Metabolomics Elucidates Dose-Dependent Molecular Beneficial Effects of Hesperidin Supplementation in Rats Fed an Obesogenic Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hesperidin Source
2.2. Animals, Diets and Treatments
2.3. Blood Pressure Measurement
2.4. Body Composition and Adiposity Index
2.5. Biochemical Analyses
2.6. 1H nuclear Magnetic Resonance Spectroscopy-Based Metabolic Profiling
2.7. Univariate Statistical Analysis
2.8. Multivariate Statistical Analysis
3. Results
3.1. A CAF Diet Induced the MetS
3.2. Hesperidin Supplementation had no Effect on Body Composition
3.3. Hesperidin Supplementation Improved the Lipid Profile in a Dose-Dependent Manner
3.4. Hesperidin Supplementation Improved Response to Insulin in CAF-Fed Rats
3.5. The Highest dose of Hesperidin Improved SBP Following a CAF Diet
3.6. Dose-Dependent Improvement in Inflammation and Markers of Arterial Stiffness after Hesperidin Supplementation in CAF-Fed Animals
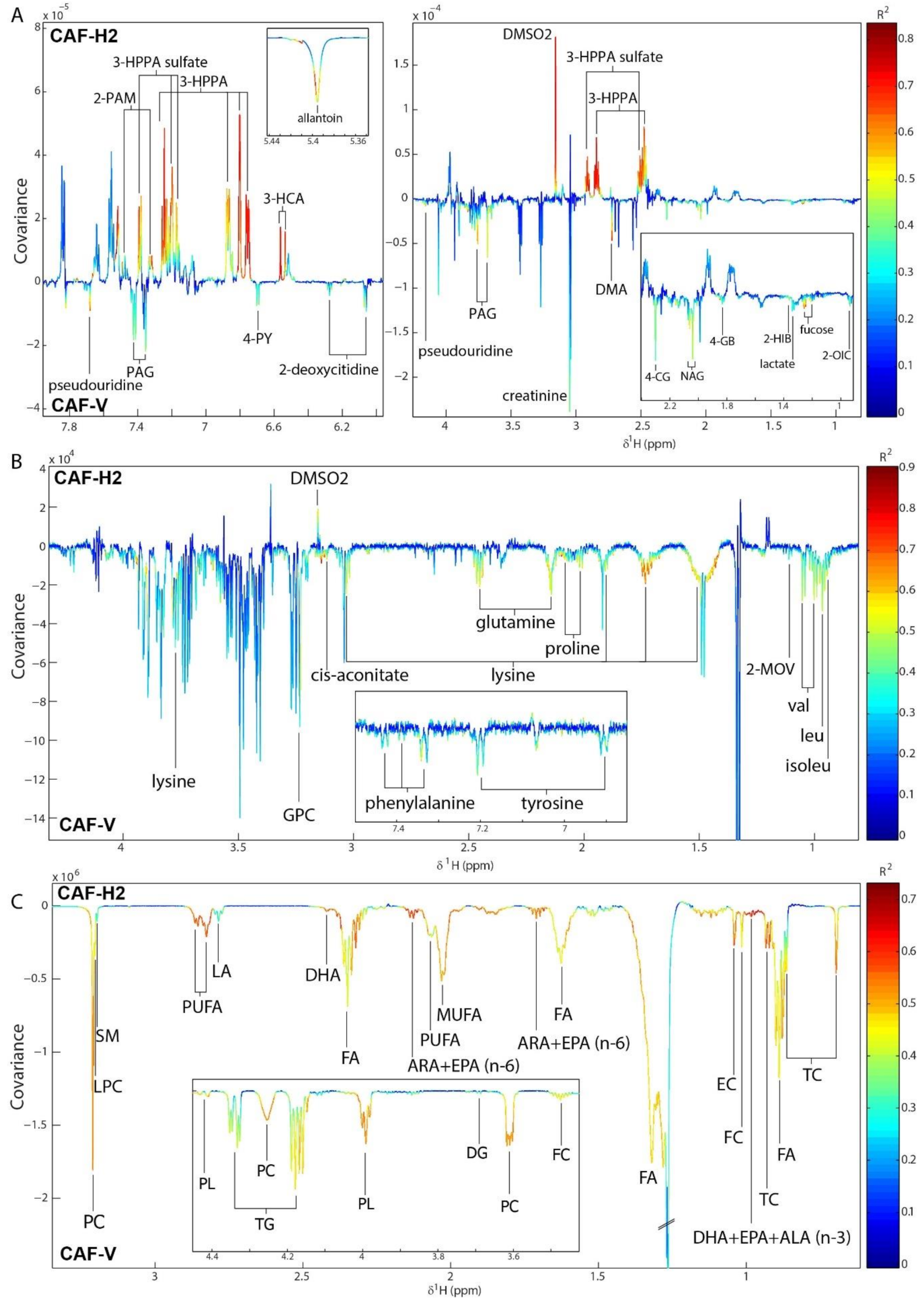
3.7. Hesperidin Supplementation Improved the Metabolic Profiles of CAF-Fed Rats in a Dose-Dependent Manner
3.8. Integration between Metabolites and the Gut Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zafar, U.; Khaliq, S.; Ahmad, H.U.; Manzoor, S.; Lone, K.P. Metabolic syndrome: an update on diagnostic criteria, pathogenesis, and genetic links. Hormones 2018, 17, 299–313. [Google Scholar] [CrossRef]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- LeBlanc, E.S.; Patnode, C.D.; Webber, E.M.; Redmond, N.; Rushkin, M.; O’Connor, E.A. Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 320, 1172–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazidi, M.; Pennathur, S.; Afshinnia, F. Link of dietary patterns with metabolic syndrome: analysis of the National Health and Nutrition Examination Survey. Nutr. Diabetes 2017, 7, e255. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxid. Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef]
- Testai, L.; Calderone, V. Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [Green Version]
- Assini, J.M.; Mulvihill, E.E.; Huff, M.W. Citrus flavonoids and lipid metabolism. Curr. Opin. Lipidol. 2013, 24, 34–40. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus Polyphenol Hesperidin Stimulates Production of Nitric Oxide in Endothelial Cells while Improving Endothelial Function and Reducing Inflammatory Markers in Patients with Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Franc, J.; Scalbert, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers 1–3. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef]
- Perche, O.; Vergnaud-Gauduchon, J.; Morand, C.; Dubray, C.; Mazur, A.; Vasson, M.-P. Orange juice and its major polyphenol hesperidin consumption do not induce immunomodulation in healthy well-nourished humans. Clin. Nutr. 2014, 33, 130–135. [Google Scholar] [CrossRef]
- Salden, B.N.; Troost, F.J.; de Groot, E.; Stevens, Y.R.; Garcés-Rimón, M.; Possemiers, S.; Winkens, B.; Masclee, A.A. Randomized clinical trial on the efficacy of hesperidin 2S on validated cardiovascular biomarkers in healthy overweight individuals. Am. J. Clin. Nutr. 2016, 104, 1523–1533. [Google Scholar] [CrossRef] [Green Version]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: the missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef] [Green Version]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Dore, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef] [Green Version]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Moco, S.; Ross, A.B. Can We Use Metabolomics to Understand Changes to Gut Microbiota Populations and Function? A Nutritional Perspective. In Metabonomics and Gut Microbiota in Nutrition and Disease; Springer: Berlin/Heidelberg, Germany, 2014; pp. 83–108. [Google Scholar]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 2011, 19, 1109–1117. [Google Scholar] [CrossRef]
- Guirro, M.; Costa, A.; Gual-Grau, A.; Mayneris-Perxachs, J.; Torrell, H.; Herrero, P.; Canela, N.; Arola, L. Multi-omics approach to elucidate the gut microbiota activity: Metaproteomics and metagenomics connection. Electrophoresis 2018, 39, 1692–1701. [Google Scholar] [CrossRef]
- Yáñez, J.A.; Andrews, P.K.; Davies, N.M. Methods of analysis and separation of chiral flavonoids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 848, 159–181. [Google Scholar] [CrossRef]
- Aturki, Z.; Brandi, V.; Sinibaldi, M. Separation of flavanone-7-O-glycoside diastereomers and analysis in citrus juices by multidimensional liquid chromatography coupled with mass spectrometry. J. Agric. Food Chem. 2004, 52, 5303–5308. [Google Scholar] [CrossRef]
- Brand, W.; Shao, J.; Hoek-Van Den Hil, E.F.; Van Elk, K.N.; Spenkelink, B.; De Haan, L.H.J.; Rein, M.J.; Dionisi, F.; Williamson, G.; Van Bladeren, P.J.; et al. Stereoselective conjugation, transport and bioactivity of S- and R-hesperetin enantiomers in vitro. J. Agric. Food Chem. 2010, 58, 6119–6125. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Homayouni, F.; Haidari, F.; Hedayati, M.; Zakerkish, M.; Ahmadi, K. Blood pressure lowering and anti-inflammatory effects of hesperidin in type 2 diabetes; a randomized double-blind controlled clinical trial. Phyther. Res. 2018, 32, 1073–1079. [Google Scholar] [CrossRef]
- Leuzzi, U.; Caristi, C.; Panzera, V.; Licandro, G. Flavonoids in Pigmented Orange Juice and Second-Pressure Extracts. J. Agric. Food Chem. 2000, 48, 5501–5506. [Google Scholar] [CrossRef]
- Mosqueda-Solís, A.; Sánchez, J.; Reynés, B.; Palou, M.; Portillo, M.P.; Palou, A.; Picó, C. Hesperidin and capsaicin, but not the combination, prevent hepatic steatosis and other metabolic syndrome-related alterations in western diet-fed rats. Sci. Rep. 2018, 8, 15100. [Google Scholar] [CrossRef]
- Sun, Y.-Z.; Chen, J.-F.; Shen, L.-M.; Zhou, J.; Wang, C.-F. Anti-atherosclerotic effect of hesperidin in LDLr-/- mice and its possible mechanism. Eur. J. Pharmacol. 2017, 815, 109–117. [Google Scholar] [CrossRef]
- Buñag, R.D. Validation in awake rats of a tail-cuff method for measuring systolic pressure. J. Appl. Physiol. 1973, 34, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Veselkov, K.A.; Lindon, J.C.; Ebbels, T.M.D.; Crockford, D.; Volynkin, V.V.; Holmes, E.; Davies, D.B.; Nicholson, J.K. Recursive segment-wise peak alignment of biological (1)h NMR spectra for improved metabolic biomarker recovery. Anal. Chem. 2009, 81, 56–66. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickard, J.M.; Chervonsky, A. V Intestinal fucose as a mediator of host-microbe symbiosis. J. Immunol. 2015, 194, 5588–5593. [Google Scholar] [CrossRef] [Green Version]
- Connelly, M.A.; Otvos, J.D.; Shalaurova, I.; Playford, M.P.; Mehta, N.N. GlycA, a novel biomarker of systemic inflammation and cardiovascular disease risk. J. Transl. Med. 2017, 15, 219. [Google Scholar] [CrossRef]
- Topp, H.; Fusch, G.; Schöch, G.; Fusch, C. Noninvasive markers of oxidative DNA stress, RNA degradation and protein degradation are differentially correlated with resting metabolic rate and energy intake in children and adolescents. Pediatr. Res. 2008, 64, 246–250. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes. Res. Clin. Pract. 2013, 7, e330–e341. [Google Scholar] [CrossRef]
- Emanuela, F.; Grazia, M.; Marco, D.R.; Maria Paola, L.; Giorgio, F.; Marco, B. Inflammation as a Link between Obesity and Metabolic Syndrome. J. Nutr. Metab. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Bakris, G.L. Non-esterified fatty acids and blood pressure elevation: a mechanism for hypertension in subjects with obesity/insulin resistance? J. Hum. Hypertens. 2007, 21, 12–19. [Google Scholar] [CrossRef]
- Soleimani, M. Insulin resistance and hypertension: New insights. Kidney Int. 2015, 87, 497–499. [Google Scholar] [CrossRef] [Green Version]
- Kolovou, G.D.; Anagnostopoulou, K.K.; Cokkinos, D. V Pathophysiology of dyslipidaemia in the metabolic syndrome. Postgrad. Med. J. 2005, 81, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Ikemura, M.; Sasaki, Y.; Giddings, J.C.; Yamamoto, J. Preventive effects of hesperidin, glucosyl hesperidin and naringin on hypertension and cerebral thrombosis in stroke-prone spontaneously hypertensive Rats. Phyther. Res. 2012, 26, 1272–1277. [Google Scholar] [CrossRef]
- Yamamoto, M.; Jokura, H.; Suzuki, A.; Hase, T.; Shimotoyodome, A. Effects of continuous ingestion of hesperidin and glucosyl hesperidin on vascular gene expression in spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2013, 59, 470–473. [Google Scholar] [CrossRef] [Green Version]
- Muniyappa, R.; Sowers, J.R. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Aroor, A.R.; DeMarco, V.G.; Martinez-Lemus, L.A.; Meininger, G.A.; Sowers, J.R. Vascular stiffness in insulin resistance and obesity. Front. Physiol. 2015, 6, 231. [Google Scholar] [CrossRef]
- Foote, C.A.; Castorena-Gonzalez, J.A.; Ramirez-Perez, F.I.; Jia, G.; Hill, M.A.; Reyes-Aldasoro, C.C.; Sowers, J.R.; Martinez-Lemus, L.A. Arterial stiffening in western diet-fed mice is associated with increased vascular elastin, transforming growth factor-ß, and plasma neuraminidase. Front. Physiol. 2016, 7, 285. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.J. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can. J. Cardiol. 2006, 22 Suppl B, 72B–80B. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-Y.; Monleon, D.; Verhamme, P.; Staessen, J.A. Branched-Chain Amino Acids as Critical Switches in Health and Disease. Hypertension 2018, 72, 1012–1022. [Google Scholar] [CrossRef]
- Harlan, S.M.; Guo, D.-F.; Morgan, D.A.; Fernandes-Santos, C.; Rahmouni, K. Hypothalamic mTORC1 Signaling Controls Sympathetic Nerve Activity and Arterial Pressure and Mediates Leptin Effects. Cell Metab. 2013, 17, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Menni, C.; Fauman, E.; Erte, I.; Perry, J.R.B.; Kastenmuller, G.; Shin, S.-Y.; Petersen, A.-K.; Hyde, C.; Psatha, M.; Ward, K.J.; et al. Biomarkers for Type 2 Diabetes and Impaired Fasting Glucose Using a Nontargeted Metabolomics Approach. Diabetes 2013, 62, 4270–4276. [Google Scholar] [CrossRef] [Green Version]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Vallabha, V.S.; Tapal, A.; Sukhdeo, S.V.; Govindaraju, K.; Tiku, P.K. Effect of arginine: Lysine ratio in free amino acid and protein form on l-NAME induced hypertension in hypercholesterolemic Wistar rats. RSC Adv. 2016, 6, 73388–73398. [Google Scholar] [CrossRef]
- Ligthart-Melis, G.C.; van de Poll, M.C.; Boelens, P.G.; Dejong, C.H.; Deutz, N.E.; van Leeuwen, P.A. Glutamine is an important precursor for de novo synthesis of arginine in humans. Am. J. Clin. Nutr. 2008, 87, 1282–1289. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Song, H.; Pan, X.; Xue, H.; Wan, Y.; Wang, T.; Tian, Z.; Hou, E.; Lanza, I.R.; Liu, P.; et al. Urinary Metabolites Associated with Blood Pressure on a Low- or High-Sodium Diet. Theranostics 2018, 8, 1468–1480. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Peng, J.; Lu, C.; Hsin, M.; Mura, M.; Wu, L.; Chu, L.; Zamel, R.; Machuca, T.; Waddell, T.; et al. Metabolomic Heterogeneity of Pulmonary Arterial Hypertension. PLoS ONE 2014, 9, e88727. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Caro, G.; Ludwig, I.A.; Polyviou, T.; Malkova, D.; García, A.; Moreno-Rojas, J.M.; Crozier, A. Identification of Plasma and Urinary Metabolites and Catabolites Derived from Orange Juice (Poly)phenols: Analysis by High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 5724–5735. [Google Scholar] [CrossRef] [Green Version]
- Najmanova, I.; Pourova, J.; Voprsalova, M.; Pilarova, V.; Semecky, V.; Novakova, L.; Mladenka, P. Flavonoid metabolite 3-(3-hydroxyphenyl)propionic acid formed by human microflora decreases arterial blood pressure in rats. Mol. Nutr. Food Res. 2016, 60, 981–991. [Google Scholar] [CrossRef]
- Akira, K.; Masu, S.; Imachi, M.; Mitome, H.; Hashimoto, M.; Hashimoto, T. 1H NMR-based metabonomic analysis of urine from young spontaneously hypertensive rats. J. Pharm. Biomed. Anal. 2008, 46, 550–556. [Google Scholar] [CrossRef]
- Gralka, E.; Luchinat, C.; Tenori, L.; Ernst, B.; Thurnheer, M.; Schultes, B. Metabolomic fingerprint of severe obesity is dynamically affected by bariatric surgery in a procedure-dependent manner. Am. J. Clin. Nutr. 2015, 102, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.G.; Yazdi, F.; Reisin, E. Bariatric Surgery and Hypertension. Am. J. Hypertens. 2018, 31, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulker, İ.; Yildiran, H. The effects of bariatric surgery on gut microbiota in patients with obesity: a review of the literature. Biosci. microbiota, food Heal. 2019, 38, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Slupsky, C.M. Metabolic fingerprint of dimethyl sulfone (DMSO2) in microbial-mammalian co-metabolism. J. Proteome Res. 2014, 13, 5281–5292. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Parameters | STD (n = 24) | CAF (n = 24) | p |
|---|---|---|---|
| Body composition | |||
| Weight (g) | 422.2 ± 5.2 | 495.1 ± 9.4 | <0.001 |
| Weight change (g) | 170.0 ± 3.9 | 245.7 ± 10.3 | <0.001 |
| Fat (%) | 6.3 ± 0.4 | 15.5 ± 1.1 | <0.001 |
| Lean (%) | 88.5 ± 0.5 | 80.0 ± 1.1 | <0.001 |
| Dietary parameters | |||
| Cumulative Energy intake (kcal) | 681.8 ± 8.1 | 1411.3 ± 28.3 | <0.001 |
| Cumulative Protein (g) | 40.9 ± 0.5 | 36.2 ± 0.8 | <0.001 |
| Cumulative Carbohydrates (g) | 97.2 ± 1.2 | 209.6 ± 4.4 | <0.001 |
| Cumulative Fat (g) | 13.6 ± 0.2 | 47.1 ± 1.5 | <0.001 |
| Cumulative Fibre (g) | 7.7 ± 0.1 | 4.4 ± 0.2 | <0.001 |
| Cumulative SFA (g) | 2.0 ± 0.0 | 18.9 ± 0.5 | <0.001 |
| Cumulative MUFA (g) | 2.9 ± 0.0 | 18.1 ± 0.7 | <0.001 |
| Cumulative PUFA (g) | 7.5 ± 0.1 | 10.2 ± 0.6 | <0.001 |
| Cholesterol (g) | - a | 0.22 ± 0.05 | <0.001 |
| Biochemical parameters | |||
| TC (mmol/L) | 22.8 ± 0.6 | 25.4 ± 0.9 | 0.013 |
| TG (mmol/L) | 1.16 ± 0.11 | 3.13 ± 0.20 | <0.001 |
| FFA(mmol/L) | 0.61 ± 0.02 | 0.82 ± 0.04 | <0.001 |
| Glucose (mmol/L) | 7.75 ± 0.17 | 9.10 ± 0.20 | <0.001 |
| SBP (mmHg) | 128.7 ± 1.1 | 143.6 ± 1.9 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guirro, M.; Gual-Grau, A.; Gibert-Ramos, A.; Alcaide-Hidalgo, J.M.; Canela, N.; Arola, L.; Mayneris-Perxachs, J. Metabolomics Elucidates Dose-Dependent Molecular Beneficial Effects of Hesperidin Supplementation in Rats Fed an Obesogenic Diet. Antioxidants 2020, 9, 79. https://doi.org/10.3390/antiox9010079
Guirro M, Gual-Grau A, Gibert-Ramos A, Alcaide-Hidalgo JM, Canela N, Arola L, Mayneris-Perxachs J. Metabolomics Elucidates Dose-Dependent Molecular Beneficial Effects of Hesperidin Supplementation in Rats Fed an Obesogenic Diet. Antioxidants. 2020; 9(1):79. https://doi.org/10.3390/antiox9010079
Chicago/Turabian StyleGuirro, Maria, Andreu Gual-Grau, Albert Gibert-Ramos, Juan Maria Alcaide-Hidalgo, Núria Canela, Lluís Arola, and Jordi Mayneris-Perxachs. 2020. "Metabolomics Elucidates Dose-Dependent Molecular Beneficial Effects of Hesperidin Supplementation in Rats Fed an Obesogenic Diet" Antioxidants 9, no. 1: 79. https://doi.org/10.3390/antiox9010079





