Antidiabetic Activity of Gold Nanoparticles Synthesized Using Wedelolactone in RIN-5F Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Cell Line
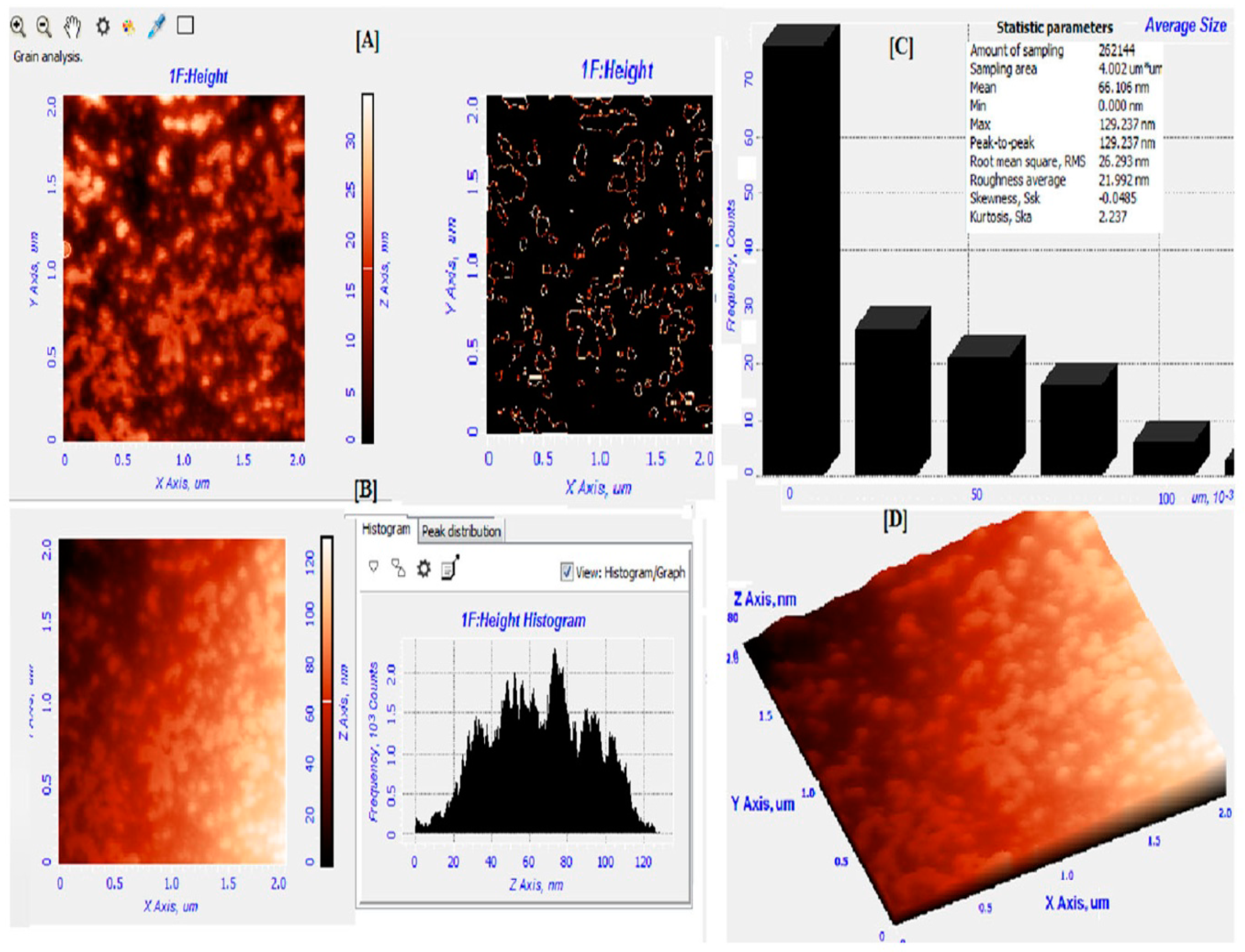
2.2. Green Synthesis and Characterization of WDL-AuNPs
2.3. Viability Assay
2.4. Glucose Stimulated Insulin Secretion
2.5. Biochemical Estimations
2.6. Staining for Apoptosis
2.7. Protein Analysis by Western Blot
2.8. RNA Isolation and Reverse Transcription PCR
2.9. Animals
2.9.1. DEHP induction
2.9.2. Experimental protocol
2.9.3. Biochemical Assay
2.10. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.P.; Cruz, M.A.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Mitragotri, S.; Tong, S. Multifunctional nanoparticles for drug delivery and molecular imaging. Annu. Rev. Biomed. Eng. 2013, 15, 253–282. [Google Scholar] [CrossRef] [PubMed]
- Alegria, E.; Ribeiro, A.; Mendes, M.; Ferraria, A.; do Rego, A.; Pombeiro, A. Effect of phenolic compounds on the synthesis of gold nanoparticles and its catalytic activity in the reduction of nitro compounds. Nanomaterials 2018, 8, 320. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Rajeshkumar, S.; Kumar, S.V. Phyto-assisted synthesis, characterization and applications of gold nanoparticles—A review. Biochem. Biophys. Rep. 2017, 11, 46–57. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Erythropel, H.C.; Maric, M.; Nicell, J.A.; Leask, R.L.; Yargeau, V. Leaching of the plasticizer di (2-ethylhexyl) phthalate (DEHP) from plastic containers and the question of human exposure. Appl. Microbiol. Biotechnol. 2014, 98, 9967–9981. [Google Scholar] [CrossRef]
- Sun, X.; Lin, Y.; Huang, Q.; Shi, J.; Qiu, L.; Kang, M.; Chen, Y.; Fang, C.; Ye, T.; Dong, S. Di (2-ethylhexyl) phthalate-induced apoptosis in rat INS-1 cells is dependent on activation of endoplasmic reticulum stress and suppression of antioxidant protection. J. Cell. Mol. Med. 2015, 19, 581–594. [Google Scholar] [CrossRef]
- Manvar, D.; Mishra, M.; Kumar, S.; Pandey, V.N. Identification and evaluation of antihepatitis C virus phytochemicals from Eclipta Alba. J. Ethnopharmacol. 2012, 144, 545–554. [Google Scholar] [CrossRef]
- Shen, P.; Yang, X.; Jiang, J.; Wang, X.; Liang, T.; He, L. Wedelolactone from Eclipta Alba inhibits lipopolysaccharide-enhanced cell proliferation of human renal mesangial cells via NF-κB signaling pathway. Am. J. Transl. Res. 2017, 9, 2132. [Google Scholar]
- Liu, Y.Q.; Han, X.F.; Bo, J.X.; Ma, H.P. Wedelolactone enhances osteoblastogenesis but inhibits osteoclastogenesis through Sema3A/NRP1/PlexinA1 pathway. Front. Pharmacol. 2016, 7, 375. [Google Scholar] [CrossRef]
- Zhao, Y.; Peng, L.; Yang, L.C.; Xu, X.D.; Li, W.J.; Luo, X.M.; Jin, X. Wedelolactone regulates lipid metabolism and improves hepatic steatosis partly by AMPK activation and up-regulation of expression of PPARα/LPL and LDLR. PLoS ONE 2015, 10, e0132720. [Google Scholar] [CrossRef]
- Peng, Y.G.; Zhang, L. Wedelolactone suppresses cell proliferation and migration through AKT and AMPK signaling in melanoma. J. Dermatol. Treat. 2019, 30, 389–395. [Google Scholar] [CrossRef]
- Delgadillo-Silva, L.F.; Tsakmaki, A.; Akhtar, N.; Franklin, Z.J.; Konantz, J.; Bewick, G.A.; Ninov, N. Modelling pancreatic β-cell inflammation in zebrafish identifies the natural product wedelolactone for human islet protection. Dis. Models Mech. 2019, 12, dmm036004. [Google Scholar] [CrossRef]
- Fraga, C.G.; Leibovitz, B.E.; Tappel, A.L. Lipid peroxidation measured as thiobarbituric acid-reactive substances in tissue slices: Characterization and comparison with homogenates and microsomes. Free Radic. Biol. Med. 1988, 4, 155–161. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of Lipid hydroperoxide in low density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Kakkar, P.; Das, B.; Viswanathan, P. A modified spectrophotometric assay of superoxide dismutase. Indian J. Biochem. Biophys. 1984, 21, 130–132. [Google Scholar]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.; Hafeman, D.G.; Hoekstra, W. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Vinayagam, R.; Xu, B. 7,8-Dihydroxycoumarin (daphnetin) protects INS-1 pancreatic β-cells against streptozotocin-induced apoptosis. Phytomedicine 2017, 24, 119–126. [Google Scholar] [CrossRef]
- Morales, M.A.; Jabbay, A.J.; Tenenzi, H.F. Mutation affecting accumulation of glycogen. Fungal Genet. Rep. 1975, 20, 24–25. [Google Scholar] [CrossRef]
- Pan, S.Y.; Zhou, S.F.; Gao, S.H.; Yu, Z.L.; Zhang, S.F.; Tang, M.K.; Sun, J.N.; Ma, D.L.; Han, Y.F.; Fong, W.F. New perspectives on how to discover drugs from herbal medicines: CAM’s outstanding contribution to modern therapeutics. Evid. Based Complementary Altern. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Hwang, S.J.; Jun, S.H.; Park, Y.; Cha, S.H.; Yoon, M.; Cho, S.; Lee, H.J.; Park, Y. Green synthesis of gold nanoparticles using chlorogenic acid and their enhanced performance for inflammation. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1677–1688. [Google Scholar] [CrossRef]
- Gulka, C.P.; Wong, A.C.; Wright, D.W. Spontaneous self-assembly and disassembly of colloidal gold nanoparticles induced by tetrakis (hydroxymethyl) phosphonium chloride. Chem. Commun. 2016, 52, 1266–1269. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.Y.; Zhou, H.; Hwang, S.; Koh, K.; Han, D.W.; Lee, J. Green synthesis of phytochemical-stabilized Au nanoparticles under ambient conditions and their biocompatibility and antioxidative activity. J. Mater. Chem. 2011, 21, 13316–13326. [Google Scholar] [CrossRef]
- Karuppiah, C.; Palanisamy, S.; Chen, S.M.; Emmanuel, R.; Muthupandi, K.; Prakash, P. Green synthesis of gold nanoparticles and its application for the trace level determination of painter’s colic. RSC Adv. 2015, 5, 16284–16291. [Google Scholar] [CrossRef]
- Sindhu, K.; Rajaram, A.; Sreeram, K.; Rajaram, R. Curcumin conjugated gold nanoparticle synthesis and its biocompatibility. RSC Adv. 2014, 4, 1808–1818. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Liu, J.; Liu, Y.; Zhang, J.; Lin, J.; Zhao, Z.; Chen, D. Effect and mechanism of wedelolactone as antioxidant-coumestan on OH-treated mesenchymal stem cells. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Prentki, M.; Nolan, C.J. Islet β cell failure in type 2 diabetes. J. Clin. Investig. 2006, 116, 1802–1812. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, H.Y.; Bae, S.; Lim, Y.H.; Hong, Y.C. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: A panel study. PLoS ONE 2013, 8, e71392. [Google Scholar] [CrossRef]
- She, Y.; Jiang, L.; Zheng, L.; Zuo, H.; Chen, M.; Sun, X.; Li, Q.; Geng, C.; Yang, G.; Jiang, L.; et al. The role of oxidative stress in DNA damage in pancreatic β cells induced by di-(2-ethylhexyl) phthalate. Chem. Biol. Interact. 2017, 265, 8–15. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Rafiq Zahid, K.; Wang, K.; Qian, Y.; Ma, P.; Ding, S.; Yang, X.; Wang, X. Adverse effect of DEHP exposure on the serum insulin level of Balb/c mice. Mol. Cell. Toxicol. 2016, 12, 83–91. [Google Scholar] [CrossRef]
- Kasahara, E.; Sato, E.F.; Miyoshi, M.; Konaka, R.; Hiramoto, K.; Sasaki, J.; Tokuda, M.; Nakano, Y.; Inoue, M. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate. Biochem. J. 2002, 365, 849–856. [Google Scholar] [CrossRef]
- Atale, N.; Gupta, S.; Yadav, U.C.; Rani, V. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. J. Microsc. 2014, 255, 7–19. [Google Scholar] [CrossRef]
- Elmore, S. A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Mickelson Weldingh, N.; Jørgensen-Kaur, L.; Becher, R.; Holme, J.; Bodin, J.; Nygaard, U.; Kocbach Bølling, A. Bisphenol A Is More Potent than Phthalate Metabolites in Reducing Pancreatic β-Cell Function. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Cain, K.; Bratton, S.B.; Cohen, G.M. The Apaf-1 apoptosome: A large caspase-activating complex. Biochimie 2002, 84, 203–214. [Google Scholar] [CrossRef]
- Fu, Y.; Dong, J.; Wang, J.; You, M.; Wei, L.; Fu, H.; Wang, Y.; Chen, J. Developmental Exposure to Di-(2-ethylhexyl) Phthalate Induces Cerebellar Granule Cell Apoptosis via the PI3K/AKT Signaling Pathway. Exp. Neurobiol. 2018, 27, 472. [Google Scholar] [CrossRef]
- Kloting, N.; Hesselbarth, N.; Gericke, M.; Kunath, A.; Biemann, R.; Chakaroun, R.; Kosacka, J.; Kovacs, P.; Kern, M.; Stumvoll, M.; et al. Di-(2-Ethylhexyl)-Phthalate (DEHP) Causes Impaired Adipocyte Function and Alters Serum Metabolites. PloS ONE 2015, 10, e0143190. [Google Scholar] [CrossRef]
- Rajesh, P.; Balasubramanian, K. Gestational exposure to di(2-ethylhexyl) phthalate (DEHP) impairs pancreatic β-cell function in F1 rat offspring. Toxicol. Lett. 2015, 232, 46–57. [Google Scholar] [CrossRef]
- Newsholme, E.A.; Dimitriadis, G. Integration of biochemical and physiologiceffects of insulin on glucose metabolism. Exp. Clin. Endocrinol. Diabetes 2001, 109, S122–S134. [Google Scholar] [CrossRef]
- Gayathri, N.S.; Dhanya, C.R.; Indu, A.R.; Kurup, P.A. Changes in somehormones by low doses of di (2-ethyl hexyl) phthalate (DEHP), a commonly used plasticizer in PVC blood storage bags & medical tubing. Indian J. Med. Res. 2004, 119, 139–144. [Google Scholar]
- Rajesh, P.; Balasubramanian, K. Phthalate exposure in utero causes epigenetic changes and impairs insulin signalling. J. Endocrinol. 2014, 223, 47–66. [Google Scholar] [CrossRef]
- Shahab, U.; Faisal, M.; Alatar, A.A.; Ahmad, S. Impact of wedelolactone in the anti-glycation and anti-diabetic activity in experimental diabetic animals. IUBMB Life 2018, 70, 547–552. [Google Scholar] [CrossRef]
- Mushtaq, M.; Srivastava, S.P.; Seth, P.K. EVect of di-2-ethylhexyl phthalate (DEHP) on glycogen metabolism in rat liver. Toxicology 1980, 16, 153–161. [Google Scholar] [CrossRef]
- Rajesh, P.; Sathish, S.; Srinivasan, C.; Selvaraj, J.; Balasubramanian, K. Phthalate is associated with insulin resistance in adipose tissue of male rat: Role of antioxidant vitamins. J. Cell. Biochem. 2013, 114, 558–569. [Google Scholar] [CrossRef]








| Enzymes | Normal Cells | DEHP (625 µM) | DEHP + WDL-AuNPS (40 µg/mL) | DEHP + WDL-AuNPS (80 µg/mL) | WDL-AuNPS (80 µg/mL) |
|---|---|---|---|---|---|
| TBARS (nmol/mg protein) | 1.50 ± 0.10 a | 5.52 ± 0.44 b | 3.36 ± 0.26 c | 2.10 ± 0.17 d | 1.72 ± 0.15 ad |
| SOD (U∗/mg of protein) | 65.01 ± 4.05 a | 23.8 ± 1.9 b | 45.89 ± 4.45 c | 59.10 ± 5.13 a | 62.01 ± 3.85 a |
| CAT (U∗∗/mg of protein) | 1.5 ± 0.10 a | 0.18 ± 0.01 b | 0.72 ± 0.06 c | 0.98 ± 0.08 d | 1.4 ± 0.12 a |
| GPx (U# /mg of protein) | 4.2 ± 0.35 a | 1.87 ± 0.15 b | 3.12 ± 0.25 c | 3.5 ± 0.28 c | 3.8 ± 0.29 a |
| Group | Glucose (mg/dL) | Insulin (µU/mL) | Glycogen (mg/100g Tissues) |
|---|---|---|---|
| Control | 102.01 ± 9.25 ad | 11.61 ± 1.09 a | 51.94 ± 4.30 a |
| WDL-AuNPs (40 mg/kg bw) | 94.81 ± 8.41 a | 10.98 ± 0.91 a | 53.01 ± 3.51 a |
| DEHP | 190.44 ± 15.65 b | 5.16 ± 0.85 b | 24.34 ± 2.01 b |
| DEHP + WDL-AuNPs (10 mg/kg bw) | 170.63 ± 13.54 c | 6.44 ± 0.45 c | 35.11 ± 2.89 c |
| DEHP + WDL-AuNPs (20 mg/kg bw) | 165.84 ± 12.54 c | 7.09 ± 0.78 cd | 39.18 ± 3.14 d |
| DEHP + WDL-AuNPs (40 mg/kg bw) | 120.91 ± 11.69 d | 8.11 ± 0.69 d | 43.79 ± 4.29 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramachandran, V.; Arokia Vijaya Anand, M.; David, E.; Venkatachalam, K.; Vijayakumar, S.; Sankaran, V.; Balupillai, A.; Sangeetha, C.C.; Gothandam, K.M.; Kotakadi, V.S.; et al. Antidiabetic Activity of Gold Nanoparticles Synthesized Using Wedelolactone in RIN-5F Cell Line. Antioxidants 2020, 9, 8. https://doi.org/10.3390/antiox9010008
Ramachandran V, Arokia Vijaya Anand M, David E, Venkatachalam K, Vijayakumar S, Sankaran V, Balupillai A, Sangeetha CC, Gothandam KM, Kotakadi VS, et al. Antidiabetic Activity of Gold Nanoparticles Synthesized Using Wedelolactone in RIN-5F Cell Line. Antioxidants. 2020; 9(1):8. https://doi.org/10.3390/antiox9010008
Chicago/Turabian StyleRamachandran, Vinayagam, Mariadoss Arokia Vijaya Anand, Ernest David, Karthikkumar Venkatachalam, Shalini Vijayakumar, Vijayalakshmi Sankaran, Agilan Balupillai, Casimeer C. Sangeetha, K. M. Gothandam, Venkata Subbaiah Kotakadi, and et al. 2020. "Antidiabetic Activity of Gold Nanoparticles Synthesized Using Wedelolactone in RIN-5F Cell Line" Antioxidants 9, no. 1: 8. https://doi.org/10.3390/antiox9010008
APA StyleRamachandran, V., Arokia Vijaya Anand, M., David, E., Venkatachalam, K., Vijayakumar, S., Sankaran, V., Balupillai, A., Sangeetha, C. C., Gothandam, K. M., Kotakadi, V. S., Ghidan, A., Al Antary, T., & Xu, B. (2020). Antidiabetic Activity of Gold Nanoparticles Synthesized Using Wedelolactone in RIN-5F Cell Line. Antioxidants, 9(1), 8. https://doi.org/10.3390/antiox9010008










