Antioxidant Activity of Metal Nanoparticles Coated with Tocopherol-Like Residues—The Importance of Studies in Homo- and Heterogeneous Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. General Information
2.3. Synthesis Procedures
2.3.1. 2,2′-Diaminodiethyl disulfide dihydrochloride (2)
2.3.2. N,N’-(disulfanediylbis(ethane-2,1-diyl))bis(6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxamide) (3)
2.3.3. 6-hydroxy-N-(2-mercaptoethyl)-5,7,8-trimethylchromane-2-carboxamide (4)
2.3.4. AuNPs Stabilized with TOAB (5)
2.3.5. AuNPs 5 Functionalized with Chromanol Derivative (1A)
2.3.6. AuNPs Functionalized with Chromanol Derivative (1B)
2.4. Preparation of Micelles
2.5. Preparation of Liposomes—Large Unilamellar Vesicles (LUVs)
2.6. Methodology of Autoxidation Measurements
3. Results and Discussion
3.1. Synthesis
3.2. Methodology of Calculation of Kinetic Parameters
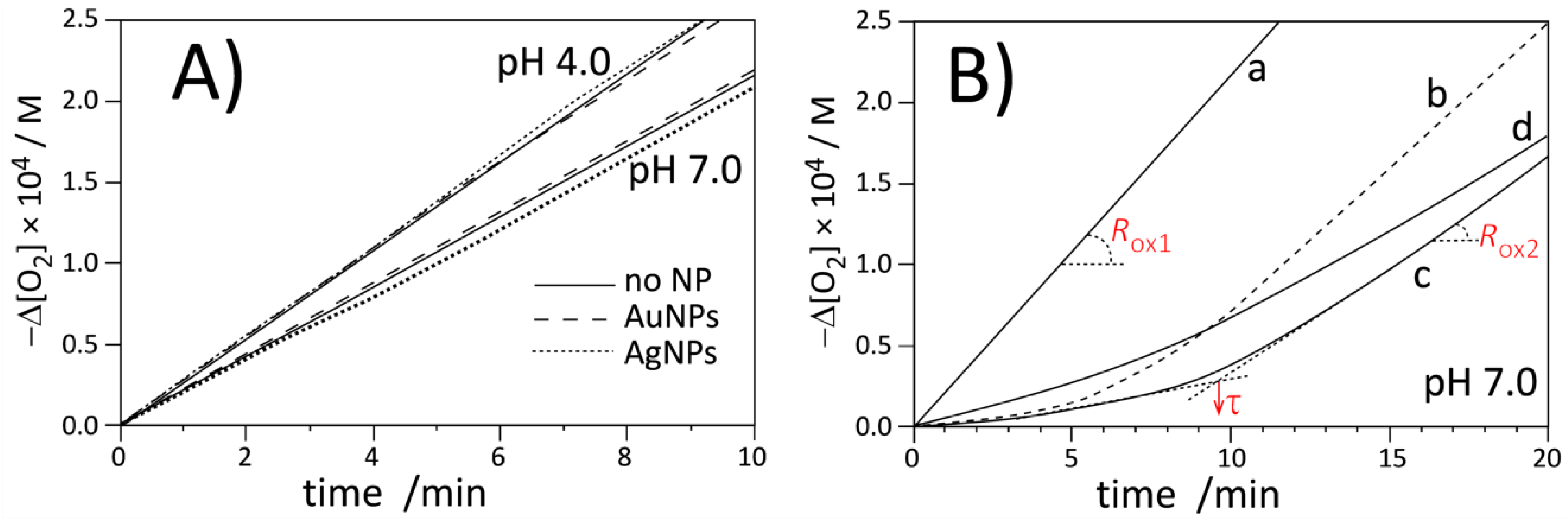
3.3. Antioxidant Activity in Homogeneous System
3.4. Antioxidant Activity in Heterogeneous Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations:
| ABAP | 2:2’-azobis(2-amidinopropane) |
| AgNPs | silver nanoparticles |
| AIBN | α,α’-azobisisobutyronitrile |
| AuNPs | gold nanoparticles |
| BOP | benzotriazol-1-yloxytris(dimethylamino) phosphonium hexafluorophosphate |
| DLS | Dynamic Light Scattering |
| DMAP | 4-dimethylaminopyridine |
| DMPC | 1,2-dimyristoyl-sn-glycero-3-phosphocholine |
| dpph• | 2,2-diphenyl-1-picrylhydrazyl radical |
| HOBt | hydroxybenzotriazole |
| LinMe | methyl linoleate |
| NPs | nanoparticles |
| PMHC | 2,2,5,7,8-pentamethylchroman-6-ol |
| R• | alkyl radical |
| ROO• | peroxyl radical |
| ROS | Reactive Oxygen Species |
| TEM | transmission electron microscopy |
| TGA | thermogravimetric analysis |
| TOAB | tetraoctylammonium bromide |
| Triton X-100 | polyethylene glycol p-(1,1,3,3-tetramethylbutyl)-phenyl ether |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid |
References
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [Green Version]
- Sims, C.M.; Hanna, S.K.; Heller, D.A.; Horoszko, C.P.; Johnson, M.E.; Montoro Bustos, A.R.; Reipa, V.; Riley, K.R.; Nelson, B.C. Redox-active nanomaterials for nanomedicine applications. Nanoscale 2017, 9, 15226–15251. [Google Scholar] [CrossRef]
- Misawa, M.; Takahashi, J. Generation of reactive oxygen species induced by gold nanoparticles under X-ray and UV Irradiations. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 604–614. [Google Scholar] [CrossRef]
- Avalos, A.; Haza, A.I.; Mateo, D.; Morales, P. Cytotoxicity and ROS production of manufactured silver nanoparticles of different sizes in hepatoma and leukemia cells. J. Appl. Toxicol. 2014, 34, 413–423. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Baschieri, A.; Del Secco, B.; Zaccheroni, N.; Valgimigli, L.; Amorati, R. The Role of Onium Salts in the Pro-Oxidant Effect of Gold Nanoparticles in Lipophilic Environments. Chem. Eur. J. 2018, 24, 9113–9119. [Google Scholar] [CrossRef] [PubMed]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.; Lynch, I. Mechanisms of silver nanoparticle release, transformation and toxicity: A critical review of current knowledge and recommendations for future studies and applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Schubert, D.; Dargusch, R.; Raitano, J.; Chan, S.W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 2006, 342, 86–91. [Google Scholar] [CrossRef]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef]
- Das, M.; Patil, S.; Bhargava, N.; Kang, J.F.; Riedel, L.M.; Seal, S.; Hickman, J.J. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 2007, 28, 1918–1925. [Google Scholar] [CrossRef] [Green Version]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 1056–1058. [Google Scholar] [CrossRef]
- Hong, R.; Han, G.; Fernandez, J.M.; Kim, B.J.; Forbes, N.S.; Rotello, V.M. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 2006, 128, 1078–1079. [Google Scholar] [CrossRef]
- Nie, Z.; Liu, K.J.; Zhong, C.J.; Wang, L.F.; Yang, Y.; Tian, Q.; Liu, Y. Enhanced radical scavenging activity by antioxidant-functionalized gold nanoparticles: A novel inspiration for development of new artificial antioxidants. Free Radic. Biol. Med. 2007, 43, 1243–1254. [Google Scholar] [CrossRef]
- Esumi, K.; Takei, N.; Yoshimura, T. Antioxidant-potentiality of gold-chitosan nanocomposites. Colloids Surf. B Biointerfaces 2003, 32, 117–123. [Google Scholar] [CrossRef]
- Hamelian, M.; Varmira, K.; Veisi, H. Green synthesis and characterizations of gold nanoparticles using Thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J. Photochem. Photobiol. B Biol. 2018, 184, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Baruwati, B.; Polshettiwar, V.; Varma, R.S. Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem. 2009, 11, 926–930. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Sannomiya, T.; Teleki, A.; Krumeich, F.; Voros, J.; Pratsinis, S.E. Non-toxic dry-coated nanosilver for plasmonic biosensors. Adv. Funct. Mater. 2010, 20, 4250–4257. [Google Scholar] [CrossRef] [Green Version]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and antiradical SiO2 nanoparticles covalently functionalized with gallic acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Blattmann, C.O.; Deligiannakis, Y. Nanoantioxidant-driven plasmon enhanced proton-coupled electron transfer. Nanoscale 2016, 8, 796–803. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Dal Poggetto, G.; Crescente, G.; Piccolella, S.; Pacifico, S. Chlorogenic Acid Entrapped in Hybrid Materials with High PEG Content: A Strategy to Obtain Antioxidant Functionalized Biomaterials? Materials 2019, 12, 148. [Google Scholar] [CrossRef] [Green Version]
- Viglianisi, C.; Di Pilla, V.; Menichetti, S.; Rotello, V.M.; Candiani, G.; Malloggi, C.; Amorati, R. Linking an α-tocopherol derivative to cobalt(0) nanomagnets: Magnetically responsive antioxidants with superior radical trapping activity and reduced cytotoxicity. Chem. Eur. J. 2014, 20, 6857–6860. [Google Scholar] [CrossRef] [Green Version]
- Baschieri, A.; Amorati, R.; Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Valgimigli, L. Enhanced Antioxidant Activity under Biomimetic Settings of Ascorbic Acid Included in Halloysite Nanotubes. Antioxidants 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Riela, S.; Guernelli, S.; Parisi, F.; Lazzara, G.; Baschieri, A.; Valgimigli, L.; Amorati, R. A synergic nanoantioxidant based on covalently modified halloysite-trolox nanotubes with intra-lumen loaded quercetin. J. Mater. Chem. B 2016, 4, 2229–2241. [Google Scholar] [CrossRef] [Green Version]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidant activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef]
- Czochara, R.; Kusio, J.; Symonowicz, M.; Litwinienko, G. Fullerene C-60 Derivatives as High-Temperature Inhibitors of Oxidative Degradation of Saturated Hydrocarbons. Ind. Eng. Chem. Res. 2016, 55, 9887–9894. [Google Scholar] [CrossRef]
- Czochara, R.; Kusio, J.; Litwinienko, G. Fullerene C60 conjugated with phenols as new hybrid antioxidants to improve the oxidative stability of polymers at elevated temperatures. RSC Adv. 2017, 7, 44021–44025. [Google Scholar] [CrossRef] [Green Version]
- Czochara, R.; Grajda, M.; Kusio, J.; Litwinienko, G. Expanding the antioxidant activity into higher temperatures—Fullerene C60 conjugated with α-Tocopherol analogue as a hybrid antioxidant in saturated lipid systems. Bulg. Chem. Commun. 2018, 50, 268–274. [Google Scholar]
- Czochara, R.; Ziaja, P.; Piotrowski, P.; Pokrop, R.; Litwinienko, G. Fullerene C-60 as an inhibitor of high temperature lipid oxidation. Carbon 2012, 50, 3943–3946. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Ortiz-Acevedo, A.; Dieckmann, G.R. Synthesis of reversible cyclic peptides. Tetrahedron Lett. 2004, 45, 6795–6798. [Google Scholar] [CrossRef]
- Swiech, O.; Bilewicz, R.; Megiel, E. TEMPO coated Au nanoparticles: Synthesis and tethering to gold surfaces. RSC Adv. 2013, 3, 5979–5986. [Google Scholar] [CrossRef]
- Jodko-Piorecka, K.; Litwinienko, G. Antioxidant activity of dopamine and l-DOPA in lipid micelles and their cooperation with an analogue of alpha-tocopherol. Free Radic. Biol. Med. 2015, 83, 1–11. [Google Scholar] [CrossRef]
- Jodko-Piorecka, K.; Litwinienko, G. First Experimental Evidence of Dopamine Interactions with Negatively Charged Model Biomembranes. ACS Chem. Neurosci. 2013, 4, 1114–1122. [Google Scholar] [CrossRef] [Green Version]
- Lucarini, M.; Pedulli, G.F. Free radical intermediates in the inhibition of the autoxidation reaction. Chem. Soc. Rev. 2010, 39, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Jodko-Piorecka, K.; Cedrowski, J.; Litwinienko, G. Physico-chemical principles of antioxidant action, including solvent and matrix dependence and interfacial phenomena. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Apak, R., Capanoglu, E., Shahidi, F., Eds.; Wiley & Sons Ltd: London, UK, 2018; pp. 225–272. [Google Scholar]
- Amorati, R.; Baschieri, A.; Valgimigli, L. Measuring Antioxidant Activity in Bioorganic Samples by the Differential Oxygen Uptake Apparatus: Recent Advances. J. Chem. 2017, 2017, 6369358. [Google Scholar] [CrossRef]
- Van Wenum, E.; Jurczakowski, R.; Litwinienko, G. Media Effects on the Mechanism of Antioxidant Action of Silybin and 2,3-Dehydrosilybin: Role of the Enol Group. J. Org. Chem. 2013, 78, 9102–9112. [Google Scholar] [CrossRef] [PubMed]
- Barclay, L.R.C.; Ingold, K.U. Autoxidation of biological molecules. 2. Autoxidation of a model membrane. Comparison of the autoxidation of egg lecithin phosphatidylcholine in water and in chlorobenzene. J. Am. Chem. Soc. 1981, 103, 6478–6485. [Google Scholar] [CrossRef]
- Hammond, G.S.; Boozer, C.E.; Hamilton, C.E.; Sen, J.N. Air Oxidation of Hydrocarbons. 3. Mechanism of Inhibitor Action in Benzene and Chlorobenzene Solutions. J. Am. Chem. Soc. 1955, 77, 3238–3244. [Google Scholar] [CrossRef]
- Howard, J.A.; Ingold, K.U. Absolute Rate Constants for Hydrocarbon Autoxidation: I. Styrene. Can. J. Chem. 1965, 43, 2729–2736. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Baskin, K.A.; Dakin, K.A.; Locke, S.J.; Vinqvist, M.R. The Antioxidant Activities of Phenolic Antioxidants in Free-Radical Peroxidation of Phospholipid-Membranes. Can. J. Chem. 1990, 68, 2258–2269. [Google Scholar] [CrossRef] [Green Version]
- Barclay, L.R.C.; Locke, S.J.; MacNeil, J.M.; Vankessel, J. Quantitative studies of the autoxidation of linoleate monomers sequestered in phosphatidylcholine bilayers. Absolute rate constants in bilayers. Can. J. Chem. 1985, 63, 2633–2638. [Google Scholar] [CrossRef]
- Mulder, P.; Litwinienko, G.; Lin, S.Q.; MacLean, P.D.; Barclay, L.R.C.; Ingold, K.U. The l-type calcium channel blockers, Hantzsch 1,4-dihydropyridines, are not peroxyl radical-trapping, chain-breaking antioxidants. Chem. Res. Toxicol. 2006, 19, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Ravi, V.; Binz, J.M.; Rioux, R.M. Thermodynamic Profiles at the Solvated Inorganic-Organic Interface: The Case of Gold-Thiolate Monolayers. Nano Lett. 2013, 13, 4442–4448. [Google Scholar] [CrossRef]
- Reik, M.; Calabro, M.; Griesemer, S.; Barry, E.; Bu, W.; Lin, B.; Rice, S.A. The influence of fractional surface coverage on the core–core separation in ordered monolayers of thiol-ligated Au nanoparticles. Soft Matter 2019, 15, 8800–8807. [Google Scholar] [CrossRef] [PubMed]
- Musialik, M.; Kita, M.; Litwinienko, G. Initiation of lipid autoxidation by ABAP at pH 4–10 in SDS micelles. Org. Biomol. Chem. 2008, 6, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Snelgrove, D.W.; Lusztyk, J.; Banks, J.T.; Mulder, P.; Ingold, K.U. Kinetic Solvent Effects on Hydrogen-Atom Abstractions: Reliable, Quantitative Predictions via a Single Empirical Equation1. J. Am. Chem. Soc. 2001, 123, 469–477. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Vinqvist, M.R. Membrane peroxidation: Inhibiting effects of water- soluble antioxidants on phospholipids of different charge types. Free Radic. Biol. Med. 1994, 16, 779–788. [Google Scholar] [CrossRef]
- Bowry, V.W.; Ingold, K.U.; Stocker, R. Vitamin E in human low-density lipoprotein. When and how this antioxidant becomes a pro-oxidant. Biochem. J. 1992, 288, 341–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingold, K.U.; Bowry, V.W.; Stocker, R.; Walling, C. Autoxidation of lipids and antioxidation by alpha-tocopherol and ubiquinol in homogeneous solution and in aqueous dispersions of lipids: Unrecognized consequences of lipid particle size as exemplified by oxidation of human low density lipoprotein. Proc. Natl. Acad. Sci. USA 1993, 90, 45–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]


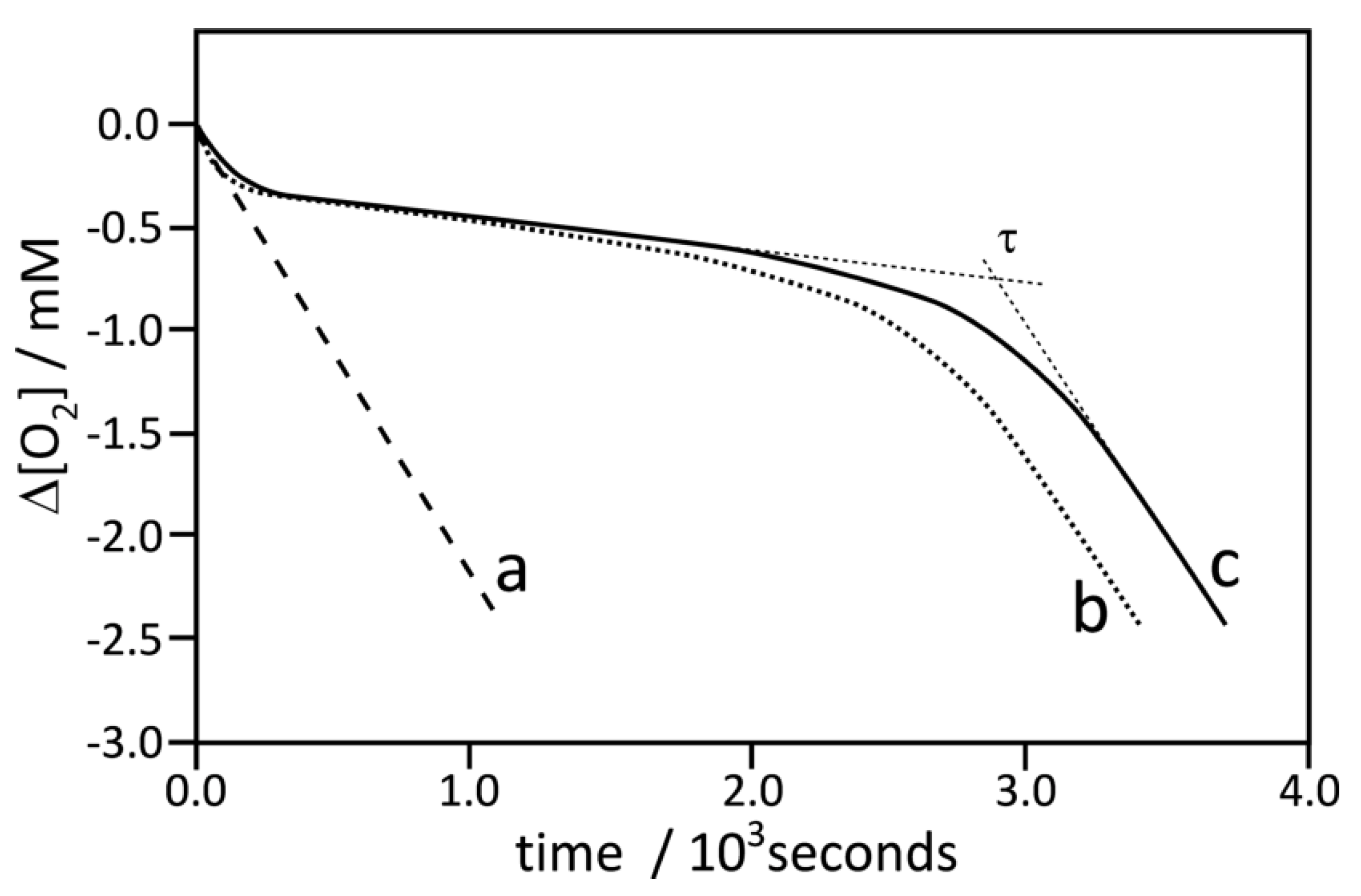

| Compound | Concentration | τ/min | 10−5 × kinh/M−1 s−1 |
|---|---|---|---|
| 3 | 5 µM | 48 ± 5 a | 6.93 ± 0.31 |
| 1A | 10.3 ppm b | 40 ± 3 a | 6.95 ± 0.36 |
| Experimental System | τ/min | Ria/nMs−1 | Rinh/nMs−1 | Rox/Rinhb | 10−3 × kinh/M−1 s−1 |
|---|---|---|---|---|---|
| micelles | |||||
| PMHC | 7.6 ± 0.7 | 4.4 ± 0.4 | 37 ± 5 | 9.0 | 18.8 ± 3.8 |
| Trolox | 9.6 ± 0.4 | 4.4 ± 0.4 | 47 ± 8 | 7.4 | 4.5 ± 0.9 |
| 1B | 9.3 ± 0.6 | 4.4 ± 0.4 | 87 ± 5 | 4.0 | 2.2 ± 0.4 |
| liposomes | |||||
| PMHC | 8.6 ± 0.6 | 3.8 ± 0.4 | 20 ± 6 | 5.0 | 13.9 ± 2.7 |
| Trolox | 8.7 ± 0.3 | 3.8 ± 0.4 | 24 ± 2 | 4.2 | 13.4 ± 2.7 |
| 1B | - | 3.8 ± 0.4 | 67 ± 3 c | 1.5 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopko, A.; Kusio, J.; Litwinienko, G. Antioxidant Activity of Metal Nanoparticles Coated with Tocopherol-Like Residues—The Importance of Studies in Homo- and Heterogeneous Systems. Antioxidants 2020, 9, 5. https://doi.org/10.3390/antiox9010005
Konopko A, Kusio J, Litwinienko G. Antioxidant Activity of Metal Nanoparticles Coated with Tocopherol-Like Residues—The Importance of Studies in Homo- and Heterogeneous Systems. Antioxidants. 2020; 9(1):5. https://doi.org/10.3390/antiox9010005
Chicago/Turabian StyleKonopko, Adrian, Jaroslaw Kusio, and Grzegorz Litwinienko. 2020. "Antioxidant Activity of Metal Nanoparticles Coated with Tocopherol-Like Residues—The Importance of Studies in Homo- and Heterogeneous Systems" Antioxidants 9, no. 1: 5. https://doi.org/10.3390/antiox9010005
APA StyleKonopko, A., Kusio, J., & Litwinienko, G. (2020). Antioxidant Activity of Metal Nanoparticles Coated with Tocopherol-Like Residues—The Importance of Studies in Homo- and Heterogeneous Systems. Antioxidants, 9(1), 5. https://doi.org/10.3390/antiox9010005





