Effect of Acacia Polyphenol Supplementation on Exercise-Induced Oxidative Stress in Mice Liver and Skeletal Muscle
Abstract
1. Introduction
2. Methods
2.1. Experimental Animals and Sample Processing
2.2. Measurement
2.3. Statistical Analysis
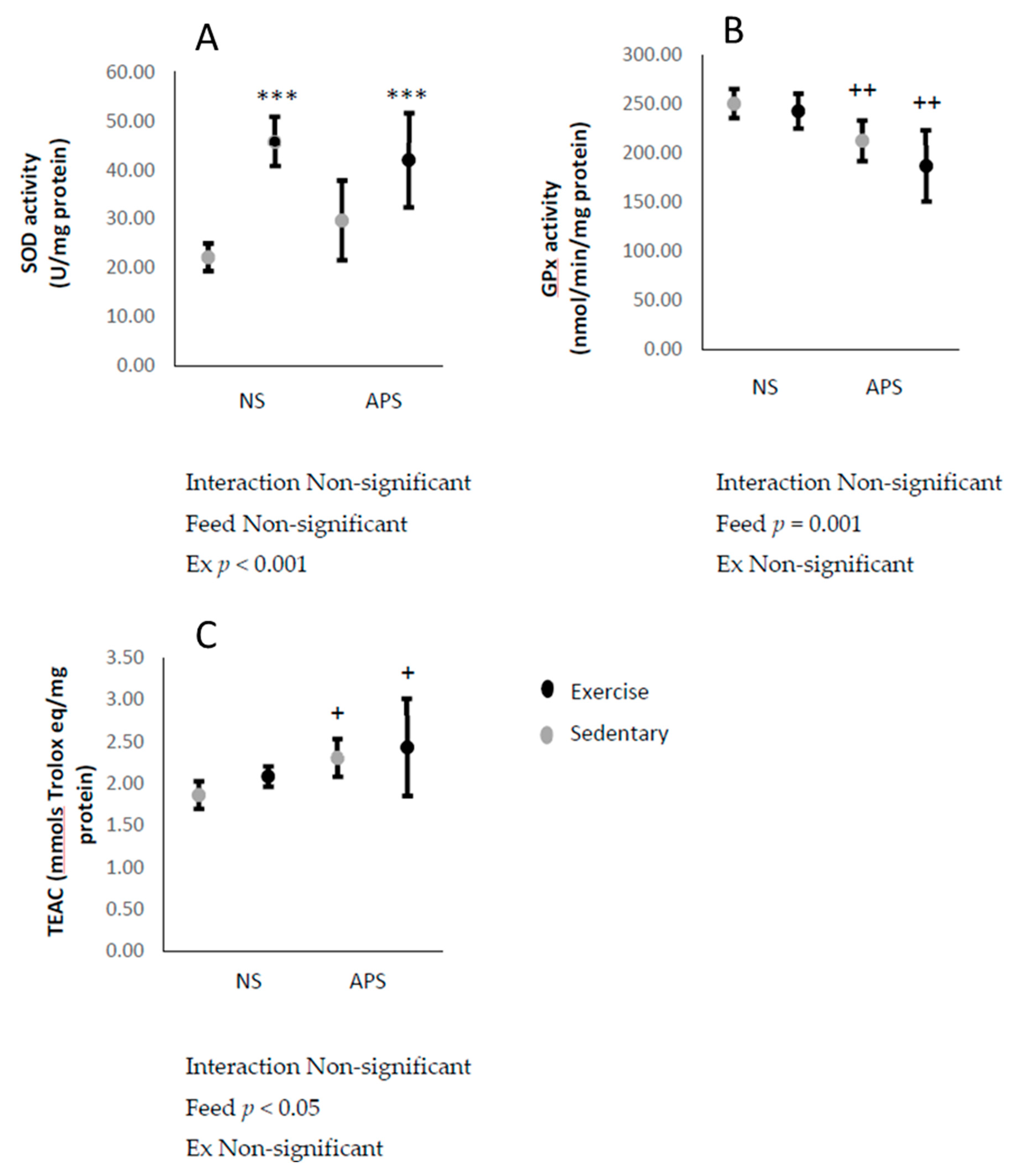
3. Results
3.1. Running Time until Exhaustion
3.2. Markers of Hepatic Toxicity
3.3. Markers of Oxidative Stress in Liver
3.4. Markers of Antioxidant Capacity in Liver
3.5. Markers of Oxidative Stress in Skeletal Muscle
3.6. Markers of Antioxidant Capacity in Skeletal Muscle
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, F.B.; Sigal, R.J.; Rich-Edwards, J.W.; Colditz, G.A.; Solomon, C.G.; Willett, W.C.; Speizer, F.E.; Manson, J.E. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: A prospective study. JAMA 1999, 282, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.C.; Lee, I.-M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; de Gonzalez, A.B.; Hartge, P. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Gimeno, A.; Sastre, J.; Desco, C.; Asensi, M.; Pallardó, F.V.; Cuesta, A.; Ferrero, J.A.; Terada, L.S.; Repine, J.E. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life 2000, 49, 539–544. [Google Scholar]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Mandal, A.; Chanda, D.; Chakraborti, S. Oxidant, antioxidant and physical exercise. Mol. Cell. Biochem. 2003, 253, 307–312. [Google Scholar] [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakaji, S.; Yamada, M.; Liu, Q.; Kurakake, S.; Okamura, N.; Kumae, T.; Umeda, T.; Sugawara, K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med. Sci. Sports Exerc. 2003, 35, 348–355. [Google Scholar] [CrossRef]
- Bentley, D.J.; Dank, S.; Coupland, R.; Midgley, A.; Spence, I. Acute antioxidant supplementation improves endurance performance in trained athletes. Res. Sports Med. 2012, 20, 1–12. [Google Scholar] [CrossRef]
- Ikarashi, N.; Toda, T.; Okaniwa, T.; Ito, K.; Ochiai, W.; Sugiyama, K. Anti-obesity and anti-diabetic effects of acacia polyphenol in obese diabetic KKAy mice fed high-fat diet. Evid.-Based Complementary Altern. Med. 2011, 2011. [Google Scholar] [CrossRef]
- Sundaram, R.; Mitra, S. Antioxidant activity of ethyl acetate soluble fraction of Acacia arabica bark in rats. Indian J. Pharmacol. 2007, 39, 33. [Google Scholar]
- Salehi, B.; Ata, A.; V Anil Kumar, N.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z. Antidiabetic potential of medicinal plants and their active components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Jarald, E.; Joshi, S.B.; Jain, D.C. Biochemical study on the hypoglycaemic effects of extract and fraction of Acacia catechu willd in alloxan-induced diabetic rats. Int. J. Diabetes Metab. 2009, 17, 63–69. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Takanami, Y.; Kawai, Y.; Sakuma, K.; Ichikawa, H.; Yoshida, N.; Yoshikawa, T. Oxidative stress and delayed-onset muscle damage after exercise. Free Radic. Biol. Med. 2004, 37, 480–487. [Google Scholar] [CrossRef]
- Korivi, M.; Hou, C.-W.; Huang, C.-Y.; Lee, S.-D.; Hsu, M.-F.; Yu, S.-H.; Chen, C.-Y.; Liu, Y.-Y.; Kuo, C.-H. Ginsenoside-Rg1 protects the liver against exhaustive exercise-induced oxidative stress in rats. Evid.-Based Complementary Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Radák, Z.; Asano, K.; Inoue, M.; Kizaki, T.; Oh-Ishi, S.; Suzuki, K.; Taniguchi, N.; Ohno, H. Superoxide dismutase derivative prevents oxidative damage in liver and kidney of rats induced by exhausting exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 72, 189–194. [Google Scholar] [CrossRef]
- Ramos, D.; Martins, E.G.; Viana-Gomes, D.; Casimiro-Lopes, G.; Salerno, V.P. Biomarkers of oxidative stress and tissue damage released by muscle and liver after a single bout of swimming exercise. Appl. Physiol. Nutr. Metab. 2013, 38, 507–511. [Google Scholar] [CrossRef]
- Suzuki, K. Involvement of neutrophils in exercise-induced muscle damage. Gen. Intern. Med. Clin. Innov. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Groussard, C.; Machefer, G.; Rannou, F.; Faure, H.; Zouhal, H.; Sergent, O.; Chevanne, M.; Cillard, J.; Gratas-Delamarche, A. Physical fitness and plasma non-enzymatic antioxidant status at rest and after a wingate test. Can. J. Appl. Physiol. 2003, 28, 79–92. [Google Scholar] [CrossRef]
- Higuchi, M.; Cartier, L.-j.; Chen, M.; Holloszy, J.O. Superoxide dismutase and catalase in skeletal muscle: Adaptive response to exercise. J. Gerontol. 1985, 40, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Antioxidant enzyme response to exercise and aging. Med. Sci. Sports Exerc. 1993, 25, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Higdon, J.V. Antioxidant activity of tea polyphenols in vivo: Evidence from animal studies. J. Nutr. 2003, 133, 3275S–3284S. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed]
- Adzet, T.; Camarasa, J.; Laguna, J.C. Hepatoprotective activity of polyphenolic compounds from Cynara scolymus against CCl4 toxicity in isolated rat hepatocytes. J. Nat. Prod. 1987, 50, 612–617. [Google Scholar] [CrossRef]
- El-Beshbishy, H.A. Hepatoprotective effect of green tea (Camellia sinensis) extract against tamoxifen-induced liver injury in rats. BMB Rep. 2005, 38, 563–570. [Google Scholar] [CrossRef]
- Shimoda, H.; Tanaka, J.; Kikuchi, M.; Fukuda, T.; Ito, H.; Hatano, T.; Yoshida, T. Walnut polyphenols prevent liver damage induced by carbon tetrachloride and d-galactosamine: Hepatoprotective hydrolyzable tannins in the kernel pellicles of walnut. J. Agric. Food Chem. 2008, 56, 4444–4449. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wang, F.; Wu, C. Hepatoprotective effects of apple polyphenols on CCl4-induced acute liver damage in mice. J. Agric. Food Chem. 2010, 58, 6525–6531. [Google Scholar] [CrossRef]
- Chang, S.-T.; Wu, J.-H.; Wang, S.-Y.; Kang, P.-L.; Yang, N.-S.; Shyur, L.-F. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef]
- Wu, J.-H.; Tung, Y.-T.; Wang, S.-Y.; Shyur, L.-F.; Kuo, Y.-H.; Chang, S.-T. Phenolic antioxidants from the heartwood of Acacia confusa. J. Agric. Food Chem. 2005, 53, 5917–5921. [Google Scholar] [CrossRef] [PubMed]
- Jayasekhar, P.; Mohanan, P.; Rathinam, K. Hepatoprotective activity of ethyl acetate extract of Acacia catechu. Indian J. Pharmacol. 1997, 29, 426–428. [Google Scholar]
- Tung, Y.-T.; Wu, J.-H.; Huang, C.-C.; Peng, H.-C.; Chen, Y.-L.; Yang, S.-C.; Chang, S.-T. Protective effect of Acacia confusa bark extract and its active compound gallic acid against carbon tetrachloride-induced chronic liver injury in rats. Food Chem. Toxicol. 2009, 47, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Kennett, M.J.; Sang, S.; Reuhl, K.R.; Ju, J.; Yang, C.S. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2010, 48, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Wan, X.; Yang, C.S.; Zhang, J. Green tea polyphenol (−)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol. Appl. Pharmacol. 2015, 283, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, Z.; Zhang, L.; Yamamoto, M.; Misra, H.P.; Trush, M.A.; Li, Y. Antioxidants and phase 2 enzymes in macrophages: Regulation by Nrf2 signaling and protection against oxidative and electrophilic stress. Exp. Biol. Med. 2008, 233, 463–474. [Google Scholar] [CrossRef]
- Sai, K.; Kai, S.; Umemura, T.; Tanimura, A.; Hasegawa, R.; Inoue, T.; Kurokawa, Y. Protective effects of green tea on hepatotoxicity, oxidative DNA damage and cell proliferation in the rat liver induced by repeated oral administration of 2-nitropropane. Food Chem. Toxicol. 1998, 36, 1043–1051. [Google Scholar] [CrossRef]
- Haramizu, S.; Ota, N.; Hase, T.; Murase, T. Catechins attenuate eccentric exercise-induced inflammation and loss of force production in muscle in senescence-accelerated mice. J. Appl. Physiol. 2011, 111, 1654–1663. [Google Scholar] [CrossRef]
- Morillas-Ruiz, J.; García, J.V.; López, F.; Vidal-Guevara, M.; Zafrilla, P. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin. Nutr. 2006, 25, 444–453. [Google Scholar] [CrossRef]
- Yada, K.; Suzuki, K.; Oginome, N.; Ma, S.; Fukuda, Y.; Iida, A.; Radak, Z. Single dose administration of taheebo polyphenol enhances endurance capacity in mice. Sci. Rep. 2018, 8, 14625. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Criswell, D.; Lawler, J.; Ji, L.L.; Martin, D.; Herb, R.A.; Dudley, G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1994, 266, R375–R380. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yada, K.; Roberts, L.A.; Oginome, N.; Suzuki, K. Effect of Acacia Polyphenol Supplementation on Exercise-Induced Oxidative Stress in Mice Liver and Skeletal Muscle. Antioxidants 2020, 9, 29. https://doi.org/10.3390/antiox9010029
Yada K, Roberts LA, Oginome N, Suzuki K. Effect of Acacia Polyphenol Supplementation on Exercise-Induced Oxidative Stress in Mice Liver and Skeletal Muscle. Antioxidants. 2020; 9(1):29. https://doi.org/10.3390/antiox9010029
Chicago/Turabian StyleYada, Koichi, Llion Arwyn Roberts, Natsumi Oginome, and Katsuhiko Suzuki. 2020. "Effect of Acacia Polyphenol Supplementation on Exercise-Induced Oxidative Stress in Mice Liver and Skeletal Muscle" Antioxidants 9, no. 1: 29. https://doi.org/10.3390/antiox9010029
APA StyleYada, K., Roberts, L. A., Oginome, N., & Suzuki, K. (2020). Effect of Acacia Polyphenol Supplementation on Exercise-Induced Oxidative Stress in Mice Liver and Skeletal Muscle. Antioxidants, 9(1), 29. https://doi.org/10.3390/antiox9010029





