Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of a Red Cabbage Extract Chemically Profiled through UHPLC-Q-Orbitrap HRMS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Sampling
2.3. Red Cabbage Polyphenolic Extraction
2.4. Determination of Total Phenolic Content (TPC)
2.5. UHPLC-Q-Orbitrap HRMS Analysis
2.6. Antioxidant Activity
2.6.1. ABTS Radical Cation Scavenging Assay
2.6.2. DPPH Free Radical-Scavenging Assay
2.6.3. Ferric Reducing Antioxidant Power
2.7. In Vitro Simulated Gastrointestinal Digestion
2.8. Statistical Analysis
3. Results and Discussion
3.1. Red Cabbage Extract Bioactivity
3.2. In Vitro Bioaccessibility of Red Cabbage Polyphenols
3.3. Identification and Quantification of Red Cabbage Bioactive Compounds Analyzed through UHPLC-Q-Orbitrap HRMS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shankar, S.; Segaran, G.; Sundar, R.D.V.; Settu, S.; Sathiavelu, M. Brassicaceae-A Classical Review on Its Pharmacological Activities. Int. J. Pharm. Sci. Rev. Res. 2019, 55, 107–113. [Google Scholar]
- Araceli, C.; MadeLourdes, P.-H.; Maelena, P.; JoseA, R.; Carlosandrés, G. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant. Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Seis, H. Oxidative stress:from basic research to clinical application. Am. J. Med. 1991, 91, S31–S38. [Google Scholar] [CrossRef]
- Bellocco, E.; Barreca, D.; Laganà, G.; Calderaro, A.; El Lekhlifi, Z.; Chebaibi, S.; Smeriglio, A.; Trombetta, D. Cyanidin-3-O-galactoside in ripe pistachio (Pistachia vera L. variety Bronte) hulls: Identification and evaluation of its antioxidant and cytoprotective activities. J. Funct. Foods 2016, 27, 376–385. [Google Scholar] [CrossRef]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B. Strategies for reducing or preventing the generation of oxidative stress. Oxidative Med. Cell. Longev. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Nita, M.; Grzybowski, A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Sandoval-Ramírez, B.A.E.; Catalán, U.R.; Fernández-Castillejo, S.; Rubió, L.; Macià, A.; Solà, R. Anthocyanin tissue bioavailability in animals: Possible implications for human health. A systematic review. J. Agric. Food Chem. 2018, 66, 11531–11543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.F.; Visser, R.G.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Wahyuningsih, S.; Wulandari, L.; Wartono, M.; Munawaroh, H.; Ramelan, A. The Effect of pH and Color Stability of Anthocyanin on Food Colorant; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 012047. [Google Scholar] [CrossRef] [Green Version]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin–Ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Estévez, M.; Barba, F.J.; Thirumdas, R.; Franco, D.; Munekata PE, S. Polyphenols: Bioaccessibility and bioavailability of bioactive components. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–332. [Google Scholar] [CrossRef]
- Grace, M.H.; Esposito, D.; Dunlap, K.L.; Lila, M.A. Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild Alaskan and commercial Vaccinium berries. J. Agric. Food Chem. 2014, 62, 4007–4017. [Google Scholar] [CrossRef] [Green Version]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef] [Green Version]
- Luz, C.; Izzo, L.; Graziani, G.; Gaspari, A.; Ritieni, A.; Mañes, J.; Meca, G. Evaluation of biological and antimicrobial properties of freeze-dried whey fermented by different strains of Lactobacillus plantarum. Food Funct. 2018, 9, 3688–3697. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lwt Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapornik, B.; Prošek, M.; Wondra, A.G. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Murador, D.C.; Mercadante, A.Z.; de Rosso, V.V. Cooking techniques improve the levels of bioactive compounds and antioxidant activity in kale and red cabbage. Food Chem. 2016, 196, 1101–1107. [Google Scholar] [CrossRef]
- Tabart, J.; Pincemail, J.; Kevers, C.; Defraigne, J.-O.; Dommes, J. Processing effects on antioxidant, glucosinolate, and sulforaphane contents in broccoli and red cabbage. Eur. Food Res. Technol. 2018, 244, 2085–2094. [Google Scholar] [CrossRef]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.P.; Marciano, S.; Monaco, P. Seasonal variation in phenolic composition and antioxidant and anti-inflammatory activities of Calamintha nepeta (L.) Savi. Food Res. Int. 2015, 69, 121–132. [Google Scholar] [CrossRef]
- Leja, M.; Kamińska, I.; Kołton, A. Phenolic compounds as the major antioxidants in red cabbage. Folia Hortic. 2010, 22, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, R.; Sehwag, S.; Singh, S.P. Antioxidant activity and polyphenol content of Brassica oleracea varieties. Int. J. Veg. Sci. 2016, 22, 353–363. [Google Scholar] [CrossRef]
- Fusari, C.M.; Nazareno, M.A.; Locatelli, D.A.; Fontana, A.; Beretta, V.; Camargo, A.B. Phytochemical profile and functionality of Brassicaceae species. Food Biosci. 2020, 100606. [Google Scholar] [CrossRef]
- Erken, O.; Kaya, S. Free radical scavenging activity, phenolic. Fresenius Environ. Bull. 2017, 26, 4383–4389. [Google Scholar]
- Podsędek, A.; Majewska, I.; Kucharska, A.Z. Inhibitory potential of red cabbage against digestive enzymes linked to obesity and type 2 diabetes. J. Agric. Food Chem. 2017, 65, 7192–7199. [Google Scholar] [CrossRef] [PubMed]
- Podsedek, A.; Sosnowska, D.; Redzynia, M.; Anders, B. Antioxidant capacity and content of Brassica oleracea dietary antioxidants. Int. J. Food Sci. Technol. 2006, 41, 49–58. [Google Scholar] [CrossRef]
- Oroian, M.; Leahu, A.; Dutuc, A.; Dabija, A. Optimization of Total Monomeric Anthocyanin (TMA) and Total Phenolic Content (TPC) Extractions from Red Cabbage (Brassica oleracea varcapitata f. rubra): Response Surface Methodology versus Artificial Neural Network. Int. J. Food Eng. 2017, 13, 1–11. [Google Scholar] [CrossRef]
- Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Effect of processing on antioxidants and their activity in dietary fiber powder from cabbage outer leaves. Dry. Technol. 2010, 28, 1063–1071. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Gupta, S.; Abu-Ghannam, N. Kinetic evaluation of colour, texture, polyphenols and antioxidant capacity of Irish York cabbage after blanching treatment. Food Chem. 2012, 131, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Kusznierewicz, B.; Bartoszek, A.; Wolska, L.; Drzewiecki, J.; Gorinstein, S.; Namieśnik, J. Partial characterization of white cabbages (Brassica oleracea varcapitata f. alba) from different regions by glucosinolates, bioactive compounds, total antioxidant activities and proteins. Lwt Food Sci. Technol. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Cruz, A.B.; Pitz, H.D.S.; Veber, B.; Bini, L.A.; Maraschin, M.; Zeni, A.L.B. Assessment of bioactive metabolites and hypolipidemic effect of polyphenolic-rich red cabbage extract. Pharm. Biol. 2016, 54, 3033–3039. [Google Scholar] [CrossRef] [Green Version]
- Caramês, E.T.; Alamar, P.D.; Pallone, J.A.L. Bioactive Compounds and Antioxidant Capacity in Freeze-Dried Red Cabbage by FT-NIR and MIR Spectroscopy and Chemometric Tools. Food Anal. Methods 2020, 13, 78–85. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Topolska, J.; Honke, J. Anthocyanins profile and antioxidant capacity of red cabbages are influenced by genotype and vegetation period. J. Funct. Foods 2014, 7, 201–211. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Ritieni, A. In Vitro Bioaccessibility and Antioxidant Activity of Coffee Silverskin Polyphenolic Extract and Characterization of Bioactive Compounds Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 2132. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Daliu, P.; Narciso, V.; Tenore, G.C.; Novellino, E. Colon bioaccessibility and antioxidant activity of white, green and black tea polyphenols extract after in vitro simulated gastrointestinal digestion. Nutrients 2018, 10, 1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas-González, R.; Viuda-Martos, M.; Álvarez JA, P.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef]
- Podsędek, A.; Redzynia, M.; Klewicka, E.; Koziołkiewicz, M. Matrix effects on the stability and antioxidant activity of red cabbage anthocyanins under simulated gastrointestinal digestion. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Sodagari, H.R.; Farzaei, M.H.; Bahramsoltani, R.; Abdolghaffari, A.H.; Mahmoudi, M.; Rezaei, N. Dietary anthocyanins as a complementary medicinal approach for management of inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 807–820. [Google Scholar] [CrossRef]
- Khalifa, I.; Zhu, W.; Li, K.-K.; Li, C.-M. Polyphenols of mulberry fruits as multifaceted compounds: Compositions, metabolism, health benefits, and stability—A structural review. J. Funct. Foods 2018, 40, 28–43. [Google Scholar] [CrossRef]
- Chen, H.; Chen, J.; Yang, H.; Chen, W.; Gao, H.; Lu, W. Variation in total anthocyanin, phenolic contents, antioxidant enzyme and antioxidant capacity among different mulberry (Morus sp.) cultivars in China. Sci. Hortic. 2016, 213, 186–192. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Sang, S. Biotransformation of tea polyphenols by gut microbiota. J. Funct. Foods 2014, 7, 26–42. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; González-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliazucchi, D.; Verzelloni, E.; Conte, A. The first tract of alimentary canal as an extractor. Release of phytochemicals from solid food matrices during simulated digestion. J. Food Biochem. 2012, 36, 555–568. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.-J.; Tomás-Barberán, F.-A.; García-Conesa, M.-T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Henning, S.M.; Zhang, Y.; Rontoyanni, V.G.; Huang, J.; Lee, R.-P.; Trang, A.; Nuernberger, G.; Heber, D. Variability in the antioxidant activity of dietary supplements from pomegranate, milk thistle, green tea, grape seed, goji, and acai: Effects of in vitro digestion. J. Agric. Food Chem. 2014, 62, 4313–4321. [Google Scholar] [CrossRef]
- Parisi, O.I.; Puoci, F.; Restuccia, D.; Farina, G.; Iemma, F.; Picci, N. Polyphenols and their formulations: Different strategies to overcome the drawbacks associated with their poor stability and bioavailability. In Polyphenols in Human Health and Disease; Academic Press: London, UK, 2014; pp. 29–45. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Esfanjani, A.F.; Assadpour, E.; Jafari, S. M Improving the bioavailability of phenolic compounds by loading them within lipid-based nanocarriers. Trends Food Sci. Technol. 2018, 76, 56–66. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Martín-Belloso, O.; Soliva-Fortuny, R.; Elez-Martínez, P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2531–2548. [Google Scholar] [CrossRef] [Green Version]
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309. [Google Scholar] [CrossRef]
- Arapitsas, P.; Sjöberg, P.J.; Turner, C. Characterisation of anthocyanins in red cabbage using high resolution liquid chromatography coupled with photodiode array detection and electrospray ionization-linear ion trap mass spectrometry. Food Chem. 2008, 109, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Prior, R.L. Identification and characterization of anthocyanins by high-performance liquid chromatography−electrospray ionization−tandem mass spectrometry in common foods in the United States: Vegetables, nuts, and grains. J. Agric. Food Chem. 2005, 53, 3101–3113. [Google Scholar] [CrossRef] [PubMed]
- Charron, C.S.; Kurilich, A.C.; Clevidence, B.A.; Simon, P.W.; Harrison, D.J.; Britz, S.J.; Baer, D.J.; Novotny, J.A. Bioavailability of anthocyanins from purple carrot juice: Effects of acylation and plant matrix. J. Agric. Food Chem. 2009, 57, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Mizgier, P.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kolniak-Ostek, J.; Kidoń, M.; Fecka, I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J. Funct. Foods 2016, 21, 133–146. [Google Scholar] [CrossRef]
- Ahmadiani, N.; Robbins, R.J.; Collins, T.M.; Giusti, M.M. Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. J. Agric. Food Chem. 2014, 62, 7524–7531. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. Lwt Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Voća, S.; Šic Žlabur, J.; Dobričević, N.; Benko, B.; Pliestić, S.; Filipović, M.; Galić, A. Bioactive compounds, pigment content and antioxidant capacity of selected cabbage cultivars. J. Cent. Eur. Agric. 2018, 19, 593–606. [Google Scholar] [CrossRef] [Green Version]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Wiczkowski, W.; Płatosz, N.; Bartoszek, A. Phytochemical composition and biological activities of differently pigmented cabbage (Brassica oleracea varcapitata) and cauliflower (Brassica oleracea var. botrytis) varieties. J. Sci. Food Agric. 2019, 99, 5499–5507. [Google Scholar] [CrossRef]

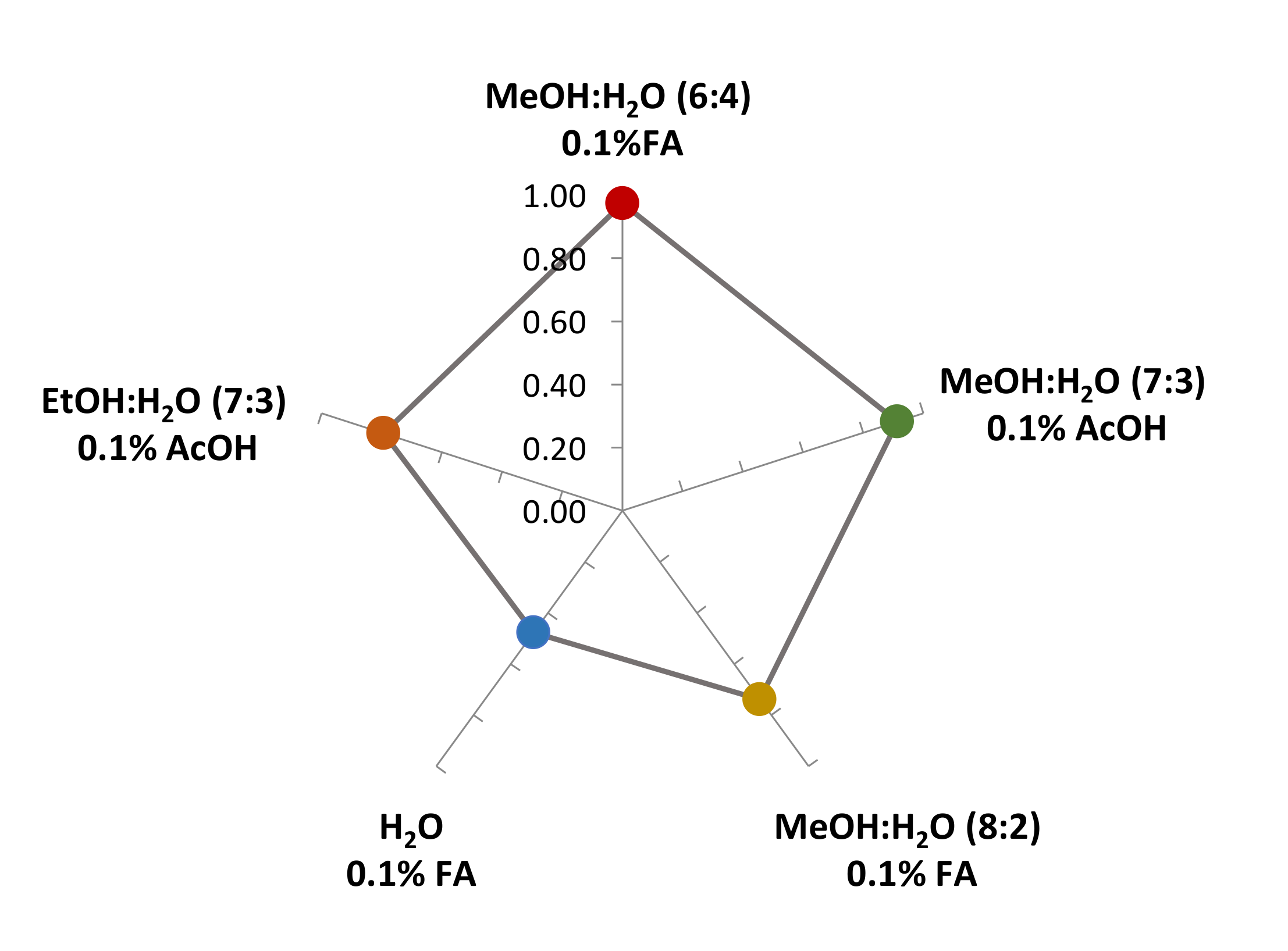
| Red Cabbage Extract | TPC * (mg GAE/g) | ABTS * | DPPH * (mmol Trolox/kg) | FRAP * |
|---|---|---|---|---|
| MeOH:H2O (6:4) 0.1% FA | 19.986 ± 0.132 a | 50.849 ± 2.955 d | 36.242 ± 0.068 f | 87.095 ± 0.699 h |
| MeOH:H2O (7:3) 0.1% AcOH | 17.591 ± 0.721 b | 49.978 ± 1.408 d | 34.466 ± 0.168 f | 85.379 ± 0.349 h |
| MeOH:H2O (8:2) 0.1% FA | 17.541 ± 0.304 b | 45.128 ± 1.065 e | 24.003 ± 0.056 g | 68.759 ± 0.349 i |
| H2O 0.1% FA | 15.798 ± 0.566 c | 47.906 ± 1.411 d | 23.498 ± 0.345 g | 67.833 ± 1.325 i |
| EtOH:H2O (7:3) 0.1% AcOH | 16.262 ± 0.528 c | 49.324 ± 0.761 d | 24.701 ± 0.166 g | 81.884 ± 1.277 h |
| References | TPC (mg GAE/g) dw * | Extraction Solvent | Sample Origin |
|---|---|---|---|
| Leja et al., 2010 [30] | 3.90–31.11 | 80% MeOH | Poland |
| Upadhyay et al., 2016 [31] | 28.25 | 80% MeOH | India |
| Fusari et al., 2020 [32] | 0.099 | Ultrapure H2O | Argentina |
| Erken et al., 2017 [33] | 313.73 | EtOH/acetone | Turkey |
| Murador et al., 2016 [27] | 3.56 | 0.5% HCl in MeOH | Brazil |
| Podsędek et al., 2017 [34] | 10.1–19.6 | 70% MeOH | Poland |
| Podsędek et al., 2006 [35] | 16.8–21.42 | 70% MeOH | Poland |
| Oroian et al., 2017 [36] | 84.75 | MeOH | Romania |
| Tanongkankit et al., 2010 [37] | 496.92–739.24 | acetone–H2O (1:1, v/v) | Thailand |
| Jaiswal et al., 2012 [38] | 18.45 | 70% MeOH | Ireland |
| Kusznierewicz et al., 2007 [39] | 2.4–4.9 | 0.1% HCl (1 N) in MeOH | Poland/Belgium Germany/England |
| Tabart et al., 2018 [28] | 18.51 | acetone/H2O/AcOH, (70:28:2, v/v/v) | Belgium |
| Cruz et al., 2016 [40] | 89.33–116 | 70% MeOH/ H2O boiled | Brazil |
| Caramês et al., 2020 [41] | 48.37–87.12 | MeOH/H2O/AcOH (0.58:0.38:0.04, v/v/v) | Brazil |
| Red Cabbage In Vitro GI Digestion | TPC (mg GAE/g) | ||
|---|---|---|---|
| Phase | Extract | Capsule * | |
| 1 | Intestinal phase | 22.287 ± 0.295 a | 22.738 ± 0.339 a |
| 2 | Pronase E | 0.124 ± 0.003 b | 4.434 ± 0.069 c |
| 3 | Viscozyme L | 0.994 ± 0.060 d | 0.102 ± 0.022 e |
| Red Cabbage In Vitro GI Phase | ||||
|---|---|---|---|---|
| 1 Intestinal phase | 2 Pronase E | 3 Viscozyme L | ||
| ABTS (mmol Trolox/kg) | Capsule | 76.755 ± 1.483 a | 0.682 ± 0.044 b | 2.600 ± 0.220 d |
| Extract | 78.513 ± 1.783 a | 12.820 ± 0.949 c | 2.626 ± 0.067 d | |
| DPPH (mmol Trolox/kg) | Capsule | 44.985 ± 2.547 e | 0.150 ± 0.003 f | 4.074 ± 0.126 h |
| Extract | 45.762 ± 1.773 e | 7.268 ± 1.095 g | 0.008 ± 0.001 i | |
| FRAP (mmol Trolox/kg) | Capsule | 90.778 ± 2.128 j | 2.055 ± 0.355 k | 28.954 ± 1.793 m |
| Extract | 91.958 ± 1.502 j | 132.931 ± 0.939 l | 0.528 ± 0.074 b | |
| Compound | Red Cabbage Content (mg/kg) | SD |
|---|---|---|
| Anthocyanins | ||
| Cyanidin 3-diglucoside-5-glucoside | 2344.684 | 9.198 |
| Cyanidin 3-soph-5-xyloside | 24.660 | 5.115 |
| Cyanidin 3,5-diglucoside | 306.750 | 12.450 |
| Cyanidin 3-galactoside | 7.000 | 1.170 |
| Cyanidin 3-(sin)soph-5-glucoside | 514.153 | 11.001 |
| Cyanidin 3-(sin)triglucoside-5-glucoside | 131.554 | 4.225 |
| Cyanidin 3-(glucofer)-diglucoside-5-glucoside | 10.549 | 0.617 |
| Cyanidin 3-(p-coum)-glucoside-5-glucoside | 13.395 | 3.542 |
| Cyanidin 3-(caf)-diglucoside-5-glucoside | 22.115 | 2.892 |
| Cyanidin 3-(fer)-glucoside-5-glucoside | 28.009 | 2.759 |
| Cyanidin 3-(sin)glucoside-5-glucoside | 153.803 | 11.370 |
| Cyanidin 3-(p-coum)-diglucoside-5-glucoside | 340.812 | 8.372 |
| Cyanidin 3-(fer)soph-5-glucoside | 268.096 | 9.925 |
| Cyanidin | 112.793 | 2.745 |
| Cyanidin 3-(sin)-diglucoside-5-glucoside | 727.842 | 13.723 |
| Cyanidin 3-(caf)(p-coum)-diglucoside-5-glucoside | 5.680 | 0.056 |
| Cyanidin 3-(caf)(sin)soph-5-glucoside | 31.820 | 8.544 |
| Cyanidin 3-(sin)(p-coum)soph-5-glucoside | 186.841 | 11.523 |
| Cyanidin 3-(sin)(fer)soph-5-glucoside | 195.990 | 11.289 |
| Cyanidin 3-(sin)(sin)soph-5-glucoside | 506.567 | 8.600 |
| Other flavonoids | ||
| Epicatechin | 1.544 | 0.232 |
| Epigallocatechin | 23.965 | 0.161 |
| Rutin | 15.302 | 0.963 |
| Kaempferol | 17.371 | 0.228 |
| Quercetin | 4.359 | 0.326 |
| Genistein | 24.122 | 1.471 |
| Phenolic acids | ||
| Caffeic acid | 39.356 | 1.250 |
| Chlorogenic acid | 55.316 | 5.581 |
| p-Coumaric acid | 3689.235 | 2.356 |
| Ferulic acid | 2533.965 | 3.161 |
| Protocatechuic acid | 2056.230 | 2.102 |
| Sinapic acid | 6325.025 | 3.568 |
| Syringic acid | 17.069 | 0.532 |
| Vanillic acid | 2136.250 | 3.256 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izzo, L.; Rodríguez-Carrasco, Y.; Pacifico, S.; Castaldo, L.; Narváez, A.; Ritieni, A. Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of a Red Cabbage Extract Chemically Profiled through UHPLC-Q-Orbitrap HRMS. Antioxidants 2020, 9, 955. https://doi.org/10.3390/antiox9100955
Izzo L, Rodríguez-Carrasco Y, Pacifico S, Castaldo L, Narváez A, Ritieni A. Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of a Red Cabbage Extract Chemically Profiled through UHPLC-Q-Orbitrap HRMS. Antioxidants. 2020; 9(10):955. https://doi.org/10.3390/antiox9100955
Chicago/Turabian StyleIzzo, Luana, Yelko Rodríguez-Carrasco, Severina Pacifico, Luigi Castaldo, Alfonso Narváez, and Alberto Ritieni. 2020. "Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of a Red Cabbage Extract Chemically Profiled through UHPLC-Q-Orbitrap HRMS" Antioxidants 9, no. 10: 955. https://doi.org/10.3390/antiox9100955
APA StyleIzzo, L., Rodríguez-Carrasco, Y., Pacifico, S., Castaldo, L., Narváez, A., & Ritieni, A. (2020). Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of a Red Cabbage Extract Chemically Profiled through UHPLC-Q-Orbitrap HRMS. Antioxidants, 9(10), 955. https://doi.org/10.3390/antiox9100955