Positive Association of Ascorbate and Inverse Association of Urate with Cognitive Function in People with Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Parameters of the Study Sample
2.2. Neuropsychological Testing
2.3. Montreal Cognitive Assessment (MoCA)
2.4. Hoehn and Yahr Scale
2.5. Unified Parkinson’s Disease Rating Scale (UPDRS)
2.6. Blood Sample Collection and Processing
2.7. Ascorbate and Urate Measurement by HPLC
2.8. Protein Carbonyl Measurement by ELISA
2.9. Statistical Analyses
3. Results
3.1. Participant Characteristics
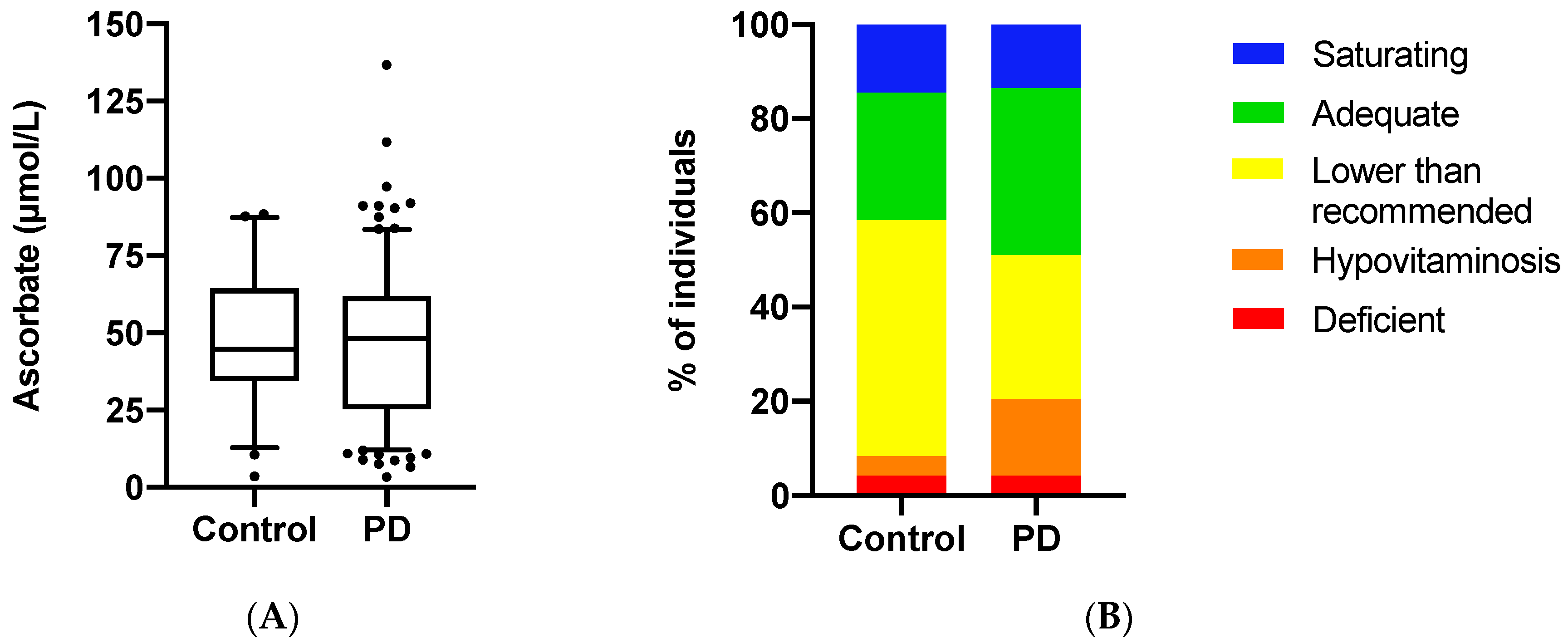
3.2. Ascorbate Status of the Study Population
3.3. Ascorbate Status Relative to Cognitive Function and Clinical Parameters
3.4. Urate Concentrations Relative to Cognitive Function and Clinical Parameters
3.5. Protein Carbonyl Concentrations Relative to Cognitive Function and Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ping, L.; Duong, D.M.; Yin, L.; Gearing, M.; Lah, J.J.; Levey, A.I.; Seyfried, N.T. Global quantitative analysis of the human brain proteome in Alzheimer’s and Parkinson’s Disease. Sci. Data 2018, 5, 180036. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.; Teo, W.-P.; Chandra, S.; Chapman, J. Parkinson’s Disease and the Environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloemd, B.R. The emerging evidence of the Parkinson pandemic. J. Parkinsons Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Erro, R.; Stamelou, M. The motor syndrome of Parkinson’s Disease. In International Review of Neurobiology; Academic Press Inc.: Cambridge, MA, USA, 2017; pp. 25–32. [Google Scholar]
- Williams-Gray, C.H.; Worth, P.F. Parkinson’s Disease, Medicine (United Kingdom); Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 44, pp. 542–546. [Google Scholar]
- Chen-Plotkin, A.S.; Hu, W.T.; Siderowf, A.; Weintraub, D.; Goldmann Gross, R.; Hurtig, H.I.; Xie, S.X.; Arnold, S.E.; Grossman, M.; Clark, C.M.; et al. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann. Neurol. 2011, 69, 655–663. [Google Scholar] [CrossRef]
- Wen, M.; Zhou, B.; Chen, Y.H.; Ma, Z.L.; Gou, Y.; Zhang, C.L.; Yu, W.; Jiao, L. Serum uric acid levels in patients with Parkinson’s disease: A meta-analysis. PLoS ONE 2017, 12, e0173731. [Google Scholar] [CrossRef]
- Glantzounis, G.; Tsimoyiannis, E.; Kappas, A.; Galaris, D. Uric Acid and Oxidative Stress. Curr. Pharm. Des. 2005, 11, 4145–4151. [Google Scholar] [CrossRef]
- Schlesinger, I.; Schlesinger, N. Uric acid in Parkinson’s disease. Mov. Disord. 2008, 23, 1653–1657. [Google Scholar] [CrossRef]
- Tana, C.; Ticinesi, A.; Prati, B.; Nouvenne, A.; Meschi, T. Uric acid and cognitive function in older individuals. Nutrients 2018, 10, 975. [Google Scholar] [CrossRef]
- Travica, N.; Ried, K.; Sali, A.; Scholey, A.; Hudson, I.; Pipingas, A. Vitamin c status and cognitive function: A systematic review. Nutrients 2017, 9, 960. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Kocot, J.; Luchowska-Kocot, D.; Kiełczykowska, M.; Musik, I.; Kurzepa, J. Does vitamin C influence neurodegenerative diseases and psychiatric disorders? Nutrients 2017, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Fraga, D.B.; Rodrigues, A.L.S. Preventive and therapeutic potential of ascorbic acid in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.S.; Schumacher-Schuh, A.; Cardoso, A.M.; Bochi, G.V.; Baldissarelli, J.; Kegler, A.; Santana, D.; Chaves, C.M.M.B.S.; Schetinger, M.R.C.; Moresco, R.N.; et al. Iron and oxidative stress in Parkinson’s disease: An observational study of injury biomarkers. PLoS ONE 2016, 11, e0146129. [Google Scholar] [CrossRef] [PubMed]
- Sudha, K.; Rao, A.V.; Rao, S.; Rao, A. Free radical toxicity and antioxidants in Parkinson’s disease. Neurol. India 2003, 51, 60–62. [Google Scholar] [PubMed]
- Ide, K.; Yamada, H.; Umegaki, K.; Mizuno, K.; Kawakami, N.; Hagiwara, Y.; Matsumoto, M.; Yoshida, H.; Kim, K.; Shiosaki, E.; et al. Lymphocyte vitamin C levels as potential biomarker for progression of Parkinson’s disease. Nutrition 2015, 31, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef]
- Wood, K.L.; Myall, D.J.; Livingston, L.; Melzer, T.R.; Pitcher, T.L.; MacAskill, M.R.; Geurtsen, G.J.; Anderson, T.J.; Dalrymple-Alford, J.C. Different PD-MCI criteria and risk of dementia in Parkinson’s disease: 4-year longitudinal study. npj Park Dis. 2016, 2, 1–8. [Google Scholar] [CrossRef]
- Litvan, I.; Goldman, J.G.; Tröster, A.I.; Schmand, B.A.; Weintraub, D.; Petersen, R.C.; Mollenhauer, B.; Adler, C.H.; Marder, K.; Williams-Gray, C.H.; et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 2012, 27, 349–356. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple-Alford, J.C.; MacAskill, M.R.; Nakas, C.T.; Livingston, L.; Graham, C.; Crucian, G.P.; Melzer, T.R.; Kirwan, J.; Keenan, R.; Wells, S.; et al. The MoCA: Well-suited screen for cognitive impairment in Parkinson disease. Neurology 2010, 75, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martin, P.; Chaudhuri, K.R.; Rojo-Abuin, J.M.; Rodriguez-Blazquez, C.; Alvarez-Sanchez, M.; Arakaki, T.; Bergareche-Yarza, A.; Chade, A.; Garretto, N.; Gershanik, O.; et al. Assessing the non-motor symptoms of Parkinson’s disease: MDS-UPDRS and NMS Scale. Eur. J. Neurol. 2015, 22, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.M.; Bayer, S.; Carr, A.C. Appropriate handling, processing and analysis of blood samples is essential to avoid oxidation of vitamin C to dehydroascorbic acid. Antioxidants 2018, 7, 29. [Google Scholar] [CrossRef]
- Carr, A.C.; Pullar, J.M.; Moran, S.; Vissers, M.C.M. Bioavailability of vitamin C from kiwifruit in non-smoking males: Determination of “healthy” and “optimal” intakes. J. Nutr. Sci. 2012, 1, 1–9. [Google Scholar] [CrossRef]
- Buss, H.; Chan, T.P.; Sluis, K.B.; Domigan, N.M.; Winterbourn, C.C. Protein carbonyl measurement by a sensitive ELISA method. Free Radic. Biol. Med. 1997, 23, 361–366. [Google Scholar] [CrossRef]
- Wilson, R.; Willis, J.; Gearry, R.; Skidmore, P.; Fleming, E.; Frampton, C.; Carr, A. Inadequate vitamin C status in prediabetes and type 2 diabetes mellitus: Associations with glycaemic control, obesity, and smoking. Nutrients 2017, 9, 997. [Google Scholar] [CrossRef]
- Férnandez-Calle, P.; Jiménez-Jiménez, F.J.; Molina, J.; Cabrera-Valdivia, F.; Vázquez, A.; Urra, D.G.; Bermejo, F.; Matallana, M.C.; Codoceo, R. Serum levels of ascorbic acid (vitamin C) in patients with Parkinson’s disease. J. Neurol. Sci. 1993, 118, 25–28. [Google Scholar] [CrossRef]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef]
- Carr, A.C.; Rowe, S. Factors affecting vitamin C status and prevalence of deficiency: A global health perspective. Nutrients 2020, 12, 1963. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.F.; Pullar, J.M.; Wilson, R.; Spittlehouse, J.K.; Vissers, M.C.M.; Skidmore, P.M.L.; Willis, J.; Cameron, V.A.; Carr, A.C. Vitamin C status correlates with markers of metabolic and cognitive health in 50-year-olds: Findings of the CHALICE cohort study. Nutrients 2017, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- May, J.M. Vitamin C transport and its role in the central nervous system. Subcell Biochem. 2012, 56, 85–103. [Google Scholar] [PubMed]
- Meredith, M.E.; May, J.M. Regulation of embryonic neurotransmitter and tyrosine hydroxylase protein levels by ascorbic acid. Brain Res. 2013, 1539, 7–14. [Google Scholar] [CrossRef]
- Young, J.I.; Züchner, S.; Wang, G. Regulation of the Epigenome by Vitamin C. Ann. Rev. Nutr. 2015, 35, 545–564. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Kim, S.; Han, M.H.; Lee, S.B. Epigenetic changes in neurodegenerative diseases. Mol. Cells 2016, 39, 783–789. [Google Scholar] [CrossRef]
- Kaut, O.; Kuchelmeister, K.; Moehl, C.; Wüllner, U. 5-methylcytosine and 5-hydroxymethylcytosine in brains of patients with multiple system atrophy and patients with Parkinson’s disease. J. Chem. Neuroanat. 2019, 96, 41–48. [Google Scholar] [CrossRef]
- Xiang, L.; Huang, G.; Shu, W.; Gong, C.; Cao, N.; Chen, R.; Li, J.; Lu, H.; Jiang, G. Role of Chromatin Remodeling Genes and TETs in the Development of Human Midbrain Dopaminergic Neurons. Stem Cell Rev. Rep. 2020. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Canas, J.A.; Dore, G.A.; Beydoun, H.A.; Rostant, O.S.; Fanelli-Kuczmarski, M.T.; Evans, M.K.; Zonderman, A.B. Serum Uric Acid and Its Association with Longitudinal Cognitive Change among Urban Adults. J. Alzheimer’s Dis. 2016, 52, 1415–1430. [Google Scholar] [CrossRef]
- Alam, A.B.; Wu, A.; Power, M.C.; West, N.A.; Alonso, A. Associations of serum uric acid with incident dementia and cognitive decline in the ARIC-NCS cohort. J. Neurol. Sci. 2020, 414, 116866. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Quinn, T.J.; Hewitt, J.; Fan, Y.; Dawson, J. Serum uric acid level and association with cognitive impairment and dementia: Systematic review and meta-analysis. Age (Omaha) 2016, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Bardin, T.; Richette, P. Uric acid and cognitive decline: A double-edge sword? Curr. Opin. Rheumatol. 2018, 30, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Afsar, B.; Elsurer, R.; Covic, A.; Johnson, R.J.; Kanbay, M. Relationship between uric acid and subtle cognitive dysfunction in chronic kidney disease. Am. J. Nephrol. 2011, 34, 49–54. [Google Scholar] [CrossRef]
- Perna, L.; Mons, U.; Schöttker, B.; Brenner, H. Association of cognitive function and serum uric acid: Are cardiovascular diseases a mediator among women? Exp. Gerontol. 2016, 81, 37–41. [Google Scholar] [CrossRef]
- Huang, H.Y.; Appel, L.J.; Choi, M.J.; Gelber, A.C.; Charleston, J.; Norkus, E.P.; Miller, E.R., III. The effects of vitamin C supplementation on serum concentrations of uric acid: Results of a randomized controlled trial. Arthritis Rheum. 2005, 52, 1843–1847. [Google Scholar] [CrossRef]
- Gao, X.; Curhan, G.; Forman, J.P.; Ascherio, A.; Choi, H.K. Vitamin C intake and serum uric acid concentration in men. J. Rheumatol. 2008, 35, 1853–1858. [Google Scholar]
- Berger, L.; Gerson, C.D.; Yü, T.F. The effect of ascorbic acid on uric acid excretion with a commentary on the renal handling of ascorbic acid. Am. J. Med. 1977, 62, 71–76. [Google Scholar] [CrossRef]
- Feigelson, P. The inhibition of xanthine oxidase in vitro by trace amounts of l-ascorbic acid. J. Biol. Chem. 1952, 197, 843–850. [Google Scholar]
- Block, G.; Norkus, E.; Hudes, M.; Mandel, S.; Helzlsouer, K. Which plasma antioxidants are most related to fruit and vegetable consumption? Am. J. Epidemiol. 2001, 154, 1113–1118. [Google Scholar] [CrossRef]
- Weber, D.; Davies, M.J.; Grune, T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biol. 2015, 5, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Perrotte, M.; Le Page, A.; Fournet, M.; Le Sayec, M.; Rassart, É.; Fulop, T.; Ramassamy, C. Blood-based redox-signature and their association to the cognitive scores in MCI and Alzheimer’s disease patients. Free Radic. Biol. Med. 2019, 130, 499–511. [Google Scholar] [CrossRef]
- Cristalli, D.O.; Arnal, N.; Marra, F.A.; De Alaniz, M.J.T.; Marra, C.A. Peripheral markers in neurodegenerative patients and their first-degree relatives. J. Neurol. Sci. 2012, 314, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Watfa, G.; Dragonas, C.; Brosche, T.; Dittrich, R.; Sieber, C.C.; Alecu, C.; Benetos, A.; Nzietchueng, R. Study of telomere length and different markers of oxidative stress in patients with Parkinson’s disease. J. Nutr. Heal. Aging 2011, 15, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Licker, V.; Kövari, E.; Hochstrasser, D.F.; Burkhard, P.R. Proteomics in human Parkinson’s disease research. J. Proteom. 2009, 73, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Rutishauser, I.H.E.; Bates, C.J.; Paul, A.A.; Black, A.E.; Mandal, A.R.; Patnaikt, B.K. Long-term vitamin status and dietary intake of healthy elderly subjects. Br. J. Nutr. 1979, 42, 33–42. [Google Scholar] [CrossRef]
- Wilson, C.W.M. Clinical pharmacological aspects of ascorbic acid. Ann. N. Y. Acad. Sci. 1975, 258, 355–376. [Google Scholar] [CrossRef]



| Parameters | Control Group (n = 48) | PD Group (n = 215) | PD-N Subgroup (n = 95) | PD-MCI Subgroup (n = 84) | PD-D Subgroup (n = 35) |
|---|---|---|---|---|---|
| Age, years 1 | 72 ± 7 | 73 ± 7 | 70 ± 7 | 72 ± 7 | 76 ± 5 |
| Male, n (%) | 29 (60) | 150 (70) | 59 (62) | 62 (74) | 29 (83) |
| Cognitive status, n (%) 2 | |||||
| Normal | 45 (94) | 95 (44) | - | - | - |
| MCI | 2 (4) | 84 (39) | - | - | - |
| Dementia | 1 (2) | 35 (16) | - | - | - |
| MoCA score | 27 ± 3 | 24 ± 5 | 27 ± 2 | 23 ± 3 | 17 ± 5 |
| Hoehn and Yahr scale | - | 2.6 ± 0.6 | 2.4 ± 0.5 | 2.7 ± 0.7 | 3.0 ± 0.7 |
| UPDRS part III score | - | 40 ± 14 | 34 ± 13 | 45 ± 13 | 49 ± 13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spencer, E.S.; Pitcher, T.; Veron, G.; Hannam, T.; MacAskill, M.; Anderson, T.; Dalrymple-Alford, J.; Carr, A.C. Positive Association of Ascorbate and Inverse Association of Urate with Cognitive Function in People with Parkinson’s Disease. Antioxidants 2020, 9, 906. https://doi.org/10.3390/antiox9100906
Spencer ES, Pitcher T, Veron G, Hannam T, MacAskill M, Anderson T, Dalrymple-Alford J, Carr AC. Positive Association of Ascorbate and Inverse Association of Urate with Cognitive Function in People with Parkinson’s Disease. Antioxidants. 2020; 9(10):906. https://doi.org/10.3390/antiox9100906
Chicago/Turabian StyleSpencer, Emma S., Toni Pitcher, Gabriel Veron, Tracey Hannam, Michael MacAskill, Tim Anderson, John Dalrymple-Alford, and Anitra C. Carr. 2020. "Positive Association of Ascorbate and Inverse Association of Urate with Cognitive Function in People with Parkinson’s Disease" Antioxidants 9, no. 10: 906. https://doi.org/10.3390/antiox9100906
APA StyleSpencer, E. S., Pitcher, T., Veron, G., Hannam, T., MacAskill, M., Anderson, T., Dalrymple-Alford, J., & Carr, A. C. (2020). Positive Association of Ascorbate and Inverse Association of Urate with Cognitive Function in People with Parkinson’s Disease. Antioxidants, 9(10), 906. https://doi.org/10.3390/antiox9100906






