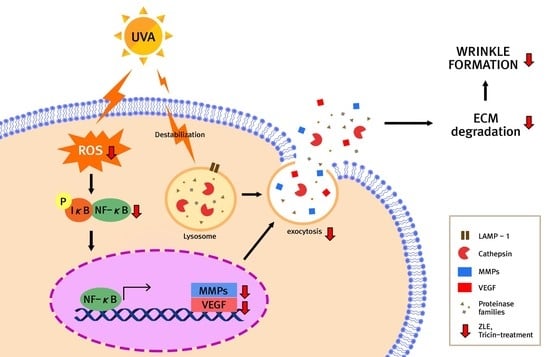
Enzyme-Treated Zizania latifolia Ethanol Extract Protects from UVA Irradiation-Induced Wrinkle Formation via Inhibition of Lysosome Exocytosis and Reactive Oxygen Species Generation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. High Performance Liquid Chromatography (HPLC)
2.3. Cell Viability
2.4. Determination of Intracellular ROS Generation
2.5. Calcium Influx Assay
2.6. Gelatin Zymography
2.7. Immunofluorescence
2.8. Experimental Animals and UVA Irradiation
2.9. Serum Biochemical Analysis
2.10. Analysis of Dorsal Skin Surface
2.11. Histological Analysis
2.12. Enzyme-Linked Immunosorbent Assay
2.13. Western Blot Analysis
2.14. Statistical Analysis
3. Results
3.1. ZLE and Tricin Protect HDFs from UVA-Induced Cell Death and Plasma Membrane Disruption
3.2. ZLE and Tricin Suppress UVA-Induced Lysosomal Exocytosis
3.3. ZLE and Tricin Alleviate UVA-Induced Oxidative Stress
3.4. Body Weight and Serum Analysis in UVA-Irradiated SKH-1 Hairless Mice Treated with ZLE
3.5. ZLE and Tricin Protect the Skin Surface in UVA-Irradiated SKH-1 Hairless Mice
3.6. ZLE and Tricin Suppress Wrinkle Formation in UVA-Irradiated SKH-1 Hairless Mice
3.7. Effects of ZLE and Tricin on Secretion of MMP-2, MMP-3, VEGF and Cathepsin B in the Serum of UVA-Irradiated SKH-1 Hairless Mice
3.8. ZLE and Tricin Protect Skin Damage in UVA-Irradiated SKH-1 Hairless Mice
3.9. ZLE and Tricin Alter Expression of LAMP-1 and Collagen-1 in UVA-Irradiated SKH-1 Hairless Mice
3.10. ZLE and Tricin Alter the Localization of LAMP-1 and Collagen-1 in UVA-Irradiated SKH-1 Hairless Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar]
- Wilson, B.D.; Moon, S.; Armstrong, F. Comprehensive review of ultraviolet radiation and the current status on sunscreens. J. Clin. Aesthet. Dermatol. 2012, 5, 18–23. [Google Scholar]
- Karran, P.; Brem, R. Protein oxidation, UVA and human DNA repair. DNA Repair 2016, 44, 178–185. [Google Scholar] [PubMed]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [PubMed]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [PubMed]
- Hsieh, C.-L.; Liu, C.-M.; Chen, H.-A.; Yang, S.-T.; Shigemura, K.; Kitagawa, K.; Yamamichi, F.; Fujisawa, M.; Liu, Y.-R.; Lee, W.-H. Reactive oxygen species–mediated switching expression of MMP-3 in stromal fibroblasts and cancer cells during prostate cancer progression. Sci. Rep. 2017, 7, 1–14. [Google Scholar]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar]
- Lazarus, G.S.; Hatcher, V.B.; Levine, N. Lysosomes and the skin. J. Investig. Dermatol. 1975, 65, 259–271. [Google Scholar]
- Wäster, P.; Eriksson, I.; Vainikka, L.; Rosdahl, I.; Öllinger, K. Extracellular vesicles are transferred from melanocytes to keratinocytes after UVA irradiation. Sci. Rep. 2016, 6, 1–13. [Google Scholar]
- Brunk, U.T.; Dalen, H.; Roberg, K.; Hellquist, H.B. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic. Biol. Med. 1997, 23, 616–626. [Google Scholar]
- Tam, C.; Idone, V.; Devlin, C.; Fernandes, M.C.; Flannery, A.; He, X.; Schuchman, E.; Tabas, I.; Andrews, N.W. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J. Cell Biol. 2010, 189, 1027–1038. [Google Scholar] [PubMed]
- Schwake, M.; Schröder, B.; Saftig, P. Lysosomal membrane proteins and their central role in physiology. Traffic 2013, 14, 739–748. [Google Scholar]
- Ge, W.; Li, D.; Gao, Y.; Cao, X. The roles of lysosomes in inflammation and autoimmune diseases. Int. Rev. Immunol. 2015, 34, 415–431. [Google Scholar] [PubMed]
- Yamauchi, T.; Hirose, T.; Sato, K.; Iwai, K.; Takahashi, N.; Minaguchi, J.; Ueno, T.; Tangkawattana, P.; Takehana, K. Changes in skin structure of the Zip13-KO mouse by Makomo (Zizania latifolia) feeding. J. Vet. Med. Sci. 2017, 79, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, H.; Qin, L.; Zhai, C. Effects of dietary carbohydrate replaced with wild rice (Zizania latifolia (Griseb) Turcz) on insulin resistance in rats fed with a high-fat/cholesterol diet. Nutrients 2013, 5, 552–564. [Google Scholar] [CrossRef]
- Jain, A.; Singh, H.B.; Bhattacharyya, P. The ethnobotany and nutritional values of wild rice [Zizania latifolia (Griseb.) Turcz. ex Stapf](Poaceae) in Manipur. Indian J. Tradit. Knowl. 2012, 11, 66–69. [Google Scholar]
- Surendiran, G.; Goh, C.; Le, K.; Zhao, Z.; Askarian, F.; Othman, R.; Nicholson, T.; Moghadasian, P.; Wang, Y.-J.; Aliani, M. Wild rice (Zizania palustris L.) prevents atherogenesis in LDL receptor knockout mice. Atherosclerosis 2013, 230, 284–292. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, S.-S.; Bang, M.-H.; Jo, S.K.; Jhee, K.-H.; Yang, S.-A. Protection against UVB-induced damages in human dermal fibroblasts: Efficacy of tricin isolated from enzyme-treated Zizania latifolia extract. Biosci. Botechnol. Biochem. 2019, 83, 551–560. [Google Scholar] [CrossRef]
- Moon, J.-M.; Park, S.-H.; Jhee, K.-H.; Yang, S.-A. Protection against UVB-induced wrinkle formation in SKH-1 hairless mice: Efficacy of tricin isolated from enzyme-treated Zizania latifolia extract. Molecules 2018, 23, 2254. [Google Scholar] [CrossRef]
- Singh, N.; Rani, M.; Sharmila, R. Flavonoids in rice, their role in health benefits. MOJ Food Process. Technol. 2017, 4, 96–99. [Google Scholar]
- Shalini, V.; Pushpan, C.K.; Sindhu, G.; Jayalekshmy, A.; Helen, A. Tricin, flavonoid from Njavara reduces inflammatory responses in hPBMCs by modulating the p38MAPK and PI3K/Akt pathways and prevents inflammation associated endothelial dysfunction in HUVECs. Immunobiology 2016, 221, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Appelqvist, H.; Wäster, P.; Eriksson, I.; Rosdahl, I.; Öllinger, K. Lysosomal exocytosis and caspase-8-mediated apoptosis in UVA-irradiated keratinocytes. J. Cell Sci. 2013, 126, 5578–5584. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.; Zhu, K. Inhibition of glioma cell lysosome exocytosis inhibits glioma invasion. PLoS ONE 2012, 7, e45910. [Google Scholar] [CrossRef] [PubMed]
- Gondi, C.S.; Rao, J.S. Cathepsin B as a cancer target. Expert Opin. Ther. Targets 2013, 17, 281–291. [Google Scholar] [CrossRef]
- Liabakk, N.-B.; Talbot, I.; Smith, R.A.; Wilkinson, K.; Balkwill, F. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenases in colorectal cancer. Cancer Res. 1996, 56, 190–196. [Google Scholar]
- Tamura, Y.; Watanabe, F.; Nakatani, T.; Yasui, K.; Fuji, M.; Komurasaki, T.; Tsuzuki, H.; Maekawa, R.; Yoshioka, T.; Kawada, K. Highly selective and orally active inhibitors of type IV collagenase (MMP-9 and MMP-2): N-sulfonylamino acid derivatives. J. Med. Chem. 1998, 41, 640–649. [Google Scholar] [CrossRef]
- Michniak-Kohn, B.; Leonardi, G.R. An overview about oxidation in clinical practice of skin aging. An. Bras. Dermatol. 2017, 92, 367–374. [Google Scholar]
- Kosmadaki, M.G.; Yaar, M.; Arble, B.L.; Gilchrest, B.A. UV induces VEGF through a TNF-alpha independent pathway. FASEB J. 2003, 17, 446–448. [Google Scholar] [CrossRef]
- Bivik, C.A.; Larsson, P.K.; Kågedal, K.M.; Rosdahl, I.K.; Öllinger, K.M. UVA/B-induced apoptosis in human melanocytes involves translocation of cathepsins and Bcl-2 family members. J. Investig. Dermatol. 2006, 126, 1119–1127. [Google Scholar] [CrossRef]
- Pearse, A.D.; Gaskell, S.A.; Marks, R. Epidermal changes in human skin following irradiation with either UVB or UVA. J. Investig. Dermatol. 1987, 88, 83–87. [Google Scholar] [CrossRef]
- Eskelinen, E.-L. Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol. Asp. Med. 2006, 27, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.J.; Jenkins, A.J. Glycation, oxidation, and lipoxidation in the development of the complications of diabetes: A carbonyl stress hypothesis. Diabetes Rev. 1997, 5, 365–391. [Google Scholar]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Meinke, M.C.; Nowbary, C.K.; Schanzer, S.; Vollert, H.; Lademann, J.; Darvin, M.E. Influences of Orally Taken Carotenoid-Rich Curly Kale Extract on Collagen I/Elastin Index of the Skin. Nutrients 2017, 9, 775. [Google Scholar] [CrossRef] [PubMed]
- Yap, W.N. Tocotrienol-rich fraction attenuates UV-induced inflammaging: A bench to bedside study. J. Cosmet. Dermatol. 2018, 17, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.E.P.S.; Pedroso, D.M.M. UV light and skin aging. Rev. Environ. Health 2014, 29, 243–254. [Google Scholar]
- Ma, W.; Wlaschek, M.; Tantcheva-Poor, I.; Schneider, L.; Naderi, L.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Chronological ageing and photoageing of the fibroblasts and the dermal connective tissue. Clin. Exp. Dermatol. 2001, 26, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.-J.; Liu, X.-M.; Yan, N.; Wang, F.-Z.; Du, Y.-M.; Zhang, Z.-F. Partial purification, identification, and quantitation of antioxidants from wild rice (Zizania latifolia). Molecules 2018, 23, 2782. [Google Scholar] [CrossRef]
- Rabe, J.H.; Mamelak, A.J.; McElgunn, P.J.; Morison, W.L.; Sauder, D.N. Photoaging: Mechanisms and repair. J. Am. Acad. Dermatol. 2006, 55, 1–19. [Google Scholar] [CrossRef]
- Eding, C.B.; Domert, J.; Wäster, P.; Jerhammar, F.; Rosdahl, I.; Öllinger, K. Melanoma growth and progression after ultraviolet an irradiation: Impact of lysosomal exocytosis and cathepsin proteases. Acta Derm. Venereol. 2015, 95, 792–797. [Google Scholar] [CrossRef]
- Reddy, A.; Caler, E.V.; Andrews, N.W. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 2001, 106, 157–169. [Google Scholar] [PubMed]
- Kundu, S.T.; Grzeskowiak, C.L.; Fradette, J.J.; Gibson, L.A.; Rodriguez, L.B.; Creighton, C.J.; Scott, K.L.; Gibbons, D.L. TMEM106B drives lung cancer metastasis by inducing TFEB-dependent lysosome synthesis and secretion of cathepsins. Nat. Commun. 2018, 9, 1–16. [Google Scholar]
- Wlaschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B Biol. 2001, 63, 41–51. [Google Scholar] [CrossRef]
- Scharffetter–Kochanek, K.; Brenneisen, P.; Wenk, J.; Herrmann, G.; Ma, W.; Kuhr, L.; Meewes, C.; Wlaschek, M. Photoaging of the skin from phenotype to mechanisms. Exp. Gerontol. 2000, 35, 307–316. [Google Scholar]
- Scharffetter-Kochanek, K.; Wlaschek, M.; Brenneisen, P.; Schauen, M.; Blaudschun, R.; Wenk, J. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol. Chem. 1997, 378, 1247–1258. [Google Scholar]
- Ichihashi, M.; Ueda, M.; Budiyanto, A.; Bito, T.; Oka, M.; Fukunaga, M.; Tsuru, K.; Horikawa, T. UV-induced skin damage. Toxicology 2003, 189, 21–39. [Google Scholar]
- Qin, Y.; Cao, X.; Yang, Y.; Shi, G.-P. Cysteine protease cathepsins and matrix metalloproteinases in the development of abdominal aortic aneurysms. Future Cardiol. 2013, 9, 89–103. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Garbers, C.; Rose-John, S. ADAM17: A molecular switch to control inflammation and tissue regeneration. Trends Immunol. 2011, 32, 380–387. [Google Scholar]











| Biomarker | Normal Control | UVA Control | ZLE 100 | ZLE 200 | Tri | AA |
|---|---|---|---|---|---|---|
| (U/L) | ||||||
| GOT | 124.8 ± 17.7 | 147.9 ± 17.2 | 125.6 ± 17.8 | 132.4 ± 11.2 | 126.9 ± 16.4 | 149.7 ± 20.7 |
| GPT | 30.4 ±3.2 | 32.7 ±3.9 | 25.8 ± 3.2 | 31.5 ±4.2 | 29 ± 3.6 | 28.5 ± 3.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, M.; Kim, H.; Moon, J.-M.; Ko, H.-S.; Clayton, P.; Lim, Y.-H. Enzyme-Treated Zizania latifolia Ethanol Extract Protects from UVA Irradiation-Induced Wrinkle Formation via Inhibition of Lysosome Exocytosis and Reactive Oxygen Species Generation. Antioxidants 2020, 9, 912. https://doi.org/10.3390/antiox9100912
An M, Kim H, Moon J-M, Ko H-S, Clayton P, Lim Y-H. Enzyme-Treated Zizania latifolia Ethanol Extract Protects from UVA Irradiation-Induced Wrinkle Formation via Inhibition of Lysosome Exocytosis and Reactive Oxygen Species Generation. Antioxidants. 2020; 9(10):912. https://doi.org/10.3390/antiox9100912
Chicago/Turabian StyleAn, Mirae, Hyungkeun Kim, Joo-Myung Moon, Hyun-Soo Ko, Paul Clayton, and Young-Hee Lim. 2020. "Enzyme-Treated Zizania latifolia Ethanol Extract Protects from UVA Irradiation-Induced Wrinkle Formation via Inhibition of Lysosome Exocytosis and Reactive Oxygen Species Generation" Antioxidants 9, no. 10: 912. https://doi.org/10.3390/antiox9100912
APA StyleAn, M., Kim, H., Moon, J.-M., Ko, H.-S., Clayton, P., & Lim, Y.-H. (2020). Enzyme-Treated Zizania latifolia Ethanol Extract Protects from UVA Irradiation-Induced Wrinkle Formation via Inhibition of Lysosome Exocytosis and Reactive Oxygen Species Generation. Antioxidants, 9(10), 912. https://doi.org/10.3390/antiox9100912