Intrarenal Transplantation of Hypoxic Preconditioned Mesenchymal Stem Cells Improves Glomerulonephritis through Anti-Oxidation, Anti-ER Stress, Anti-Inflammation, Anti-Apoptosis, and Anti-Autophagy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Preparations (MSCS Isolation, Characterization, and Culture)
2.3. HIF-1α Determination and Growth Factors Array Assay
2.4. Experimental Model and Design
2.5. Tracking of Intrarenal Arterial Injected MSCs in Rat Kidneys
2.6. Measurements of Proteinuria and Hydroxyproline Degree
2.7. Renal Morphology
2.8. Immunohistochemistry
2.9. Western Blot and Nuclear Extraction
2.10. Statistical Analysis
3. Results
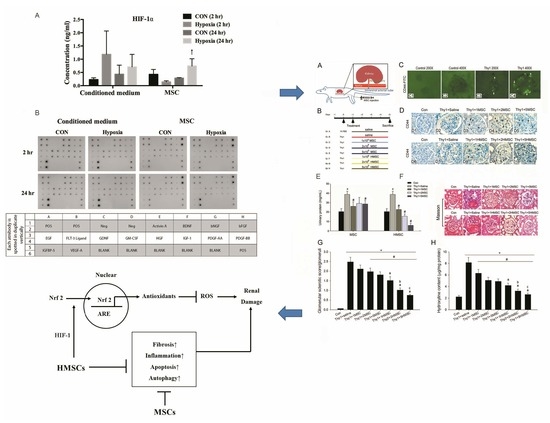
3.1. Recruitment of MSCs and HMSCs into Nephritic Not Normal Kidneys
3.2. MSC or HMSC Ameliorates Nephritic Severity in the Rat GN Model
3.3. Hypoxic Preconditioning Upregulated HIF-1α and VEGF Expression
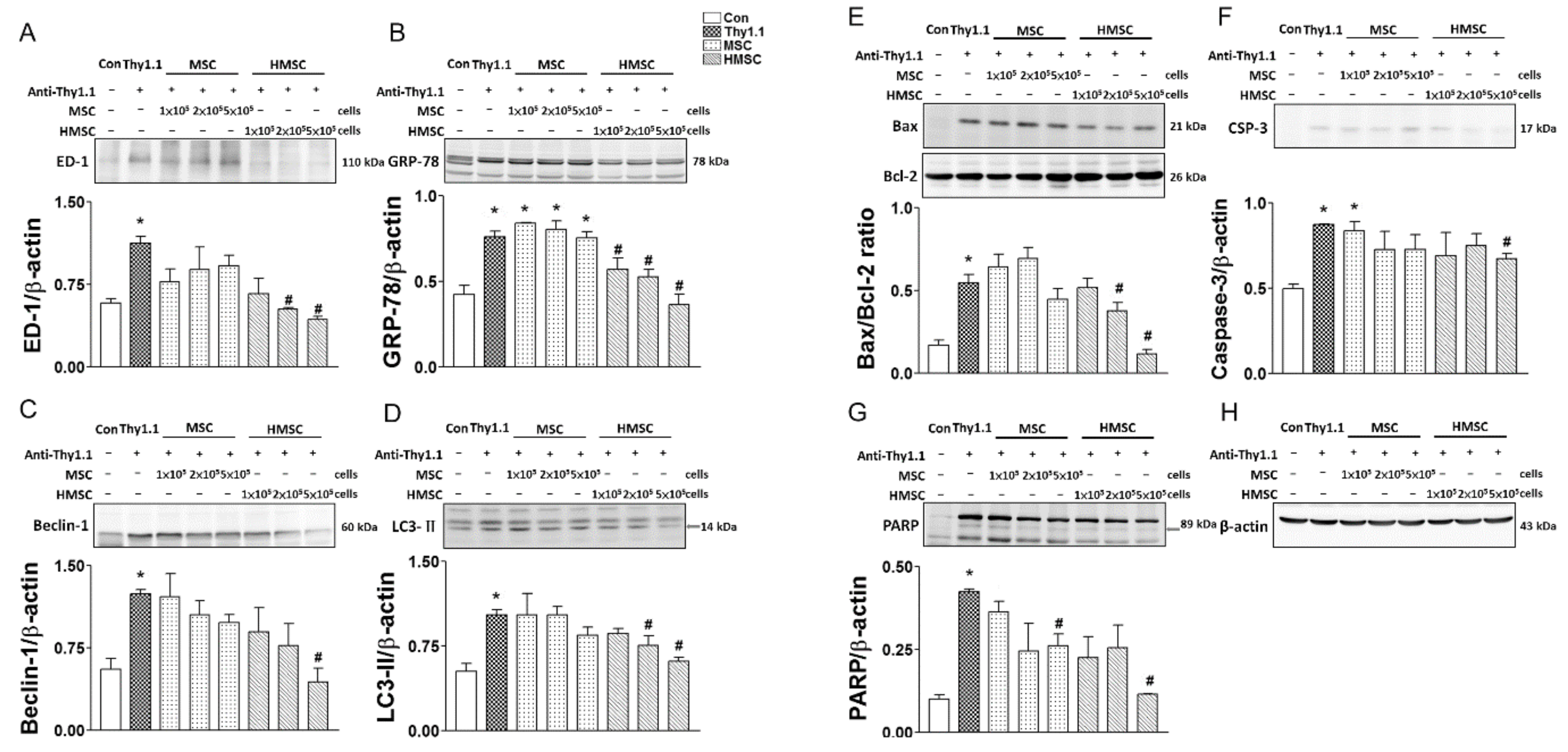
3.4. MSCs or HMSCs Reduce ED1, ER Stress, Autophagy, and Apoptosis with Western Blot
3.5. MSCs or HMSCs Reduce ED-1 Infiltration, ER Stress, Autophagy, and Apoptosis by IHC
3.6. HMSCs Promote Nuclear Nrf2 Expression, Reduce NF-kB Expression, Rescue ROS Enzymatic Scavengers and Elevate Anti-Oxidative Response Element Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ED-1 | macrophage/monocyte |
| ER stress | endoplasmic reticulum stress |
| ESRD | end stage renal disease |
| GN | glomerulonephritis |
| HIF-1α | hypoxia induced factor-1α |
| HMSCs | hypoxic-preconditioned mesenchymal stem cells |
| MSCs | mesenchymal stem cells |
| NF-kB | nuclear factor kappa B |
| Nrf2 | nuclear factor (erythroid-derived 2) |
| ROS | reactive oxygen species |
| VEGF | vascular endothelial growth factor |
| TUNEL | terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling |
References
- Couser, W.G. Glomerulonephritis. Lancet 1999, 353, 1509–1515. [Google Scholar] [CrossRef]
- Kunter, U.; Rong, S.; Djuric, Z.; Boor, P.; Müller-Newen, G.; Yu, D.; Floege, J. Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J. Am. Soc. Nephrol. 2006, 17, 2202–2212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, G. Influence of clinical and histological features on actuarial renal survival in adult patients with idiopathic IgA nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis: Survey of the recent literature. Am. J. Kidney Dis. 1992, 20, 315–323. [Google Scholar] [CrossRef]
- Deegens, J.K.; Wetzels, J.F. Diagnosis and treatment of primary glomerular diseases. Membranous nephropathy, focal segmental glomerulosclerosis and IgA nephropathy. Minerva Urol. Nefrol. 2005, 57, 211–236. [Google Scholar]
- Heaf, J.; Lokkegaard, H.; Larsen, S. The epidemiology and prognosis of glomerulonephritis in Denmark 1985–1997. Nephrol. Dial. Transpl. 1999, 14, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- Moranne, O.; Watier, L.; Rossert, J.; Stengel, B. N-Progress Study Group. Primary glomerulonephritis: An update on renal survival and determinants of progression. QJM 2008, 101, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Pippias, M.; Jager, K.J.; Kramer, A.; Leivestad, T.; Sanchez, M.B.; Caskey, F.J.; Collart, F.; Couchoud, C.; Dekker, F.W.; Finne, P.; et al. The changing trends and outcomes in renal replacement therapy: Data from the ERA-EDTA Registry. Nephrol. Dial. Transpl. 2015, 31, 831–841. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Xie, Y.; Li, Q.; Chen, X. Stem cell-based cell therapy for glomerulonephritis. Biomed. Res. Int. 2014, 2014, 124730. [Google Scholar] [CrossRef] [Green Version]
- El-Ansary, M.; Saadi, G.; Abd El-Hamid, S.M. Mesenchymal stem cells are a rescue approach for recovery of deteriorating kidney function. Nephrology 2012, 17, 650–657. [Google Scholar] [CrossRef]
- Gu, F.; Wang, D.; Zhang, H.; Feng, X.; Gilkeson, G.S.; Shi, S.; Sun, L. Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clin. Rheumatol. 2014, 33, 1611–1619. [Google Scholar] [CrossRef]
- Wang, D.; Li, J.; Zhang, Y.; Zhang, M.; Chen, J.; Li, X.; Hu, X.; Jiang, S.; Shi, S.; Sun, L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: A multicenter clinical study. Arthritis Res. Ther. 2014, 16, R79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer-Schwesinger, C.; Lange, C.; Bröcker, V.; Agustian, P.A.; Lehmann, U.; Raabe, A.; Brinkmeyer, M.; Kobayashi, E.; Schiffer, M.; Büsche, G.; et al. Bone marrow-derived progenitor cells do not contribute to podocyte turnover in the puromycin aminoglycoside and renal ablation models in rats. Am. J. Pathol. 2011, 178, 494–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budisavljevic, M.N.; Hodge, L.; Barber, K.; Fulmer, J.R.; Durazo-Arvizu, R.A.; Self, S.E.; Kuhlmann, M.; Raymond, J.R.; Greene, E.L. Oxidative stress in the pathogenesis of experimental mesangial proliferative glomerulonephritis. Am. J. Physiol. Renal. Physiol. 2003, 285, F1138–F1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Tian, X.; Bai, S.; Fan, J.; Hou, W.; Tong, H.; Li, D. Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int. J. Mol. Med. 2012, 30, 85–92. [Google Scholar] [PubMed]
- Zeng, W.; Xiao, J.; Zheng, G.; Xing, F.; Tipoe, G.L.; Wang, X.; He, C.; Chen, Z.; Liu, Y. Antioxidant treatment enhances human mesenchymal stem cell anti-stress ability and therapeutic efficacy in an acute liver failure model. Sci. Rep. 2015, 5, 11100. [Google Scholar] [CrossRef] [Green Version]
- Petrangeli, E.; Coroniti, G.; Brini, A.T.; De Girolamo, L.; Stanco, D.; Niada, S.; Silecchia, G.; Morgante, E.; Lubrano, C.; Russo, M.A.; et al. Hypoxia promotes the inflammatory response and stemness features in visceral fat stem cells from obese subjects. J. Cell Physiol. 2016, 231, 668–679. [Google Scholar] [CrossRef]
- Liu, H.; Xue, W.; Ge, G.; Luo, X.; Li, Y.; Xiang, H.; Ding, X.; Tian, P.; Tian, X. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1alpha in MSCs. Biochem. Biophys. Res. Commun. 2010, 401, 509–515. [Google Scholar] [CrossRef]
- Huang, T.F.; Yew, T.L.; Chiang, E.R.; Ma, H.L.; Hsu, C.Y.; Hsu, S.H.; Hsu, Y.T.; Hung, S.C. Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am. J. Sport. Med. 2013, 41, 1117–1125. [Google Scholar] [CrossRef]
- Yew, T.L.; Chang, M.C.; Hsu, Y.T.; He, F.Y.; Weng, W.H.; Tsai, C.C.; Chiu, F.Y.; Hung, S.C. Efficient expansion of mesenchymal stem cells from mouse bone marrow under hypoxic conditions. J. Tissue Eng. Regen. Med. 2013, 7, 984–993. [Google Scholar] [CrossRef]
- Chen, Y.M.; Chien, C.T.; Hu-Tsai, M.I.; Wu, K.D.; Tsai, C.C.; Wu, M.S.; Tsai, T.J. Pentoxifylline attenuates experimental mesangial proliferative glomerulonephritis. Kidney Int. 1999, 56, 932–943. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Sumithran, E.; Glasgow, E.F.; Atkins, R.C. The enhancement of aminonucleoside nephrosis by the co-administration of protamine. Kidney Int. 1987, 32, 691–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 119, 493–501. [Google Scholar] [PubMed]
- Hu, X.; Yu, S.P.; Fraser, J.L.; Lu, Z.; Ogle, M.E.; Wang, J.A.; Wei, L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac. Cardiovasc. Surg. 2008, 135, 799–808. [Google Scholar] [PubMed] [Green Version]
- Yu, X.; Lu, C.; Liu, H.; Rao, S.; Cai, J.; Liu, S.; Kriegel, A.J.; Greene, A.S.; Liang, M.; Ding, X. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS ONE 2013, 8, e62703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Morioka, T.; Uchiyama, M.; Oite, T. Bone marrow cell infusion ameliorates progressive glomerulosclerosis in an experimental rat model. Kidney Int. 2006, 69, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rampino, T.; Gregorini, M.; Bedino, G.; Piotti, G.; Gabanti, E.; Ibatici, A.; Sessarego, N.; Piacenza, C.; Balenzano, C.T.; Esposito, P.; et al. Mesenchymal stromal cells improve renal injury in anti-Thy 1 nephritis by modulating inflammatory cytokines and scatter factors. Clin. Sci. 2011, 120, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakr, S.; Rashed, L.; Zarouk, W.; El-Shamy, R. Effect of mesenchymal stem cells on anti-Thy1,1 induced kidney injury in albino rats. Asian Pac. J. Trop. Biomed. 2013, 3, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, H.; Yamahara, K.; Ishikane, S.; Otani, K.; Nakamura, A.; Sawai, K.; Ichimaru, N.; Sada, M.; Taguchi, A.; Hosoda, H.; et al. Allogenic fetal membrane-derived mesenchymal stem cells contribute to renal repair in experimental glomerulonephritis. Am. J. Physiol. Renal. Physiol. 2010, 299, F1004–F1013. [Google Scholar] [CrossRef] [Green Version]
- Uchimura, H.; Marumo, T.; Takase, O.; Kawachi, H.; Shimizu, F.; Hayashi, M.; Saruta, T.; Hishikawa, K.; Fujita, T. Intrarenal injection of bone marrow-derived angiogenic cells reduces endothelial injury and mesangial cell activation in experimental glomerulonephritis. J. Am. Soc. Nephrol. 2005, 16, 997–1004. [Google Scholar] [CrossRef]
- Abe-Yoshio, Y.; Abe, K.; Miyazaki, M.; Furusu, A.; Nishino, T.; Harada, T.; Koji, T.; Kohno, S. Involvement of bone marrow-derived endothelial progenitor cells in glomerular capillary repair in habu snake venom-induced glomerulonephritis. Virchows Archiv 2008, 453, 97–106. [Google Scholar] [CrossRef]
- Li, D.; Wang, N.; Zhang, L.; Hanyu, Z.; Xueyuan, B.; Fu, B.; Shaoyuan, C.; Zhang, W.; Xuefeng, S.; Li, R.; et al. Mesenchymal stem cells protect podocytes from apoptosis induced by high glucose via secretion of epithelial growth factor. Stem Cell Res. Ther. 2013, 4, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoja, C.; Garcia, P.B.; Rota, C.; Conti, S.; Gagliardini, E.; Corna, D.; Zanchi, C.; Bigini, P.; Benigni, A.; Remuzzi, G.; et al. Mesenchymal stem cell therapy promotes renal repair by limiting glomerular podocyte and progenitor cell dysfunction in adriamycin-induced nephropathy. Am. J. Physiol. Renal. Physiol. 2012, 303, F1370–F1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezquer, M.E.; Ezquer, F.E.; Arango-Rodríguez, M.L.; Conget, P.A. MSC transplantation: A promising therapeutic strategy to manage the onset and progression of diabetic nephropathy. Biol. Res. 2012, 45, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmon, A.B.; Perez, V.I.; Bokov, A.; Jernigan, A.; Kim, G.; Zhao, H.; Levine, R.L.; Richardson, A. Lack of methionine sulfoxide reductase A in mice increases sensitivity to oxidative stress but does not diminish life span. FASEB J. 2009, 23, 3601–3608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle-Prieto, A.; Conget, P.A. Human mesenchymal stem cells efficiently manage oxidative stress. Stem Cells Dev. 2010, 19, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Liu, C.; Yan, M.; Raman, I.; Du, Y.; Fang, X.; Zhou, X.J.; Mohan, C.; Li, Q.Z. Delivering Oxidation Resistance-1 (OXR1) to Mouse Kidney by Genetic Modified Mesenchymal Stem Cells Exhibited Enhanced Protection against Nephrotoxic Serum Induced Renal Injury and Lupus Nephritis. J. Stem Cell Res. Ther. 2014, 4. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Shimizu, A.; Pastan, I.; Taguchi, K.; Naganuma, E.; Suzuki, T.; Hosoya, T.; Yokoo, T.; Saito, A.; Miyata, T.; et al. Keap1 inhibition attenuates glomerulosclerosis. Nephrol. Dial. Transpl. 2014, 29, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Engl. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef] [Green Version]
- de Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Engl. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Hou, Y.; Lin, D. Polydatin protects bone marrow stem cells against oxidative injury: Involvement of Nrf 2/ARE Pathways. Stem Cells Int. 2016, 2016, 9394150. [Google Scholar] [CrossRef] [Green Version]
- Rosova, I.; Dao, M.; Capoccia, B.; Link, D.; Nolta, J.A. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 2008, 26, 2173–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annabi, B.; Lee, Y.T.; Turcotte, S.; Naud, E.; Desrosiers, R.R.; Champagne, M.; Eliopoulos, N.; Galipeau, J.; Beliveau, R. Hypoxia promotes murine bone-marrow-derived stromal cell migration and tube formation. Stem Cells 2003, 21, 337–347. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-H.; Hsu, S.-P.; Chien, C.-T. Intrarenal Transplantation of Hypoxic Preconditioned Mesenchymal Stem Cells Improves Glomerulonephritis through Anti-Oxidation, Anti-ER Stress, Anti-Inflammation, Anti-Apoptosis, and Anti-Autophagy. Antioxidants 2020, 9, 2. https://doi.org/10.3390/antiox9010002
Chang H-H, Hsu S-P, Chien C-T. Intrarenal Transplantation of Hypoxic Preconditioned Mesenchymal Stem Cells Improves Glomerulonephritis through Anti-Oxidation, Anti-ER Stress, Anti-Inflammation, Anti-Apoptosis, and Anti-Autophagy. Antioxidants. 2020; 9(1):2. https://doi.org/10.3390/antiox9010002
Chicago/Turabian StyleChang, Hao-Hsiang, Shih-Ping Hsu, and Chiang-Ting Chien. 2020. "Intrarenal Transplantation of Hypoxic Preconditioned Mesenchymal Stem Cells Improves Glomerulonephritis through Anti-Oxidation, Anti-ER Stress, Anti-Inflammation, Anti-Apoptosis, and Anti-Autophagy" Antioxidants 9, no. 1: 2. https://doi.org/10.3390/antiox9010002
APA StyleChang, H.-H., Hsu, S.-P., & Chien, C.-T. (2020). Intrarenal Transplantation of Hypoxic Preconditioned Mesenchymal Stem Cells Improves Glomerulonephritis through Anti-Oxidation, Anti-ER Stress, Anti-Inflammation, Anti-Apoptosis, and Anti-Autophagy. Antioxidants, 9(1), 2. https://doi.org/10.3390/antiox9010002