Rosmarinus officinalis Essential Oil Improves Scopolamine-Induced Neurobehavioral Changes via Restoration of Cholinergic Function and Brain Antioxidant Status in Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oil and Chemical Material
2.2. Gas Chromatograph–Mass Spectrometry (GC-MS) Analysis
2.3. Animals
2.4. Behavioral Analysis
2.4.1. Novel Tank Diving Test (NTT)
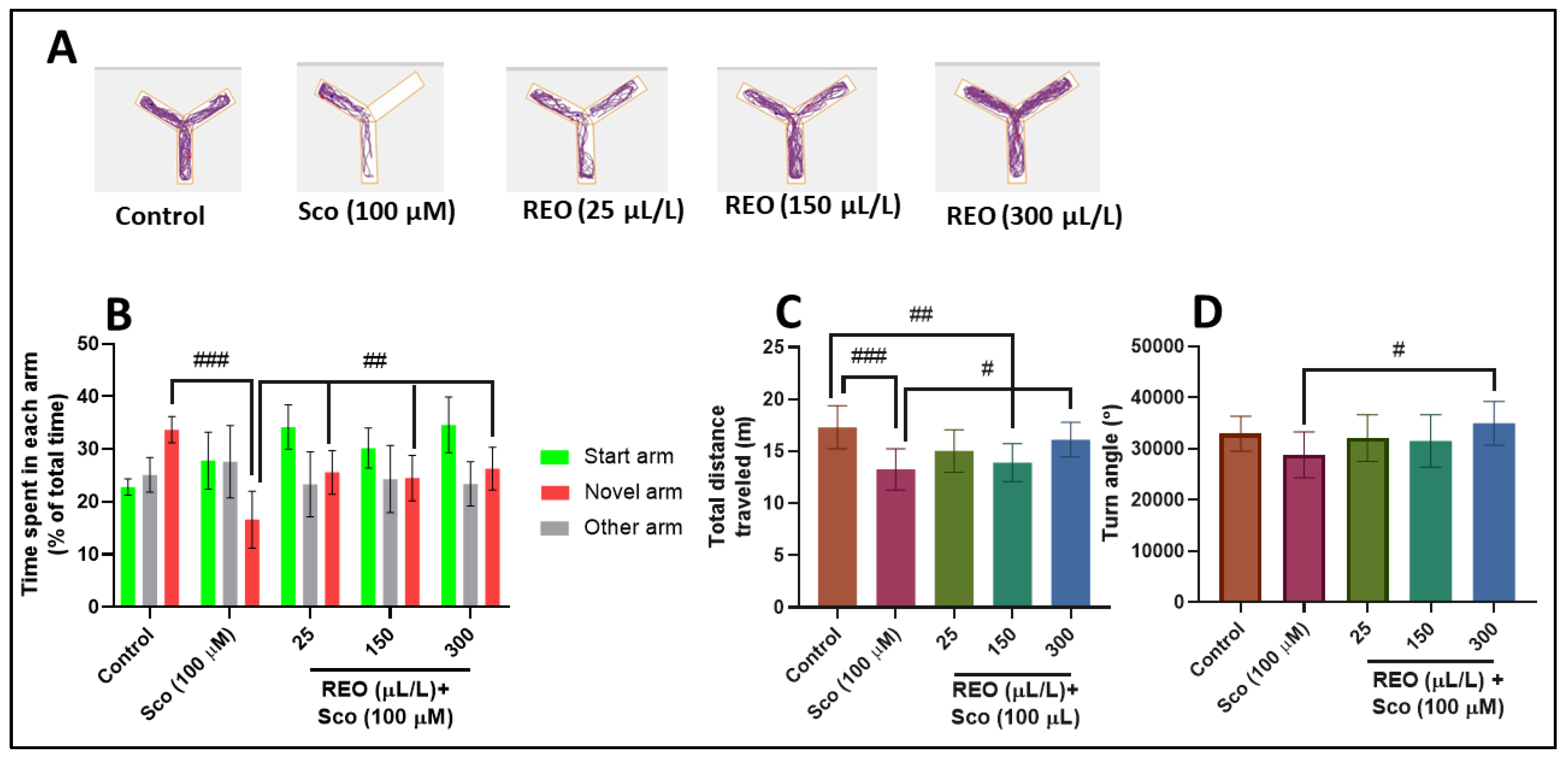
2.4.2. Y-Maze Test
2.5. Biochemical Parameters Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Chemical Composition of the Essential Oil
3.2. Effects on Anxiety-Like Behavior in NTT Test and on Y-Maze Response to Novelty and Spatial Memory
3.3. Effects on AChE Activity
3.4. Effects on SOD, CAT, and GPX Specific Activities
3.5. Effects on MDA Level
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Craig, L.A.; Hong, N.S.; Kopp, J.; McDonald, R.J. Reduced cholinergic status in hippocampus produces spatial memory deficits when combined with kainic acid induced seizure. Hippocampus 2008, 18, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Prim. 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Dunn, J.K.; Clark, C.M.; Farlow, M.; Foster, N.L.; Liao, T.; Gonzales, N.; Lai, E.; Massman, P. Chronic Donepezil Treatment Is Associated with Slowed Cognitive Decline in Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2001, 12, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Bullock, R.; Touchon, J.; Bergman, H.; Gambina, G.; He, Y.; Rapatz, G.; Nagel, J.; Lane, R. Rivastigmine and donepezil treatment in moderate to moderately-severe Alzheimer’s disease over a 2-year period. Curr. Med. Res. Opin. 2005, 21, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Jafarian, S.; Ling, K.-H.; Hassan, Z.; Perimal-Lewis, L.; Sulaiman, M.R.; Perimal, E.K. Effect of zerumbone on scopolamine-induced memory impairment and anxiety-like behaviours in rats. Alzheimer’s Dement. 2019, 5, 637–643. [Google Scholar] [CrossRef]
- Naderali, E.; Nikbakht, F.; Ofogh, S.; Rasoolijazi, H. The role of rosemary extract (40% carnosic acid) in degeneration of hippocampal neurons induced by kainic acid in the rat: The behavioral and histochemical approach. J. Integr. Neurosci. 2018, 17, 31–43. [Google Scholar] [CrossRef]
- Karim, N.; Khan, I.; Abdelhalim, A.; Abdel-Halim, H.; Hanrahan, J.R. Molecular docking and antiamnesic effects of nepitrin isolated from Rosmarinus officinalis on scopolamine-induced memory impairment in mice. Biomed. Pharmacother. 2017, 96, 700–709. [Google Scholar] [CrossRef]
- Song, H.; Xu, L.; Zhang, R.; Cao, Z.; Zhang, H.; Yang, L.; Guo, Z.; Qu, Y.; Yu, J. Rosemary extract improves cognitive deficits in a rats model of repetitive mild traumatic brain injury associated with reduction of astrocytosis and neuronal degeneration in hippocampus. Neurosci. Lett. 2016, 622, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R. Zebrafish and relational memory: Could a simple fish be useful for the analysis of biological mechanisms of complex vertebrate learning? Behav. Process. 2017, 141, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Meshalkina, D.A.; Kizlyk, M.N.; Kysil, E.V.; Collier, A.D.; Echevarria, D.J.; Abreu, M.S.; Barcellos, L.J.G.; Song, C.; Kalueff, A.V. Understanding zebrafish cognition. Behav. Process. 2017, 141, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Scott-Scheiern, T.; Kempker, L.; Simons, K. Active avoidance conditioning in zebrafish (Danio rerio). Neurobiol. Learn. Mem. 2007, 87, 72–77. [Google Scholar] [CrossRef]
- Aoki, R.; Tsuboi, T.; Okamoto, H. Y-maze avoidance: An automated and rapid associative learning paradigm in zebrafish. Neurosci. Res. 2015, 91, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Braida, D.; Ponzoni, L.; Martucci, R.; Sparatore, F.; Gotti, C.; Sala, M. Role of neuronal nicotinic acetylcholine receptors (nAChRs) on learning and memory in zebrafish. Psychopharmacology 2014, 231, 1975–1985. [Google Scholar] [CrossRef]
- Hamilton, T.J.; Morrill, A.; Lucas, K.; Gallup, J.; Harris, M.; Healey, M.; Pitman, T.; Schalomon, M.; Digweed, S.; Tresguerres, M. Establishing zebrafish as a model to study the anxiolytic effects of scopolamine. Sci. Rep. 2017, 7, 15081. [Google Scholar] [CrossRef] [Green Version]
- Nematolahi, P.; Mehrabani, M.; Karami-Mohajeri, S.; Dabaghzadeh, F. Effects of Rosmarinus officinalis L. on memory performance, anxiety, depression, and sleep quality in university students: A randomized clinical trial. Complement. Ther. Clin. Pract. 2018, 30, 24–28. [Google Scholar] [CrossRef]
- Miraj, S. An evidence-based review on herbal remedies of Rosmarinus officinalis. Der Pharm. Lett. 2016, 8, 426–436. [Google Scholar]
- Napoli, E.M.; Curcuruto, G.; Ruberto, G. Screening of the essential oil composition of wild Sicilian rosemary. Biochem. Syst. Ecol. 2010, 38, 659–670. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.M.; Potortí, A.G.; Fede, M.R.; Virga, G.; Leone, R.; D’Anna, E.; et al. Agronomical evaluation of Sicilian biotypes of Lavandula stoechas L. spp. stoechas and analysis of the essential oils. J. Essent. Oil Res. 2015, 27, 115–124. [Google Scholar] [CrossRef]
- Epa, N.; Mass, N.I.H.; Library, S.; Ei, V.Z.; Sparkman, J.A. NIST Standard Reference Database 1A. In Natl. Inst. Stand. Technol. NIST; 2004. Available online: https://www.nist.gov/system/files/documents/srd/NIST1aVer22Man.pdf (accessed on 9 December 2019).
- Sparkman, O.D. Review. J. Am. Soc. Mass Spectrom. 2007, 18, 803–806. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, A.C.; Junior, G.B.; Zago, D.C.; Zeppenfeld, C.C.; da Silva, D.T.; Heinzmann, B.M.; Baldisserotto, B.; da Cunha, M.A. Anesthesia and anesthetic action mechanism of essential oils of Aloysia triphylla and Cymbopogon flexuosus in silver catfish (Rhamdia quelen). Vet. Anaesth. Analg. 2017, 44, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cachat, J.M.; Canavello, P.R.; Elkhayat, S.I.; Bartels, B.K.; Hart, P.C.; Elegante, M.F.; Beeson, E.C.; Laffoon, A.L.; Haymore, W.A.M.; Tien, D.H.; et al. Video-aided analysis of zebrafish locomotion and anxiety-related behavioral responses. Neuromethods 2011, 51, 1–14. [Google Scholar]
- Cognato, G.d.P.; Bortolotto, J.W.; Blazina, A.R.; Christoff, R.R.; Lara, D.R.; Vianna, M.R.; Bonan, C.D. Y-Maze memory task in zebrafish (Danio rerio): The role of glutamatergic and cholinergic systems on the acquisition and consolidation periods. Neurobiol. Learn. Mem. 2012, 98, 321–328. [Google Scholar] [CrossRef]
- Zanandrea, R.; Abreu, M.S.; Piato, A.; Barcellos, L.J.G.; Giacomini, A.C.V.V. Lithium prevents scopolamine-induced memory impairment in zebrafish. Neurosci. Lett. 2018, 664, 34–37. [Google Scholar] [CrossRef]
- Batista, F.L.A.; Lima, L.M.G.; Abrante, I.A.; de Araújo, J.I.F.; Batista, F.L.A.; Abrante, I.A.; Magalhães, E.A.; de Lima, D.R.; Lima, M.d.C.L.; do Prado, B.S.; et al. Antinociceptive activity of ethanolic extract of Azadirachta indica A. Juss (Neem, Meliaceae) fruit through opioid, glutamatergic and acid-sensitive ion pathways in adult zebrafish (Danio rerio). Biomed. Pharmacother. 2018, 108, 408–416. [Google Scholar] [CrossRef]
- Dumitru, G.; El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Boiangiu, R.S.; Todirascu-Ciornea, E.; Hritcu, L.; Singab, A.N.B. Agathisflavone isolated from Schinus polygamus (Cav.) Cabrera leaves prevents scopolamine-induced memory impairment and brain oxidative stress in zebrafish (Danio rerio). Phytomedicine 2019, 58, 152889. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef]
- Elyemni, M.; Louaste, B.; Nechad, I.; Elkamli, T.; Bouia, A.; Taleb, M.; Chaouch, M.; Eloutassi, N. Extraction of Essential Oils of Rosmarinus officinalis L. by Two Different Methods: Hydrodistillation and Microwave Assisted Hydrodistillation. Sci. World J. 2019, 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozarowski, M.; Mikolajczak, P.L.; Bogacz, A.; Gryszczynska, A.; Kujawska, M.; Jodynis-Liebert, J.; Piasecka, A.; Napieczynska, H.; Szulc, M.; Kujawski, R.; et al. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia 2013, 91, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Noori Ahmad Abadi, M.; Mortazavi, M.; Kalani, N.; Marzouni, H.Z.; Kooti, W.; Ali-Akbari, S. Effect of Hydroalcoholic Extract of Rosmarinus officinalis L. Leaf on Anxiety in Mice. J. Evid. Based Complement. Altern. Med. 2016, 21, NP85–NP90. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, A.; Karim, N.; Chebib, M.; Aburjai, T.; Khan, I.; Johnston, G.A.R.; Hanrahan, J.R. Antidepressant, anxiolytic and antinociceptive activities of constituents from Rosmarinus officinalis. J. Pharm. Pharm. Sci. 2015, 18, 448–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.M.; Mo, M.S.; Xu, P.Y. Progress in mechanisms of acetylcholinesterase inhibitors and memantine for the treatment of Alzheimer’s disease. Neuroimmunol. Neuroinflamm. 2015, 2, 274–280. [Google Scholar]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Rosemary (Rosmarinus officinalis) essential oil components exhibit anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects in experimental diabetes. Pathophysiology 2017, 24, 297–303. [Google Scholar] [CrossRef]
- Takayama, C.; de-Faria, F.M.; de Almeida, A.C.A.; Dunder, R.J.; Manzo, L.P.; Socca, E.A.R.; Batista, L.M.; Salvador, M.J.; Souza-Brito, A.R.M.; Luiz-Ferreira, A. Chemical composition of Rosmarinus officinalis essential oil and antioxidant action against gastric damage induced by absolute ethanol in the rat. Asian Pac. J. Trop. Biomed. 2016, 6, 677–681. [Google Scholar] [CrossRef] [Green Version]
- El-Hadary, A.E.; Elsanhoty, R.M.; Ramadan, M.F. In vivo protective effect of Rosmarinus officinalis oil against carbon tetrachloride (CCl4)-induced hepatotoxicity in rats. PharmaNutrition 2019, 9, 100151. [Google Scholar] [CrossRef]
- Pérez-Fons, L.; GarzÓn, M.T.; Micol, V. Relationship between the Antioxidant Capacity and Effect of Rosemary (Rosmarinus officinalis L.) Polyphenols on Membrane Phospholipid Order. J. Agric. Food Chem. 2010, 58, 161–171. [Google Scholar] [CrossRef]





| No. | KI b | Compound | % |
|---|---|---|---|
| Monoterpene hydrocarbons | 40.14 | ||
| 1 | 920 | Tricyclene | 0.32 |
| 2 | 925 | α-Thujene | 0.09 |
| 3 | 934 | α-Pinene c | 19.89 |
| 4 | 949 | Camphene | 8.67 |
| 5 | 953 | Thuja-2.4(10)-diene | 0.33 |
| 6 | 972 | Sabinene | 0.02 |
| 7 | 975 | β-Pinene c | 1.56 |
| 10 | 988 | β-Myrcene | 3.97 |
| 12 | 1001 | α-Phellandrene | 0.63 |
| 13 | 1008 | α-Terpinene | 0.15 |
| 14 | 1015 | p-Cymene | 0.68 |
| 15 | 1024 | Limonene c | 2.16 |
| 17 | 1037 | cis-β-Ocimene | 0.16 |
| 18 | 1047 | trans-β-Ocimene | 0.04 |
| 19 | 1058 | γ-Terpinene | 0.74 |
| 22 | 1087 | Terpinolene | 0.75 |
| Oxygenated monoterpenes | 26.44 | ||
| 16 | 1033 | Eucalyptol c | 26.02 |
| 20 | 1070 | cis-Sabinene hydrate | 0.01 |
| 21 | 1075 | cis-Linalool oxide | 0.01 |
| 23 | 1098 | Linalool | 1.10 |
| 24 | 1109 | endo-Fenchol | 0.02 |
| 25 | 1115 | exo-Fenchol | 0.06 |
| 26 | 1125 | α-Campholenal | 0.14 |
| 27 | 1148 | Camphor c | 16.71 |
| 28 | 1151 | Camphene hydrate | 0.04 |
| 29 | 1155 | Menthone | 0.03 |
| 30 | 1158 | Isopulegol | 0.03 |
| 31 | 1162 | trans-Pinocamphone | 0.07 |
| 32 | 1164 | Pinocarvone | 0.07 |
| 33 | 1168 | Borneol | 2.50 |
| 34 | 1175 | cis-Pinocamphone | 0.17 |
| 35 | 1178 | Terpinen-4-ol | 0.78 |
| 36 | 1186 | p-Cymen-8-ol | 0.08 |
| 37 | 1191 | a-Terpineol | 1.38 |
| 38 | 1198 | Myrtenol | 0.10 |
| 39 | 1204 | trans-Dihydro Carvone | 0.06 |
| 40 | 1210 | Verbenone | 1.18 |
| 41 | 1221 | trans-Carveol | 0.01 |
| 42 | 1231 | Linalyl formate | 0.06 |
| 43 | 1242 | cis-Dihydro Carvone | 0.03 |
| 44 | 1244 | Neral | 0.01 |
| 45 | 1248 | Carvone | 0.03 |
| 46 | 1258 | Linalyl acetate | 0.35 |
| 47 | 1275 | Geranial | 0.01 |
| 48 | 1289 | Bornyl acetate | 1.10 |
| 49 | 1299 | Thymol | 0.01 |
| 50 | 1305 | Carvacrol | 0.03 |
| 51 | 1327 | Piperitenone | 0.02 |
| 52 | 1348 | Eugenol | 0.01 |
| 53 | 1361 | Neryl acetate | 0.01 |
| 54 | 1366 | Linalyl isobutanoate | 0.02 |
| 55 | 1376 | α-ylangene | 0.11 |
| 56 | 1380 | α-Copaene | 0.06 |
| 57 | 1384 | Geranyl acetate | 0.03 |
| 58 | 1403 | Methyl eugenol | 0.02 |
| Sesquiterpenes | 4.74 | ||
| 59 | 1410 | α-Caryophyllene | 0.01 |
| 60 | 1418 | α-cis-Bergamotene | 0.03 |
| 61 | 1425 | β-Caryophyllene c | 3.11 |
| 62 | 1433 | β-Ylangene | 0.02 |
| 63 | 1444 | β-Bergamotene | 0.01 |
| 64 | 1447 | β-Copaene | 0.02 |
| 65 | 1454 | Aromadendrene | 0.04 |
| 66 | 1459 | α-Humulene | 0.88 |
| 67 | 1480 | γ-Muurolene | 0.07 |
| 68 | 1484 | α-Curcumene | 0.09 |
| 69 | 1491 | β-Selinene | 0.02 |
| 70 | 1497 | γ-Amorphene | 0.06 |
| 71 | 1508 | α-Muurolene | 0.02 |
| 72 | 1510 | β-Bisabolene | 0.05 |
| 73 | 1518 | γ-Cadinene | 0.05 |
| 74 | 1527 | δ-Cadinene | 0.12 |
| 75 | 1543 | α-Cadinene | 0.01 |
| 76 | 1548 | α-Calacorene | 0.04 |
| 77 | 1589 | Caryophyllene oxide | 0.10 |
| Others | 2.16 | ||
| 8 | 977 | Octen-3-ol | 0.37 |
| 9 | 982 | 3-Octanone | 1.56 |
| 11 | 993 | 3-Octanol | 0.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capatina, L.; Boiangiu, R.S.; Dumitru, G.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Todirascu-Ciornea, E. Rosmarinus officinalis Essential Oil Improves Scopolamine-Induced Neurobehavioral Changes via Restoration of Cholinergic Function and Brain Antioxidant Status in Zebrafish (Danio rerio). Antioxidants 2020, 9, 62. https://doi.org/10.3390/antiox9010062
Capatina L, Boiangiu RS, Dumitru G, Napoli EM, Ruberto G, Hritcu L, Todirascu-Ciornea E. Rosmarinus officinalis Essential Oil Improves Scopolamine-Induced Neurobehavioral Changes via Restoration of Cholinergic Function and Brain Antioxidant Status in Zebrafish (Danio rerio). Antioxidants. 2020; 9(1):62. https://doi.org/10.3390/antiox9010062
Chicago/Turabian StyleCapatina, Luminita, Razvan Stefan Boiangiu, Gabriela Dumitru, Edoardo Marco Napoli, Giuseppe Ruberto, Lucian Hritcu, and Elena Todirascu-Ciornea. 2020. "Rosmarinus officinalis Essential Oil Improves Scopolamine-Induced Neurobehavioral Changes via Restoration of Cholinergic Function and Brain Antioxidant Status in Zebrafish (Danio rerio)" Antioxidants 9, no. 1: 62. https://doi.org/10.3390/antiox9010062
APA StyleCapatina, L., Boiangiu, R. S., Dumitru, G., Napoli, E. M., Ruberto, G., Hritcu, L., & Todirascu-Ciornea, E. (2020). Rosmarinus officinalis Essential Oil Improves Scopolamine-Induced Neurobehavioral Changes via Restoration of Cholinergic Function and Brain Antioxidant Status in Zebrafish (Danio rerio). Antioxidants, 9(1), 62. https://doi.org/10.3390/antiox9010062









