The Anti-Proliferative and Anti-Invasive Effect of Leaf Extracts of Blueberry Plants Treated with Methyl Jasmonate on Human Gastric Cancer In Vitro Is Related to Their Antioxidant Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blueberry Growth Condition and Pre-Harvest MeJA Treatment
2.2. Preparation of Blueberry Aqueous Crude Extracts
2.3. Antioxidant Properties of Crude Extracts
2.4. Assessment of the Effect of Extracts on Gastric Cancer Cells Viability and Migratory Capacity
2.5. Assessment of the Effect of Leaves Extracts on Gastric Cancer-Related Protein Expression
2.6. Obtainment of a Rich-Anthocyanin Extract from Blueberry Leaves
2.7. Study of the In Vivo Anticancer Effect of Leaves Extracts Using a Gerbil Model
2.8. Statistical Analysis
3. Results
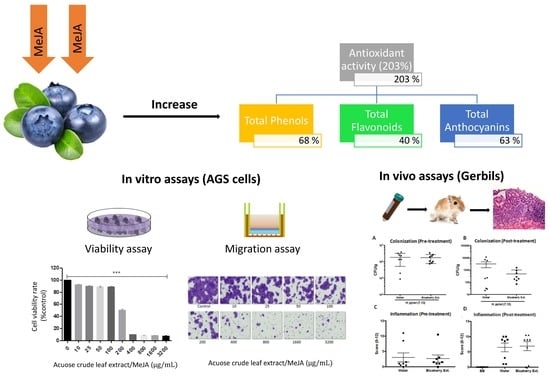
3.1. In Vitro Anticancer and Antioxidant Properties of Blueberry Crude Extracts on Gastric Cancer Cells
3.2. Anticarcinogenic and Antioxidant Properties of Leaf Extract from Blueberry Plants Treated with MeJA
3.3. Comparing the In Vitro Anticancer Effect between Crude and Rich-Anthocyanin Leaves Extracts
3.4. Evaluation of Rich Anthocyanin Extracts of Leaves from MeJA-Treated Plants Over In Vivo Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heise, K.; Bertran, E.; Andia, M.; Ferreccio, C. Incidence and survival of stomach cancer in a high-risk population of Chile. World J. Gastroenterol. 2009, 15, 1854–1862. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Gastroenterol. Rev. 2019, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Melgoza, G.; Galindo, H.; Madrid, J.; Sánchez, C.; Nervi, B.; Alvarez, M.; Orellana, E. Treatment of advanced gastric cancer with oxaliplatin plus 5-fluorouracil/leucovorin (FOLFOX-4 chemotherapy). Rev. Med. Chile 2007, 135, 1380–1387. [Google Scholar] [PubMed]
- Liu, W.; Quan, H.; Chen, X.; Ouyang, Y.; Xiao, H. Clinicopathological features and prognosis of young gastric cancer patients following radical gastrectomy: A propensity score matching analysis. Sci. Rep. 2019, 9, 5943. [Google Scholar] [CrossRef]
- Songun, I.; Bonenkamp, J.J.; Hermans, J.; van Krieken, J.H.; van de Velde, C.J. Prognostic value of resection-line involvement in patients undergoing curative resections for gastric cancer. Eur. J. Cancer 1996, 32A, 433–437. [Google Scholar] [CrossRef]
- Wu, W.; Chung, M. The gastric fluid proteome as a potential source of gastric cancer biomarkers. J. Prot. 2013, 90, 3–13. [Google Scholar] [CrossRef]
- McGee, D.J.; Mobley, H.L. Mechanisms of Helicobacter pylori infection: Bacterial factors. Curr. Top Microbiol. Immunol. 1999, 241, 155–180. [Google Scholar]
- Naito, Y.; Yoshikawa, T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radic. Biol. Med. 2002, 33, 323–336. [Google Scholar] [CrossRef]
- Díaz, P.; Valenzuela, M.; Valderrama, M.; Bravo, J.; Quest, A. Helicobacter pylori and Gastric Cancer: Adaptive Cellular Mechanisms Involved in Disease Progression. Front. Microbiol. 2018, 9, 5. [Google Scholar]
- Suzuki, H.; Marshall, B.J.; Hibi, T. Overview: Helicobacter pylori and extra gastric disease. Int. J. Hematol. 2006, 84, 291–300. [Google Scholar] [CrossRef]
- Suzuki, T.; Matsuo, K.; Sawaki, A.; Ito, H.; Hirose, K.; Wakai, K.; Sato, S.; Nakamura, T.; Yamao, K.; Ueda, R.; et al. Systematic review and meta-analysis: Importance of CagA status for successful eradication of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2006, 24, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Yusefi, A.R.; Lankarani, K.B.; Bastani, P.; Radinmanesh, M.; Kavosi, Z. Risk Factors for Gastric Cancer: A Systematic Review. Asian Pac. J. Cancer Prev. 2018, 19, 591–603. [Google Scholar] [PubMed]
- Bulger, E.M.; Helton, W.S. Nutrient antioxidants in gastrointestinal diseases. Gastroenterol. Clin. N. Am. 1998, 27, 403–419. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Bellocco, R.; Wolk, A.; Ekstrom, A. Total antioxidant potential of fruit and vegetables and risk of gastric cancer. Gastroenterology 2002, 123, 985–991. [Google Scholar] [CrossRef]
- Ajaikumar, K.B.; Asheef, M.; Babu, B.H.; Padikalla, J. The inhibition of gastric mucosal injury by Punica granatum L. (pomegranate) methanolic extract. J. Ethnopharmacol. 2005, 96, 171–176. [Google Scholar] [CrossRef]
- Lee, J.E.; Chan, A.T. Fruit, vegetables, and folate: Cultivating the evidence for cancer prevention. Gastroenterol. 2011, 141, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Weng, C.J.; Yen, G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef]
- Olas, B. Berry Phenolic Antioxidants—Implications for Human Health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef]
- Johnso, S.; Arjmandi, B. Evidence for anti-cancer properties of blueberries: A mini-review. Anti-Cancer Agents Med. Chem. 2013, 13, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Mazur, A.; Lamaison, J.L.; Rémésy, C. Bioavailability of a bilberry anthocyanin extract and its impact on plasma antioxidant capacity in rats. J. Sci. Food Agric. 2006, 86, 90–97. [Google Scholar] [CrossRef]
- Perez, A.G.; Sanz, C.; Olıas, R.; Olıas, J.M. Effect of methyl jasmonate on in vitro strawberry ripening. J. Agric. Food Chem. 1997, 45, 3733–3737. [Google Scholar] [CrossRef]
- Wang, L.; Stoner, G. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Sayyar, M.; Babalar, M.; Kalantari, S.; Martinez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011, 124, 964–970. [Google Scholar] [CrossRef]
- Yi, T.G.; Park, Y.; Park, J.E.; Park, N. Enhancement of phenolic compounds and antioxidative activities by the combination of culture medium and methyl jasmonate elicitation in hairy root cultures of Lactuca indica L. Nat. Product. Commun. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Chinnici, F.; Bendini, A.A.; Gaiani, A.; Riponi, C. Radical scavenging activities of peels and pulps from cv. Golden delicious apples as related to their phenolic composition. J. Agric. Food Chem. 2004, 52, 4684–4689. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.A. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 29–55. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Cheng, G.W.; Breen, P.J. Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J. Am. Soc. Hort. Sci. 1991, 116, 865–869. [Google Scholar] [CrossRef]
- Ribera, A.E.; Reyes-Díaz, M.; Alberdi, M.; Zuñiga, G.E.; Mora, M.L. Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Vaccinium corymbosum L.) grown in southern Chile. J. Soil Sci. Plant Nutr. 2010, 10, 509–536. [Google Scholar] [CrossRef]
- Nyman, N.A.; Kumpulainen, J.T. Determination of anthocyanidins in berries and red wine by high-performance liquid chromatography. J. Agric. Food Chem. 2001, 49, 4183–4187. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Whelan, S.L.; Ferlay, J.; Storm, H. Cancer Incidence in Five Continents; IARC Press: Lyon, France, 2005; Volume I–VIII, No. 6. [Google Scholar]
- Bazuro, G.E.; Torino, F.; Gasparini, G.; Capurso, L. Chemoprevention in gastrointestinal adenocarcinoma: For few but not for all? Minerva Gastroenterol. Dietol. 2008, 54, 429–444. [Google Scholar] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.L.; Lundgreen, A.; Torres-Mejia, G.; Wolff, R.K.; Hines, L.; Baumgartner, K.; John, E.M. Diet and lifestyle factors modify immune/inflammation response genes to alter breast cancer risk and prognosis: The breast cancer health disparities study. Mutat. Res. Fundam. Mol. Mech. Mutagen 2014, 770, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pervin, M.; Hasnat, M.A.; Ou, L.B. Antibacterial and antioxidant activities of Vaccinium corymbosum L. leaf extract. Asian Pac. J. Trop. Dis. 2013, 3, 444–453. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Gordillo, G.; Fang, H.; Khanna, S.; Harper, J.; Phillips, G.; Sen, C. Oral administration of blueberry inhibits angiogenic tumor growth and enhances survival of mice with endothelial cell neoplasm. Antiox. Redox Signal. 2009, 11, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Jeyabalan, J.; Aqil, F.; Munagala, R.; Annamalai, L.; Vadhanam, M.V.; Gupta, R.C. Chemopreventive and therapeutic activity of dietary blueberry against estrogen-mediated breast cancer. J. Agric. Food Chem. 2014, 62, 3963–3971. [Google Scholar] [CrossRef]
- Piljac-Zegarac, J.; Belscak, A.; Piljac, A. Antioxidant capacity and polyphenolic content of blueberry (Vaccinium corymbosum L.) leaf infusions. J. Med. Food 2009, 12, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Díaz, M.; Lobos, T.; Cardemil, L.; Nunes-Nesi, A.; Retamales, J.; Jaakola, L.; Alberdi, M.; Ribera-Fonseca, A. Methyl jasmonate: An alternative for improving the quality and health properties of fresh fruits. Molecules 2016, 21, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Y.; Bowman, L.; Ding, M. Methyl jasmonate enhances antioxidant activity and flavonoid content in blackberries (Rubus sp.) and promotes antiproliferation of human cancer cells. Food Chem. 2008, 107, 1261–1269. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Ehlenfeldt, M.; Prior, R. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hara, H.; Chiji, H.; Kasai, T. Gastroprotective effect of red pigments in black chokeberry fruit (Aroniamelano carpa Elliot) on acute gastric lesions in rats. J. Agric. Food Chem. 2004, 52, 2226–2229. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, G.M.; Figueroa, N.E.; Poblete, L.A.; Cherian, S.; Figueroa, C.R. Effects of preharvest applications of methyl jasmonate and chitosan on postharvest decay, quality and chemical attributes of Fragaria chiloensis fruit. Food Chem. 2016, 190, 448–453. [Google Scholar] [CrossRef]
- Ozturk, B.; Yıldız, K.; Ozkan, Y. Effects of pre-harvest methyl jasmonate treatments on bioactive compounds and peel color development of “Fuji” apples. Int. J. Food Prop. 2015, 18, 954–962. [Google Scholar] [CrossRef]
- Cocetta, G.; Rossoni, M.; Gardana, C.; Mignani, I.; Ferrante, A.; Spinardi, A. Methyl jasmonate affects phenolic metabolism and gene expression in blueberry (Vaccinium corymbosum). Physiol. Plant. 2015, 153, 269–283. [Google Scholar] [CrossRef]
- Li, L.; Dong, Y.; Ren, H.; Xue, Y.; Meng, H.; Li, M. Increased antioxidant activity and polyphenol metabolites in methyl jasmonate treated mung bean (Vigna radiata) sprouts. Food Sci. Tech. 2017, 37, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Seeram, N.; Adams, L.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.; Blanchette, M.; Barrette, S.; Moghrabi, A.; Béliveau, R. Inhibition of cancer cell proliferation and suppression of TNF-induced activation of NFkappaB by edible berry juice. Anticancer Res. 2007, 27, 937–948. [Google Scholar] [PubMed]
- Subash, S.; Essa, M.M.; Al-Adawi, S.; Memon, M.A.; Manivasagam, T.; Akbar, M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regen. Res. 2014, 9, 1557–1566. [Google Scholar] [PubMed]
- Seeram, N. Berry Fruits for Cancer Prevention: Current Status and Future Prospects. J. Agric. Food Chem. 2008, 56, 630–635. [Google Scholar] [CrossRef]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018, 58, 2491–2507. [Google Scholar] [CrossRef]
- Sezer, E.D.; Oktay, L.M.; Karadadaş, E.; Memmedov, H.; Selvi Gunel, N.; Sözmen, E. Assessing anticancer potential of blueberry flavonoids, quercetin, kaempferol, and gentisic acid, through oxidative stress and apoptosis parameters on HCT-116 Cells. J. Med. Food 2019, 22, 11. [Google Scholar] [CrossRef]
- Davidson, K.T.; Zhu, Z.; Balabanov, D.; Zhao, L.; Wakefield, M.R.; Bai, Q.; Fang, Y. Beyond conventional medicine—A look at blueberry, a cancer-fighting superfruit. Pathol. Oncol. Res. 2018, 24, 733–738. [Google Scholar] [CrossRef]
- San Miguel-Chávez, R. Phenolic Antioxidant Capacity: A Review of the State of the Art; Open Access Peer-Reviewed Chapter; Intech Open: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Vilella-Bach, M.; Bachmann, R.; Flanigan, A.; Chen, J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 2001, 294, 1942–1945. [Google Scholar] [CrossRef]
- Zhou, H.; Huang, S. Role of mTOR Signaling in tumor cell motility, invasion and metastasis. Curr. Protein Pept. Sci. 2011, 12, 30–42. [Google Scholar]
- Matsuoka, T.; Yashiro, M. The Role of PI3K/Akt/mTOR Signaling in Gastric Carcinoma. Cancers 2014, 6, 1441–1463. [Google Scholar] [CrossRef] [Green Version]
- Caron, E.; Ghosh, S.; Matsuoka, Y.; Ashton-Beaucage, D.; Therrien, M.; Lemieux, S.; Perreault, C.; Roux, P.P.; Kitano, H. A comprehensive map of the mTOR signaling network. Mol. Syst. Biol. 2010, 6, 453. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Ma, L.; Teruya-Feldstein, J.; Rojo, F.; Salmena, L.; Alimonti, A.; Egia, A.; Sasaki, A.T.; Thomas, G.; Kozma, S.C.; et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Investig. 2008, 118, 9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Lin, S.H.; Din, Z.H.; Jui-Hsin, S.; Liu, C.I. Sinulariolide Inhibits Gastric Cancer Cell Migration and Invasion through Downregulation of the EMT Process and Suppression of FAK/PI3K/AKT/mTOR and MAPKs Signaling Pathways. Mar. Drugs 2019, 17, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Chen, Z.; Huang, Z.; Chen, F.; Ye, Z.; Lin, S.; Wang, W. Effect of T-cadherin on the AKT/mTOR signaling pathway, gastric cancer cell cycle, migration and invasion, and its association with patient survival rate. Exp. Ther. Med. 2019, 17, 3607–3613. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Mei, D.; Xu, P.; Wang, H.; Wang, Y. YAP promotes gastric cancer cell survival and migration/invasion via the ERK/endoplasmic reticulum stress pathway. Oncol. Lett. 2019, 18, 6752–6758. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, M.; Dorfman, R.G.; Pan, Y.; Tang, D.; Xu, L.; Zhao, Z.; Zhou, Q.; Zhou, L.; Wang, Y.; et al. SIRT2 Promotes the Migration and Invasion of Gastric Cancer through RAS/ERK/JNK/MMP-9 Pathway by Increasing PEPCK1-Related Metabolism. Neoplasia 2018, 20, 745–756. [Google Scholar] [CrossRef]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef]
- Lee, K.W.; Lee, H.J. The roles of polyphenols in cancer chemoprevention. BioFactors 2006, 26, 105–121. [Google Scholar] [CrossRef]
- Costea, T.; Nagy, P.; Ganea, C.; Szöllὅsi, J.; Mocanu, M.M. Molecular Mechanisms and Bioavailability of Polyphenols in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 1062. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Bueso-Ramos, C.; Aggarwal, B.B. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-κB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002, 62, 4945–4954. [Google Scholar]
- Hou, D.X.; Kumamoto, T. Flavonoids as protein kinase inhibitors for cancer chemoprevention: Direct binding and molecular modeling. Antioxid. Redox Signal. 2010, 13, 691–719. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.H.; Barve, A.; Yu, S.; Huang, M.T.; Kong, A. Cancer chemoprevention by phytochemicals: Potential molecular targets, biomarkers and animal models. Acta Pharmacol. Sin. 2007, 28, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Lavy, A.; Aviram, M. Consumption of red wine with meals reduces the susceptibility of human plasma and LDL to undergo lipid peroxidation. Am. J. Clin Nutr. 1995, 61, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M. Treatment of fibrocystic disease of the breast with myrtillus anthocyanins. Minerva Ginecol. 1993, 45, 617–621. [Google Scholar] [PubMed]
- Scharrer, A.; Ober, M. Anthocyanosides in the treatment of retinopathies. Klin Monatsbl Augenheikd 1981, 178, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J. Dietary Flavonoids as Cancer Chemopreventive Agents: An Updated Review of Human Studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Russo, G.L.; Tedesco, I.; Spagnuolo, C.; Russo, M. Antioxidant polyphenols in cancer treatment: Friend, foe or foil? Semin. Cancer Biol. 2017, 46, 1–13. [Google Scholar] [CrossRef]
- Abourashed, E.A. Bioavailability of Plant-Derived Antioxidants. Antioxidants 2013, 2, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to Improve Resveratrol Systemic and Topical Bioavailability: An Update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef] [Green Version]





| Primary Antibodies | Clone | Molecular Weight (kDa) | Dilution |
|---|---|---|---|
| mTOR | 7C10 | 289 | 1/1000 |
| AKT (pan) | 11E7 | 60 | 1/1000 |
| phospho-P70S6K(Thr389) | 108D2 | 70, 85 | 1/1000 |
| p44/42 MAPK (Erk1/2) | 137F5 | 42–44 | 1/1000 |
| Phospho-p44/42 MAPK (Erk1/2) | 197G2 | 42–44 | 1/1000 |
| β-Actin | 13E5 | 45 | 1/5000 |
| Crude Extract (µg mL−1) | Antioxidant Activity (µg TE g−1 FW) | Total Phenols (mg CAE g−1 FW) | Total Flavonoids (mg Rutin g−1 FW) | Total Anthocyanins (mg c3g g−1 FW) | ||||
|---|---|---|---|---|---|---|---|---|
| MeJA Untreated Plants | MeJA Treated Plants | MeJA Untreated Plants | MeJA Treated Plants | MeJA Untreated Plants | MeJA Treated Plants | MeJA Untreated Plants | MeJA Treated Plants | |
| 25 | 30 a | 90 a* | 0.3 a | 0.6 a* | n.d. | n.d. | 2.0 a | 2.1 a |
| 50 | 118 b | 194 b* | 0.4 a | 0.7 a* | 0.3 a | 0.4 a* | 3.9 b | 4.1 b |
| 100 | 266 c | 375 c* | 0.7 b | 0.9 b* | 0.5 b | 0.6 b | 6.2 c | 8.4 c* |
| 200 | 569 d | 748 d* | 1.4 c | 1.6 c | 0.9 c | 1.2 c* | 12 d | 18 d* |
| 400 | 946 e | 1345 e* | 3.4 d | 3.4 d | 1.8 d | 2.0 d | 20 e | 32 e* |
| 800 | 1486 f | 1733 f* | 5.5 e | 8.3 e* | 3.9 e | 4.4 e* | 63 f | 74 f* |
| 1600 | 1924 g | 1969 g* | 12 f | 15 f* | 7.9 f | 9.3 f* | 88 g | 127 g* |
| 3200 | 1990 h | 1998 h | 24 g | 27 g | 16 g | 18 g* | 185 h | 250 h* |
| Phenolic Acids Concentration (mg g−1 FW) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Crude Extract (µg mL−1) | Chlorogenic Acid | Cafeic Acid | Coumaric Acid | Feluric Acid | ||||
| MeJA Untreated Plants | MeJA Treated Plants | MeJA Untreated Plants | MeJA Treated Plants | MeJA Untreated Plants | MeJA Treated Plants | MeJA Untreated Plants | MeJA Treated Plants | |
| 100 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.27 a | n.d. | 0.22 a |
| 200 | 0.11 a | n.d. | n.d. | n.d. | 0.42 a | 0.39 b | 0.36 a | 0.36 b |
| 400 | 0.53 b | n.d. | n.d. | n.d. | 0.50 a | 0.59 c | 0.46 b | 0.42 c |
| 800 | 0.69 c* | 0.26 a | 0.54 a | n.d. | 1.28 b | 1.12 d | 1.06 c* | 0.88 d |
| 1600 | 3.10 d* | 0.85 b | 1.63 b* | 0.95 a | 2.63 c* | 2.31 e | 2.27 d | 2.04 e |
| 3200 | 9.55 e* | 5.76 c | 4.97 c* | 2.32 b | 5.02 d | 5.35 f | 3.77 e | 3.77 f |
| Flavonoids Concentration (mg g−1 FW) | ||||||||
| Crude Extract (µg mL−1) | Rutin | Kaempferol-3-O | Myrecitin | |||||
| MeJA untreated plants | MeJA treated plants | MeJA untreated plants | MeJA treated plants | MeJA untreated plants | MeJA treated plants | |||
| 100 | 0.18 a | 0.33 a* | n.d. | n.d. | 0.67 a | 0.76 a* | ||
| 200 | 0.43 b | 0.48 b | n.d. | n.d. | 1.00 b | 1.21 b* | ||
| 400 | 0.48 b | 0.58 c | n.d. | n.d. | 1.66 c | 2.13 c* | ||
| 800 | 0.94 c | 1.03 d | 1.58 a | 1.43 a | 2.60 d | 3.83 d* | ||
| 1600 | 1.56 d | 1.86 e* | 3.09 b | 3.04 b | 2.54 d | 5.67 e* | ||
| 3200 | 2.45 e | 3.15 f* | 5.70 c | 5.83 c | 2.70 d | 6.91 f* | ||
| Crude Extract (mg mL-1) | Anthocyanidin Concentration (mg g−1 FW) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delphinidin | Malvidin | Cyanidin | Petunidin | Peonidin | ||||||
| MeJA Untreated Plants | MeJA Treated Plants | MeJA Untreated Plants | MeJA Treated Plants | MeJA Untreated Plants | MeJA Treated Plants | MeJA Untreated Plants | MeJA Treated Plants | MeJA Untreated Plants | MeJA Treated Plants | |
| 50 | 1.1 a | 4.4 a* | 0.25 a | 0.54 a* | 0.05 a | 0.08 a* | n.d. | n.d. | n.d. | n.d. |
| 100 | 1.5 a | 4.9 a* | 0.36 b | 0.61 b* | 0.12 b | 0.16 b* | n.d. | n.d. | n.d. | n.d. |
| 200 | 6.5 b | 11 b* | 0.96 c | 1.2 c | 0.19 c | 0.32 c* | n.d. | n.d. | n.d. | n.d. |
| 400 | 15 c | 21 c* | 2 d | 2.4 d | 0.39 d | 0.66 d* | n.d. | 0.09 a* | n.d. | n.d. |
| 800 | 31 d | 50 d* | 3.5 e | 5.1 e* | 0.79 e | 1.5 e* | 0.08 a | 0.14 a* | 0.06 a | 0.05 a |
| 1600 | 59 e | 84 e* | 6.6 f | 8.8 f* | 1.9 f | 2.8 f* | 0.16 b | 0.27 b* | 0.08 b | 0.14 b* |
| 3200 | 112 f | 153 f* | 16 g | 21 g* | 3.6 g | 5.3 g* | 0.32 c | 0.48 c* | 0.20 c | 0.24 c* |
| Compound | Leaves Crude Extracts | |
|---|---|---|
| MeJA Untreated Plants | MeJA Treated Plants | |
| Isocitric Cafeoil Acid | + | + |
| Myricetin glycoside | + | + |
| Kaempferol | + | + |
| Quercetin | + | + |
| Kineic Cafeoyl Acid | + | + |
| Quercetin-3- Glycoside | + | + |
| Malonil Acid Mono Cafeoil kineic | + | + |
| Myricetin-3-Glycoside | + | + |
| Myricetin-3-Xylose | + | − |
| Rutin | + | + |
| Quercetin-3 Oβ- Xyloside | + | + |
| Quercetin-3-Ramnoside | + | + |
| Kaempferol-3-O-Rutinoside | + | + |
| Isorhamnetine-3-Rutinoside | + | + |
| Quercetin-3-O-glucose | + | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribera-Fonseca, A.; Jiménez, D.; Leal, P.; Riquelme, I.; Roa, J.C.; Alberdi, M.; Peek, R.M.; Reyes-Díaz, M. The Anti-Proliferative and Anti-Invasive Effect of Leaf Extracts of Blueberry Plants Treated with Methyl Jasmonate on Human Gastric Cancer In Vitro Is Related to Their Antioxidant Properties. Antioxidants 2020, 9, 45. https://doi.org/10.3390/antiox9010045
Ribera-Fonseca A, Jiménez D, Leal P, Riquelme I, Roa JC, Alberdi M, Peek RM, Reyes-Díaz M. The Anti-Proliferative and Anti-Invasive Effect of Leaf Extracts of Blueberry Plants Treated with Methyl Jasmonate on Human Gastric Cancer In Vitro Is Related to Their Antioxidant Properties. Antioxidants. 2020; 9(1):45. https://doi.org/10.3390/antiox9010045
Chicago/Turabian StyleRibera-Fonseca, Alejandra, Danae Jiménez, Pamela Leal, Ismael Riquelme, Juan Carlos Roa, Miren Alberdi, Richard M. Peek, and Marjorie Reyes-Díaz. 2020. "The Anti-Proliferative and Anti-Invasive Effect of Leaf Extracts of Blueberry Plants Treated with Methyl Jasmonate on Human Gastric Cancer In Vitro Is Related to Their Antioxidant Properties" Antioxidants 9, no. 1: 45. https://doi.org/10.3390/antiox9010045
APA StyleRibera-Fonseca, A., Jiménez, D., Leal, P., Riquelme, I., Roa, J. C., Alberdi, M., Peek, R. M., & Reyes-Díaz, M. (2020). The Anti-Proliferative and Anti-Invasive Effect of Leaf Extracts of Blueberry Plants Treated with Methyl Jasmonate on Human Gastric Cancer In Vitro Is Related to Their Antioxidant Properties. Antioxidants, 9(1), 45. https://doi.org/10.3390/antiox9010045