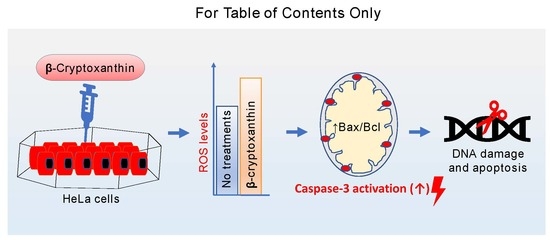
Chemopreventive Effect of β-Cryptoxanthin on Human Cervical Carcinoma (HeLa) Cells Is Modulated through Oxidative Stress-Induced Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
2.2. Carotenoid Extraction and Saponification
2.3. Purification of β-Cryptoxanthin
2.4. Spectrophotometry, HPLC, APCI-MS, and APCI-MS/MS Analysis of β-Cryptoxanthin
2.5. Cytotoxic Activities of Purified β-Cryptoxanthin
2.6. RNA Isolation and Quantitative Real-Time PCR (qPCR) Analysis
2.7. ROS Production Assay
2.8. Immunofluorescence Assay for Native Caspase-3 and the Integrity of the Mitochondrial Membrane
2.9. Expression of Active (Cleaved) Caspase-3 Proteins
2.10. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling (TUNEL) Assay
2.11. Statistical Analyses
3. Result and Discussion
3.1. Purification of β-Cryptoxanthin
3.2. Proliferation Inhibitory Effect of β-Cryptoxanthin on HeLa Cells
3.3. β-Cryptoxanthin Triggers ROS Production in HeLa Cells
3.4. β-Cryptoxanthin Regulates the Expression of Apoptosis-Related mRNA, Proteins, and Disrupt the Integrity of the Mitochondrial Membrane
3.5. β-Cryptoxanthin Induces Nuclei DNA Fragmentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adadi, P.; Barakova, N.V.; Krivoshapkina, E.F. Selected Methods of Extracting Carotenoids, Characterization, and Health Concerns: A Review. J. Agric. Food Chem. 2018, 66, 5925–5947. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [Green Version]
- Kaur, V.; Kumar, M.; Kumar, A.; Kaur, K.; Dhillon, V.S.; Kaur, S. Pharmacotherapeutic potential of phytochemicals: Implications in cancer chemoprevention and future perspectives. Biomed. Pharmacother. 2018, 97, 564–586. [Google Scholar] [CrossRef]
- Lou, S.N.; Ho, C.T. Phenolic compounds and biological activities of small-size citrus: Kumquat and calamondin. J. Food Drug Anal. 2017, 25, 162–175. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76 Pt 3, 735–750. [Google Scholar] [CrossRef]
- Yen, G.C.; Tsai, C.M.; Lu, C.C.; Weng, C.J. Recent progress in natural dietary non-phenolic bioactives on cancers metastasis. J. Food Drug Anal. 2018, 26, 940–964. [Google Scholar] [CrossRef]
- Bohn, T. Carotenoids and Markers of Oxidative Stress in Human Observational Studies and Intervention Trials: Implications for Chronic Diseases. Antioxidant 2019, 8, 179. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Niranjana, R.; Gayathri, R.; Nimish Mol, S.; Sugawara, T.; Hirata, T.; Miyashita, K.; Ganesan, P. Carotenoids modulate the hallmarks of cancer cells. J. Funct. Food. 2015, 18, 968–985. [Google Scholar] [CrossRef]
- Gansukh, E.; Mya, K.K.; Jung, M.; Keum, Y.S.; Kim, D.H.; Saini, R.K. Lutein derived from marigold (Tagetes erecta) petals triggers ROS generation and activates Bax and caspase-3 mediated apoptosis of human cervical carcinoma (HeLa) cells. Food Chem. Toxicol. 2019, 127, 11–18. [Google Scholar] [CrossRef]
- Saini, R.K.; Moon, S.H.; Gansukh, E.; Keum, Y.S. An efficient one-step scheme for the purification of major xanthophyll carotenoids from lettuce, and assessment of their comparative anticancer potential. Food Chem. 2018, 266, 56–65. [Google Scholar] [CrossRef]
- Maiani, G.; Periago Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef]
- Al-Delaimy, W.K.; van Kappel, A.L.; Ferrari, P.; Slimani, N.; Steghens, J.P.; Bingham, S.; Johansson, I.; Wallström, P.; Overvad, K.; Tjønneland, A.; et al. Plasma levels of six carotenoids in nine European countries: Report from the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr. 2004, 7, 713–722. [Google Scholar] [CrossRef]
- Pan, W.H.; Yeh, N.H.; Yang, R.Y.; Lin, W.H.; Wu, W.C.; Yeh, W.T.; Sung, M.K.; Lee, H.S.; Chang, S.J.; Huang, C.J.; et al. Vegetable, fruit, and phytonutrient consumption patterns in Taiwan. J. Food Drug Anal. 2018, 26, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Abnet, C.C.; Qiao, Y.L.; Dawsey, S.M.; Buckman, D.W.; Yang, C.S.; Blot, W.J.; Dong, Z.W.; Taylor, P.R.; Mark, S.D. Prospective study of serum retinol, β-carotene, β-cryptoxanthin, and lutein/zeaxanthin and esophageal and gastric cancers in China. Cancer Causes Cont. 2003, 14, 645–655. [Google Scholar] [CrossRef]
- Burri, B.J.; La Frano, M.R.; Zhu, C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr. Rev. 2016, 74, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.M.; Stram, D.O.; Arakawa, K.; Lee, H.P.; Yu, M.C. Dietary Cryptoxanthin and Reduced Risk of Lung Cancer: The Singapore Chinese Health Study. Cancer Epidemiol. Biomark. Prev. 2003, 12, 890–898. [Google Scholar]
- Wu, C.; Han, L.; Riaz, H.; Wang, S.; Cai, K.; Yang, L. The chemopreventive effect of β-cryptoxanthin from mandarin on human stomach cells (BGC-823). Food Chem. 2013, 136, 1122–1129. [Google Scholar] [CrossRef]
- Cilla, A.; Attanzio, A.; Barberá, R.; Tesoriere, L.; Livrea, M.A. Anti-proliferative effect of main dietary phytosterols and β-cryptoxanthin alone or combined in human colon cancer Caco-2 cells through cytosolic Ca+2—And oxidative stress-induced apoptosis. J. Funct. Food. 2015, 12, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Lian, F.; Hu, K.Q.; Russell, R.M.; Wang, X.D. β-Cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor β expression. Int. J. Cancer 2006, 119, 2084–2089. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Hurst, W.J. Methods of Analysis for Functional Foods and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Kim, D.E.; Shang, X.; Assefa, A.D.; Keum, Y.S.; Saini, R.K. Metabolite profiling of green, green/red, and red lettuce cultivars: Variation in health beneficial compounds and antioxidant potential. Food Res. Int. 2018, 105, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Gansukh, E.; Kazibwe, Z.; Pandurangan, M.; Judy, G.; Kim, D.H. Probing the impact of quercetin-7-O-glucoside on influenza virus replication influence. Phytomedicine 2016, 23, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Inbaraj, B.S.; Lu, H.; Hung, C.F.; Wu, W.B.; Lin, C.L.; Chen, B.H. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC–DAD–APCI–MS. J. Pharm. Biomed. Anal. 2008, 47, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ikoma, Y.; Kato, M.; Kuniga, T.; Nakajima, N.; Yoshida, T. Quantification of Carotenoids in Citrus Fruit by LC-MS and Comparison of Patterns of Seasonal Changes for Carotenoids among Citrus Varieties. J. Agric. Food Chem. 2007, 55, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Dong, L.; Pajkovic, N.D. Atmospheric pressure chemical ionization tandem mass spectrometry of carotenoids. Int. J. Mass Spectromet. 2012, 312, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids Affect Proliferation of Human Prostate Cancer Cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 175–176. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, Z. Increased Oxidative Stress as a Selective Anticancer Therapy. Oxid. Med. Cell. Longev. 2015, 2015, 294303. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Zhang, Y.; Zheng, J.; Pan, J. Reactive Oxygen Species in Cancer Stem Cells. Antioxidant. Redox Signal. 2012, 16, 1215–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijay, K.; Sowmya, P.R.R.; Arathi, B.P.; Shilpa, S.; Shwetha, H.J.; Raju, M.; Baskaran, V.; Lakshminarayana, R. Low-dose doxorubicin with carotenoids selectively alters redox status and upregulates oxidative stress-mediated apoptosis in breast cancer cells. Food Chem. Toxicol. 2018, 118, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Heo, S.J.; Kang, S.M.; Ahn, G.; Jeon, Y.J. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. In Vitro 2010, 24, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Arathi, B.P.; Sowmya, P.R.R.; Kuriakose, G.C.; Shilpa, S.; Shwetha, H.J.; Kumar, S.; Raju, M.; Vallikannan, B.; Lakshminarayana, R. Fractionation and characterization of lycopene oxidation products by LC-MSMS (ESI)+: Elucidation of chemoprevention potency of oxidized lycopene in breast cancer cell lines. J. Agric. Food Chem. 2018, 66, 11362–11371. [Google Scholar] [CrossRef]
- Arathi, B.P.; Sowmya, P.R.R.; Kuriakose, G.C.; Vijay, K.; Baskaran, V.; Jayabaskaran, C.; Lakshminarayana, R. Enhanced cytotoxic and apoptosis inducing activity of lycopene oxidation products in different cancer cell lines. Food Chem. Toxicol. 2016, 97, 265–276. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef]
- Choi, H.; Lee, D.G. Lycopene induces apoptosis in Candida albicans through reactive oxygen species production and mitochondrial dysfunction. Biochimie 2015, 115, 108–115. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Kumar, S. Caspase function in programmed cell death. Cell Death Differ. 2007, 14, 32–43. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowmya, P.R.R.; Arathi, B.P.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Astaxanthin from shrimp efficiently modulates oxidative stress and allied cell death progression in MCF-7 cells treated synergistically with β-carotene and lutein from greens. Food Chem. Toxicol. 2017, 106, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Danen-van Oorschot, A.; van Der Eb, A.; Noteborn, M. The chicken anemia virus-derived protein apoptin requires activation of caspases for induction of apoptosis in human tumor cells. J. Virol. 2000, 74, 7072–7078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H. A novel cleavage product formed by autoxidation of lycopene induces apoptosis in HL-60 cells. Free Radic. Biol. Med. 2003, 35, 1653–1663. [Google Scholar] [CrossRef]
- Palozza, P.; Serini, S.; Torsello, A.; Nicuolo, F.D.; Maggiano, N.; Ranelletti, F.O.; Wolf, F.I.; Calviello, G. Mechanism of Activation of Caspase Cascade During β-Carotene-Induced Apoptosis in Human Tumor Cells. Nutr. Cancer 2003, 47, 76–87. [Google Scholar] [CrossRef]
- Nagata, S. Apoptotic DNA fragmentation. Exp. Cell Res. 2000, 256, 12–18. [Google Scholar] [CrossRef]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta 2011, 1810, 497–503. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Asai, A.; Nagao, A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett. 2005, 220, 75–84. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Xu, S.; Yu, X.; Ma, D.; Hu, X.; Cao, X. In Vivo Induction of Apoptosis by Fucoxanthin, a Marine Carotenoid, Associated with Down-Regulating STAT3/EGFR Signaling in Sarcoma 180 (S180) Xenografts-Bearing Mice. Mar. Drugs 2012, 10, 2055–2068. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Sugiura, M.; Shibata, Y.; Ojima, T. Effect of β-cryptoxanthin–rich Satsuma mandarin juice supplementation on pulse wave velocity: A randomized controlled trial. J. Nutr. Intermed. Met. 2017, 8, 8–13. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gansukh, E.; Nile, A.; Sivanesan, I.; Rengasamy, K.R.R.; Kim, D.-H.; Keum, Y.-S.; Saini, R.K. Chemopreventive Effect of β-Cryptoxanthin on Human Cervical Carcinoma (HeLa) Cells Is Modulated through Oxidative Stress-Induced Apoptosis. Antioxidants 2020, 9, 28. https://doi.org/10.3390/antiox9010028
Gansukh E, Nile A, Sivanesan I, Rengasamy KRR, Kim D-H, Keum Y-S, Saini RK. Chemopreventive Effect of β-Cryptoxanthin on Human Cervical Carcinoma (HeLa) Cells Is Modulated through Oxidative Stress-Induced Apoptosis. Antioxidants. 2020; 9(1):28. https://doi.org/10.3390/antiox9010028
Chicago/Turabian StyleGansukh, Enkhtaivan, Arti Nile, Iyyakkannu Sivanesan, Kannan R. R. Rengasamy, Doo-Hwan Kim, Young-Soo Keum, and Ramesh Kumar Saini. 2020. "Chemopreventive Effect of β-Cryptoxanthin on Human Cervical Carcinoma (HeLa) Cells Is Modulated through Oxidative Stress-Induced Apoptosis" Antioxidants 9, no. 1: 28. https://doi.org/10.3390/antiox9010028
APA StyleGansukh, E., Nile, A., Sivanesan, I., Rengasamy, K. R. R., Kim, D.-H., Keum, Y.-S., & Saini, R. K. (2020). Chemopreventive Effect of β-Cryptoxanthin on Human Cervical Carcinoma (HeLa) Cells Is Modulated through Oxidative Stress-Induced Apoptosis. Antioxidants, 9(1), 28. https://doi.org/10.3390/antiox9010028