•BMPO-OOH Spin-Adduct as a Model for Study of Decomposition of Organic Hydroperoxides and the Effects of Sulfide/Selenite Derivatives. An EPR Spin-Trapping Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. EPR Study of the BMPO-Adducts
2.3. Plasmid DNA Cleavage
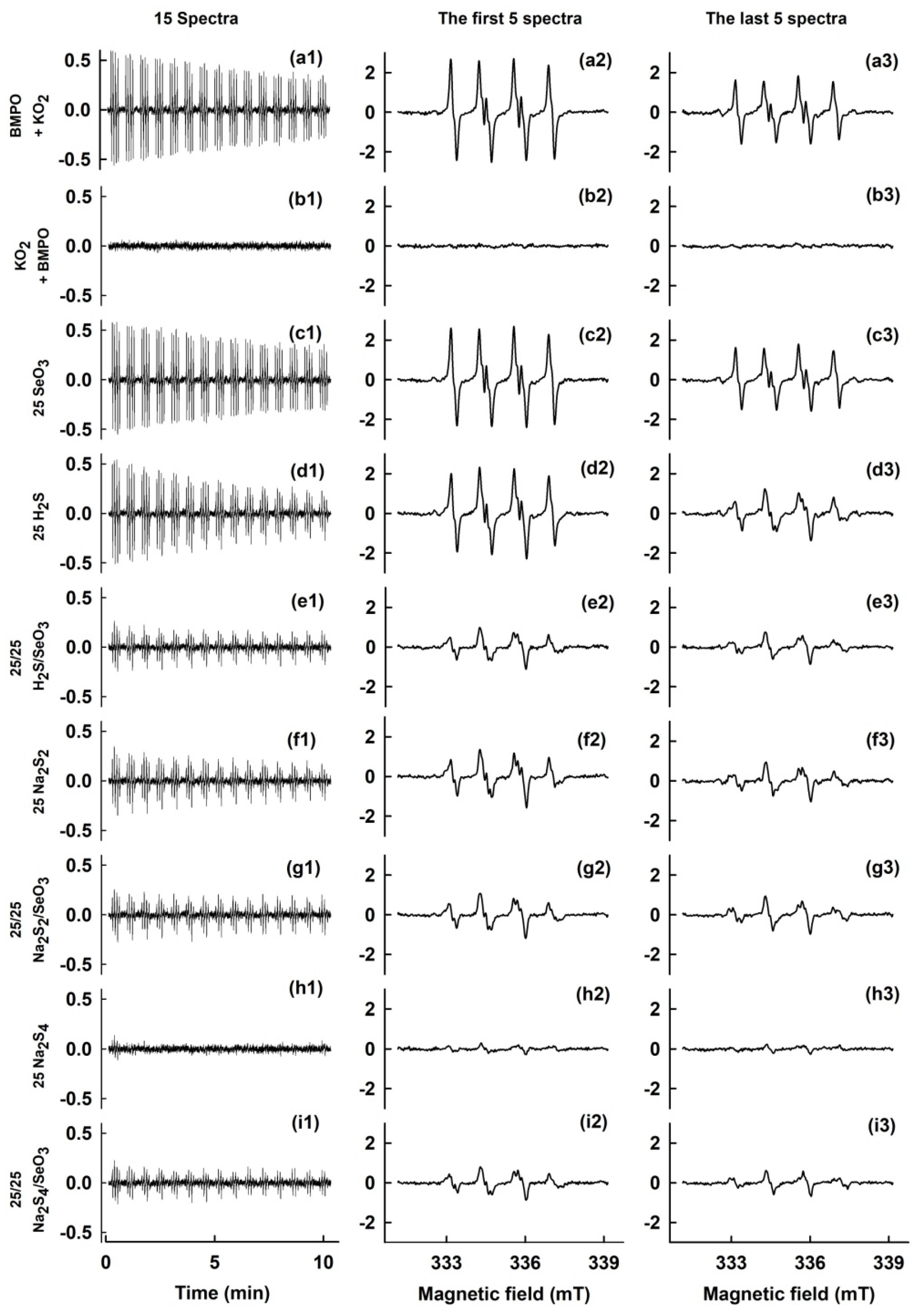
3. Results
3.1. •BMPO-OOH as a Model Hydroperoxide and the Effects of Na2Sn/Na2SeOn
3.2. Simulation of BMPO-Adducts Spectra in the Presence of Na2Sn/Na2SeO3
3.3. Cleavage of Plasmid DNA
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [Green Version]
- Tomasova, L.; Konopelski, P.; Ufnal, M. Gut bacteria and hydrogen sulfide: The new old players in circulatory system homeostasis. Molecules 2016, 21, 1558. [Google Scholar] [CrossRef]
- Cacanyiova, S.; Berenyiova, A.; Balis, P.; Kristek, F.; Grman, M.; Ondrias, K.; Breza, J.; Breza, J., Jr. Nitroso-sulfide coupled signaling triggers specific vasoactive effects in the intrarenal arteries of patients with arterial hypertension. J. Physiol. Pharmacol. 2017, 68, 527–538. [Google Scholar]
- Fukuto, J.M.; Ignarro, L.J.; Nagy, P.; Wink, D.A.; Kevil, C.G.; Feelisch, M.; Cortese-Krott, M.M.; Bianco, C.L.; Kumagai, Y.; Hobbs, A.J.; et al. Biological hydropersulfides and related polysulfides—A new concept and perspective in redox biology. FEBS Lett. 2018, 592, 2140–2152. [Google Scholar] [CrossRef] [Green Version]
- Misak, A.; Grman, M.; Bacova, Z.; Rezuchova, I.; Hudecova, S.; Ondriasova, E.; Krizanova, O.; Brezova, V.; Chovanec, M.; Ondrias, K. Polysulfides and products of H2S/S-nitrosoglutathione in comparison to H2S, glutathione and antioxidant Trolox are potent scavengers of superoxide anion radical and produce hydroxyl radical by decomposition of H2O2. Nitric Oxide 2018, 76, 136–151. [Google Scholar] [CrossRef]
- Eghbal, M.A.; Pennefather, P.S.; O’Brien, P.J. H2S cytotoxicity mechanism involves reactive oxygen species formation and mitochondrial depolarisation. Toxicology 2004, 203, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Radford, M.N.; Yang, C.T.; Chen, W.; Xian, M. Inorganic hydrogen polysulfides: Chemistry, chemical biology and detection. Br. J. Pharmacol. 2019, 176, 616–627. [Google Scholar] [CrossRef] [Green Version]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, E.; Vinceti, M. Selenium and human health: Witnessing a Copernican revolution? J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 328–368. [Google Scholar] [CrossRef] [PubMed]
- Misak, A.; Kurakova, L.; Goffa, E.; Brezova, V.; Grman, M.; Ondriasova, E.; Chovanec, M.; Ondrias, K. Sulfide (Na₂S) and Polysulfide (Na₂S₂) interacting with doxycycline produce/scavenge superoxide and hydroxyl radicals and induce/inhibit DNA cleavage. Molecules 2019, 24, 1148. [Google Scholar] [CrossRef] [Green Version]
- Kharma, A.; Grman, M.; Misak, A.; Domínguez-Álvarez, E.; Nasim, M.J.; Ondrias, K.; Chovanec, M.; Jacob, C. Inorganic Polysulfides and related reactive sulfur–selenium species from the perspective of chemistry. Molecules 2019, 24, 1359. [Google Scholar] [CrossRef] [Green Version]
- Kharma, A.; Misak, A.; Grman, M.; Brezova, V.; Kurakova, L.; Baráth, P.; Jacob, C.; Chovanec, M.; Ondrias, K.; Domínguez-Álvarez, E. Release of reactive selenium species from phthalic selenoanhydride in the presence of hydrogen sulfide and glutathione with implications for cancer research. New J. Chem. 2019, 43, 11771–11783. [Google Scholar] [CrossRef] [Green Version]
- Grman, M.; Misak, A.; Kurakova, L.; Brezova, V.; Cacanyiova, S.; Berenyiova, A.; Balis, P.; Tomasova, L.; Kharma, A.; Domínguez-Álvarez, E.; et al. Products of sulfide/selenite interaction possess antioxidant properties, scavenge superoxide-derived radicals, react with DNA, and modulate blood pressure and tension of isolated thoracic aorta. Oxidative Med. Cell. Longev. 2019, 2019, 9847650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 2018, 172, 409–422.e421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Joseph, J.; Zhang, H.; Karoui, H.; Kalyanaraman, B. Synthesis and biochemical applications of a solid cyclic nitrone spin trap: A relatively superior trap for detecting superoxide anions and glutathiyl radicals. Free Radic. Biol. Med. 2001, 31, 599–606. [Google Scholar] [CrossRef]
- Bézière, N.; Hardy, M.; Poulhès, F.; Karoui, H.; Tordo, P.; Ouari, O.; Frapart, Y.M.; Rockenbauer, A.; Boucher, J.L.; Mansuy, D.; et al. Metabolic stability of superoxide adducts derived from newly developed cyclic nitrone spin traps. Free Radic. Biol. Med. 2014, 67, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of reactive oxygen and nitrogen species by Electron Paramagnetic Resonance (EPR) technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef]
- Tsai, P.; Marra, J.M.; Pou, S.; Bowman, M.K.; Rosen, G.M. Is there stereoselectivity in spin trapping superoxide by 5-tert-butoxycarbonyl-5-methyl-1-pyrroline N-oxide? J. Org. Chem. 2005, 70, 7093–7097. [Google Scholar] [CrossRef]
- Tsai, P.; Ichikawa, K.; Mailer, C.; Pou, S.; Halpern, H.J.; Robinson, B.H.; Nielsen, R.; Rosen, G.M. Esters of 5-carboxyl-5-methyl-1-pyrroline N-oxide: A family of spin traps for superoxide. J. Org. Chem. 2003, 68, 7811–7817. [Google Scholar] [CrossRef]
- Stolze, K.; Udilova, N.; Rosenau, T.; Hofinger, A.; Nohl, H. Synthesis and characterization of EMPO-derived 5,5-disubstituted 1-pyrroline N-oxides as spin traps forming exceptionally stable superioxide spin adducts. Biol. Chem. 2003, 384, 493–500. [Google Scholar] [CrossRef]
- Chang, J.; Taylor, R.D.; Davidson, R.A.; Sharmah, A.; Guo, T. Electron paramagnetic resonance spectroscopy investigation of radical production by gold nanoparticles in aqueous solutions under X-ray irradiation. J. Phys. Chem. A 2016, 120, 2815–2823. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Merritt, M.V.; Sawyer, D.T. Electrochemical studies of the reactivity of superoxide ion with several alkyl halides in dimethyl sulfoxide. J. Org. Chem. 1970, 35, 2157–2159. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, M.; Ma, Y.; Yoshikawa, Y.; Imanaka, T.; Mori, T.; Furuta, M.; Tsuruyama, T.; Yoshikawa, K. A single-molecule assessment of the protective effect of DMSO against DNA double-strand breaks induced by photo-and γ-ray-irradiation, and freezing. Sci. Rep. 2017, 7, 8557. [Google Scholar] [CrossRef] [PubMed]






| BMPO-Adduct | aN, mT | aHβ, mT | aHγ, mT |
|---|---|---|---|
| •BMPO-OH(1) | 1.423 ± 0.011 | 1.541 ± 0.014 | 0.078 ± 0.011 |
| •BMPO-OH(2) | 1.365 ± 0.038 | 1.248 ± 0.036 | 0.073 ± 0.015 |
| •BMPO-OOH(1) | 1.339 ± 0.002 | 1.186 ± 0.007 | – |
| •BMPO-OOH(2) | 1.334 ± 0.003 | 0.958 ± 0.007 | – |
| •BMPO-CR | 1.528 | 2.221 | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misak, A.; Brezova, V.; Grman, M.; Tomasova, L.; Chovanec, M.; Ondrias, K. •BMPO-OOH Spin-Adduct as a Model for Study of Decomposition of Organic Hydroperoxides and the Effects of Sulfide/Selenite Derivatives. An EPR Spin-Trapping Approach. Antioxidants 2020, 9, 918. https://doi.org/10.3390/antiox9100918
Misak A, Brezova V, Grman M, Tomasova L, Chovanec M, Ondrias K. •BMPO-OOH Spin-Adduct as a Model for Study of Decomposition of Organic Hydroperoxides and the Effects of Sulfide/Selenite Derivatives. An EPR Spin-Trapping Approach. Antioxidants. 2020; 9(10):918. https://doi.org/10.3390/antiox9100918
Chicago/Turabian StyleMisak, Anton, Vlasta Brezova, Marian Grman, Lenka Tomasova, Miroslav Chovanec, and Karol Ondrias. 2020. "•BMPO-OOH Spin-Adduct as a Model for Study of Decomposition of Organic Hydroperoxides and the Effects of Sulfide/Selenite Derivatives. An EPR Spin-Trapping Approach" Antioxidants 9, no. 10: 918. https://doi.org/10.3390/antiox9100918





