Metab. Brain Dis. 2019, 34, 853–864. [Google Scholar]
- Odontuya, G.; Hoult, J.R.; Houghton, P.J. Structure-activity relationship for antiinflammatory effect of luteolin and its derived glycosides. Phytother. Res. 2005, 19, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Abu-Khudir, R.; Habieb, M.E.; Mohamed, M.A.; Hawas, A.M.; Mohamed, T.M. Anti-apoptotic role of spermine against lead and/or gamma irradiation-induced hepatotoxicity in male rats. Environ. Sci. Pollut. Res. 2017, 24, 24272–24283. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Li, C.; Huo, K.; Wang, Q.; Lu, L.; Zhang, Q.; Wang, Y.; Wang, W. Luteolin prevents H2O2-induced apoptosis in H9C2 cells through modulating Akt-P53/Mdm2 signaling pathway. BioMed Res. Int. 2016, 2016, 5125836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, S.-W. Structural insights into the transcription-independent apoptotic pathway of P53. BMB Rep. 2014, 47, 167. [Google Scholar] [CrossRef] [Green Version]
- Pei, D.; Zhang, Y.; Zheng, J. Regulation of P53: A collaboration between Mdm2 and Mdmx. Oncotarget 2012, 3, 228. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Zhao, P.; Li, X.; Pan, H.; Ma, S.; Ding, L. Cytotoxicity of luteolin in primary rat hepatocytes: The role of CYP3A-mediated ortho-benzoquinone metabolite formation and glutathione depletion. J. Appl. Toxicol. 2015, 35, 1372–1380. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, S.H.; Shin, J.; Harikishore, A.; Lim, J.K.; Jung, Y.; Lyu, H.N.; Baek, N.I.; Choi, K.Y.; Yoon, H.S.; et al. Luteolin suppresses cancer cell proliferation by targeting vaccinia-related kinase 1. PLoS ONE 2014, 9, e109655. [Google Scholar] [CrossRef] [Green Version]
- Lodhi, S.; Vadnere, G.P.; Patil, K.D.; Patil, T.P. Protective effects of luteolin on injury induced inflammation through reduction of tissue uric acid and pro-inflammatory cytokines in rats. J. Tradit. Complementary Med. 2019. [Google Scholar] [CrossRef]
- Xiao, C.; Xia, M.-L.; Wang, J.; Zhou, X.-R.; Lou, Y.-Y.; Tang, L.-H.; Zhang, F.-J.; Yang, J.-T.; Qian, L.-B. Luteolin attenuates cardiac ischemia/reperfusion injury in diabetic rats by modulating Nrf2 antioxidative function. Oxid. Med. Cell. Longev. 2019, 2019, 2719252. [Google Scholar] [CrossRef] [PubMed]








| Name | Accession Number | Forward Primer (5’---3’) | Reverse Primer (5’---3’) |
|---|---|---|---|
| Gapdh | NM_017008.4 | AGTGCCAGCCTCGTCTCATA | GATGGTGATGGGTTTCCCGT |
| Sod2 | NM_017051.2 | TAAGGGTGGTGGAGAACCCA | TGATGACAGTGACAGCGTCC |
| Cat | NM_012520.2 | TTTTCACCGACGAGATGGCA | AAGGTGTGTGAGCCATAGCC |
| Gpx1 | NM_030826.4 | CAGTCCACCGTGTATGCCTT | GTAAAGAGCGGGTGAGCCTT |
| Gsr | NM_053906.2 | TACTGCACTTCCCGGTAGGA | TGGATGCCAACCACCTTCTC |
| Nfe2l2 | NM_031789.2 | TTGTAGATGACCATGAGTCGC | ACTTCCAGGGGCACTGTCTA |
| Hmox1 | NM_012580 | GCGAAACAAGCAGAACCCA | GCTCAGGATGAGTACCTCCCA |
| Nos2 | NM_012611.3 | GTTCCTCAGGCTTGGGTCTT | TGGGGGAACACAGTAATGGC |
| Tnf | NM_012675.3 | GGCTTTCGGAACTCACTGGA | CCCGTAGGGCGATTACAGTC |
| Il1β | NM_031512.2 | GACTTCACCATGGAACCCGT | GGAGACTGCCCATTCTCGAC |
| Bcl2 | NM_016993 | ACTCTTCAGGGATGGGGTGA | TGACATCTCCCTGTTGACGC |
| Bax | NM_017059.2 | GGGCCTTTTTGCTACAGGGT | TTCTTGGTGGATGCGTCCTG |
| Casp3 | NM_012922.2 | GAGCTTGGAACGCGAAGAAA | TAACCGGGTGCGGTAGAGTA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL-Megrin, W.A.; Alkhuriji, A.F.; Yousef, A.O.S.; Metwally, D.M.; Habotta, O.A.; Kassab, R.B.; Abdel Moneim, A.E.; El-Khadragy, M.F. Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities. Antioxidants 2020, 9, 10. https://doi.org/10.3390/antiox9010010
AL-Megrin WA, Alkhuriji AF, Yousef AOS, Metwally DM, Habotta OA, Kassab RB, Abdel Moneim AE, El-Khadragy MF. Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities. Antioxidants. 2020; 9(1):10. https://doi.org/10.3390/antiox9010010
Chicago/Turabian StyleAL-Megrin, Wafa A., Afrah F. Alkhuriji, Al Omar S. Yousef, Dina M. Metwally, Ola A. Habotta, Rami B. Kassab, Ahmed E. Abdel Moneim, and Manal F. El-Khadragy. 2020. "Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities" Antioxidants 9, no. 1: 10. https://doi.org/10.3390/antiox9010010
APA StyleAL-Megrin, W. A., Alkhuriji, A. F., Yousef, A. O. S., Metwally, D. M., Habotta, O. A., Kassab, R. B., Abdel Moneim, A. E., & El-Khadragy, M. F. (2020). Antagonistic Efficacy of Luteolin against Lead Acetate Exposure-Associated with Hepatotoxicity is Mediated via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Activities. Antioxidants, 9(1), 10. https://doi.org/10.3390/antiox9010010





