Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa
Abstract
:1. Introduction
2. Oxidative Stress and Retinitis Pigmentosa
2.1. Animal Models of RP
2.2. Synoptic Aspects of Oxidation and Antioxidation
2.3. Oxidative Stress and RP
3. Mesenchymal Cells: Therapeutic Strategies in Retinitis Pigmentosa
- Adipose-derived stem cells (ADSCs)
- Adult adipocytes
- Platelets
- Cell differentiation and trans-differentiation for lost/damaged cell replacement
- Paracrine action for cell repair and functional stimulation
- Exosomes and microvesicle secretion
- Modulation of host immune responses in inflammation site
3.1. Transdifferentation
3.2. Paracrine Effect
3.3. Extracellular Vesicles
| MSC Effects | Mechanisms | Comments |
|---|---|---|
| Transdifferentiation | Ability to differentiate into the three germ leyers cells. | Ectoderm: epithelial cell, neuron Mesoderm: condrocyte, adipocyte, osteocyte, connective stromal cell Endoderm: muscle cell, gut epithelial cell, lung cell |
| Cell fusion | Ability to fuse with another cell forming a heterokaryon (i.e. multinuclear cell). | |
| Mitochondrial transfer | Ability to transfer mitochondria in damaged cells to increase activity of the respiratory chain complex and ATP levels. | MSC makes contact with the targeted cell and builds a gap junctional channel to transfer mitochondria. |
| Extracellular vesicles | Ability to release microvesicles and/or exososomes containing bioactive molecules, RNA, microRNA, lipids and proteins for intercellular communication. | The interaction of extracellular vesicles with the targeted cell leads to fusion, release and transfer of the vesicles’ components. |
| Paracrine effect | Ability to secrete bioactive cytokines and chemokines that act on immunomodulation, angiogenesis/arteriogenesis, antiapoptosis, antioxidation and cell migration/stimulation. | Examples: IL-6; HGF; IDO; HO-1; TGF; NO; HLA-G5; PGE2; VEGF; FGF; IGF; MCP1; SDF1; PIGF; IL-6; Bcl-2; Akt; STC1; GM-CSF; TNF; GDNF; SCF; LIF; CCL; CXCL. |
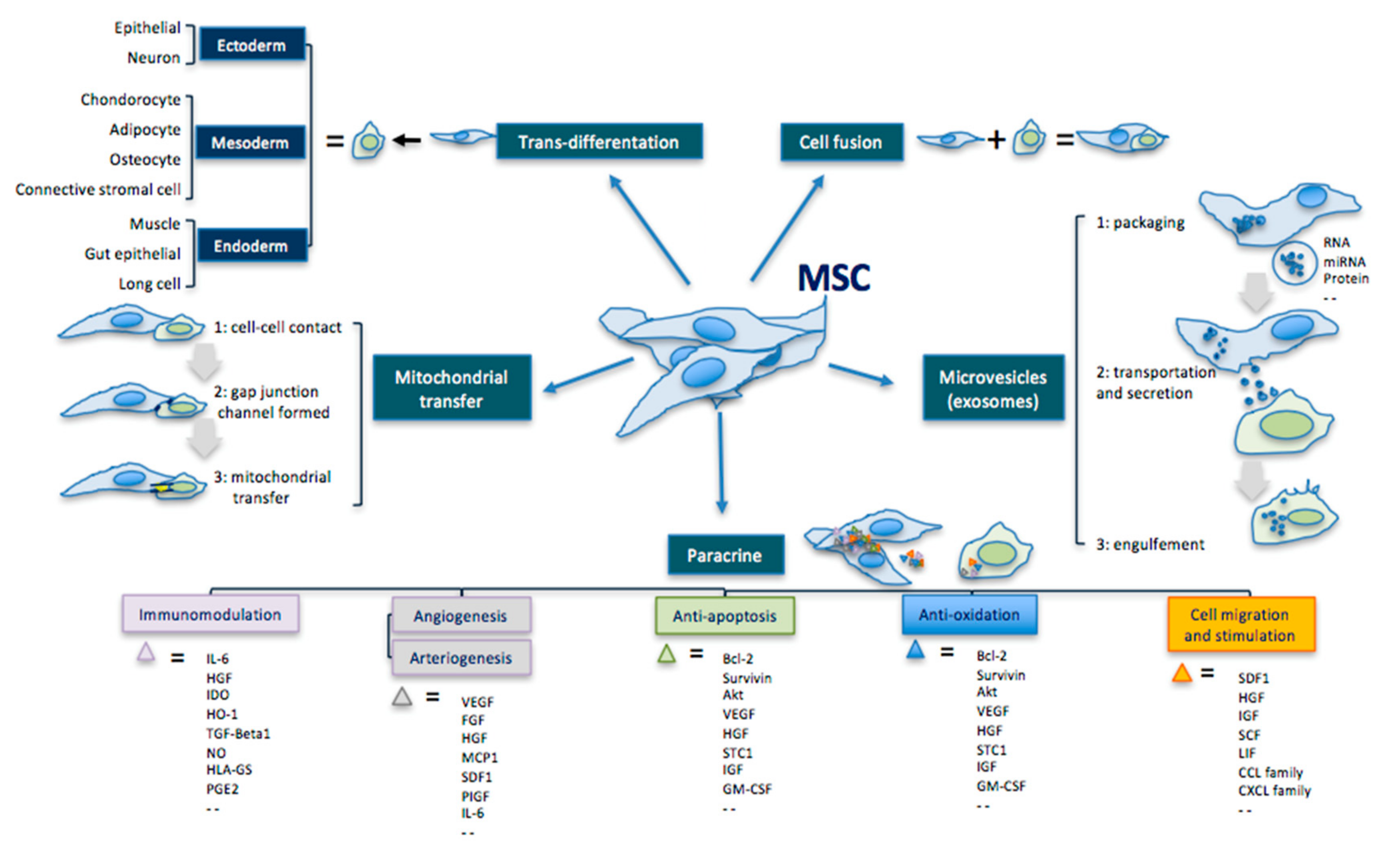
4. Cell-Mediated Biomolecular and Antioxidative Mechanisms in RP
- Hemorheological activity
- Antioxidant activity
- Anti-inflammatory activity
- Anti-apoptotic activity
- Cytoprotective activity
4.1. Hemorheological Activity
4.2. Antioxidant Activity
4.3. Anti-inflammatory Activity
4.4. Antiapoptotic Activity
4.5. Cytoprotective Activity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADSCs | Adipose Derived Stem Cells |
| AMD | Age Macular Disease |
| ASCs | Adipose Stromal Cells |
| BCEA | Bivariate Contour Ellipse Area |
| BCVA | Best Corrected Visual Acuity |
| BDNF | Brain-Derived Neurotrophic Factor |
| bFGF | Basic Fibroblast Growth Factor |
| BM-MSCs | Bone Marrow Mesenchymal Stem Cells |
| CASPasis | Cysteine Aspartate-Specific Proteinases |
| cERG | Cone ERG or Photopic ERG |
| CNS | Central Nervous System |
| CNTF | Ciliary Neurotrophic Factor |
| EGF | Epidermal Growth Factor |
| ER | endoplasmic reticulum |
| ERG | ElectroretinoGram |
| ESCs | Embrionic Stem Cells |
| GAP-43 | Growth-Associated Protein-43 |
| GDNF | Glial Derived Neurotrophic Factor |
| GF | Growth Factor |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| HGF | Hepatocyte Growth Factor |
| HIF-1alpha | Hypoxia-Inducible Factor-1alpha |
| IAP | Inhibitor of Apoptosis Protein |
| IFN-β | Interferon-β |
| IGF-1 | Insulin-like Growth Factor-1 |
| IL-1RA | IL-1 Receptor Antagonist |
| IL | Interleukin |
| IRD | Inherited Retinal Disease |
| M-CSF | Macrophage Colony-Stimulating Factor |
| MAPK1 | Mitogen-Activated Protein Kinase |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MSCs | Mesenchymal Stem Cells |
| PDGF | Platelet-Derived Growth Factor |
| PDAF | Platelet-Derived Angiogenesis Factor |
| PEDF | Pigment-Epithelium-Derived Factor |
| PGE2R | Prostaglandin E2 Receptor |
| PI3-K | Phosphatidylinositol-3-Kinase |
| PlGF | Placental Growth Factor |
| POS | Photoreceptor Segments |
| PRP | Platelet-Rich Plasma |
| PRDX2 | Peroxiredoxin 2 |
| RdCVF | Rod Cone Viability Factor |
| RGC | Retinal Ganglion Cell |
| RMG | Retinal Müller Glia |
| ROS | Reactive Oxygen Species |
| RP | Retinitis Pigmentosa |
| RPE | Retinal Pigment Epithelium |
| SOD | Superoxide Dismutase |
| SVF | Stromal Vascular Fraction |
| TGF- | Transforming Growth Factor- |
| TNF-alpha | Tumoral Necrosis Factor—alpha |
| TSP | Thrombospondin |
| UPR | Unfolded Protein Response |
| VEGF | Vascular Endothelial Growth Factor |
References
- Pagon, R.A. Retinitis pigmentosa. Surv. Ophthalmol. 1988, 33, 137–177. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Hamel, C.P. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Ikeda, Y.; Nakatake, S.; Miller, J.W.; Vavvas, D.G.; Sonoda, K.H.; Ishibashi, T. Necrotic cone photoreceptor cell death in retinitis pigmentosa. Cell Death Dis. 2015, 6, e2038. [Google Scholar] [CrossRef] [Green Version]
- Aït-Ali, N.; Fridlich, R.; Millet-Puel, G.; Clérin, E.; Delalande, F.; Jaillard, C.; Blond, F.; Perrocheau, L.; Reichman, S.; Byrne, L.C.; et al. Rod-Derived Cone Viability Factor Promotes Cone Survival by Stimulating Aerobic Glycolysis. Cell 2015, 161, 817–832. [Google Scholar] [CrossRef] [Green Version]
- Campochiaro, P.A.; Mir, T.A. The mechanism of cone cell death in Retinitis Pigmentosa. Prog. Retin. Eye Res. 2018, 62, 24–37. [Google Scholar] [CrossRef]
- Yang, Y.J.; Peng, J.; Ying, D.; Peng, Q. A Brief Review on the Pathological Role of Decreased Blood Flow Affected in Retinitis Pigmentosa. J. Ophthalmol. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Yang, X.; Dong, A.; Petters, R.M.; Peng, Y.-W.; Wong, F.; Campochiaro, P.A. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell. Physiol. 2005, 203, 457–464. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Strauss, R.W.; Lu, L.; Hafiz, G.; Wolfson, Y.; Shah, S.M.; Sophie, R.; Mir, T.A.; Scholl, H.P. Is There Excess Oxidative Stress and Damage in Eyes of Patients with Retinitis Pigmentosa? Antioxid. Redox Signal. 2015, 23, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Punzo, C.; Xiong, W.; Cepko, C.L. Loss of Daylight Vision in Retinal Degeneration: Are Oxidative Stress and Metabolic Dysregulation to Blame? J. Biol. Chem. 2014, R111, 304428. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.-L.; Mérida, S.; Bosch-Morell, F.; Miranda, M.; Villar, V.M. Autophagy Dysfunction and Oxidative Stress, Two Related Mechanisms Implicated in Retinitis Pigmentosa. Front. Physiol. 2018, 9, 1008. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Scimone, C.; Nicocia, G.; D’Angelo, R.; Sidoti, A. Retracted Article: Role of oxidative stress in Retinitis pigmentosa: New involved pathways by an RNA-Seq analysis. Cell Cycle 2018, 18, 84–104. [Google Scholar] [CrossRef] [Green Version]
- Agbaga, M.-P.; Merriman, D.K.; Brush, R.S.; Lydic, T.A.; Conley, S.M.; Naash, M.I.; Jackson, S.; Woods, A.S.; Reid, G.E.; Busik, J.V.; et al. Differential composition of DHA and very-long-chain PUFAs in rod and cone photoreceptors. J. Lipid Res. 2018, 59, 1586–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birtel, J.; Gliem, M.; Oishi, A.; Müller, P.; Herrmann, P.; Holz, F.G.; Mangold, E.; Knapp, M.; Bolz, H.J.; Issa, P.C. Genetic testing in patients with retinitis pigmentosa: Features of unsolved cases. Clin. Exp. Ophthalmol. 2019, 47, 779–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audo, I.; Mohand-Saïd, S.; Boulanger-Scemama, E.; Zanlonghi, X.; Condroyer, C.; Demontant, V.; Boyard, F.; Antonio, A.; Méjécase, C.; El Shamieh, S.; et al. MERTK mutation update in inherited retinal diseases. Hum. Mutat. 2018, 39, 887–913. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Donato, L.; Esposito, T.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. A novel RLBP1 gene geographical area-related mutation present in a young patient with retinitis punctata albescens. Hum. Genom. 2017, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Utz, V.M.; Coussa, R.G.; Antaki, F.; Traboulsi, E.I. Gene therapy for RPE65-related retinal disease. Ophthalmic Genet. 2018, 39, 671–677. [Google Scholar] [CrossRef]
- Hicks, D.; Hamel, C.P. The Retinal Pigment Epithelium in Health and Disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef]
- Nowak, J.Z. Oxidative stress, polyunsaturated fatty acids-derived oxidation products and bisretinoids as potential inducers of CNS diseases: Focus on age-related macular degeneration. Pharmacol. Rep. 2013, 65, 288–304. [Google Scholar] [CrossRef]
- Beutelspacher, S.C.; Serbecic, N.; Barash, H.; Burgansky-Eliash, Z.; Grinvald, A.; Krastel, H.; Jonas, J.B. Retinal blood flow velocity measured by retinal function imaging in retinitis pigmentosa. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1855–1858. [Google Scholar] [CrossRef]
- Langmann, T. Microglia activation in retinal degeneration. J. Leukoc. Biol. 2007, 81, 1345–1351. [Google Scholar] [CrossRef]
- Otani, A.; Dorrell, M.I.; Kinder, K.; Moreno, S.K.; Nusinowitz, S.; Banin, E.; Heckenlively, J.; Friedlander, M. Rescue of retinal degeneration by intravitreally injected adult bone marrow–derived lineage-negative hematopoietic stem cells. J. Clin. Investig. 2004, 114, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.-Q.; Aleman, T.S.; Dejneka, N.S.; Dudus, L.; Fisher, K.J.; Maguire, A.M.; Jacobson, S.G.; Bennett, J. Long-Term Protection of Retinal Structure but Not Function Using RAAV.CNTF in Animal Models of Retinitis Pigmentosa. Mol. Ther. 2001, 4, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, V.; Novelli, E.; Strettoi, E. Environmental enrichment reduces photoreceptor degeneration and retinal inflammation in a mouse model of retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4261. [Google Scholar]
- He, Y.; Zhang, Y.; Liu, X.; Ghazaryan, E.; Li, Y.; Xie, J.; Su, G. Recent Advances of Stem Cell Therapy for Retinitis Pigmentosa. Int. J. Mol. Sci. 2014, 15, 14456–14474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, B.A.; Mullins, R.F.; Stone, E.M. Stem cells for investigation and treatment of inherited retinal disease. Hum. Mol. Genet. 2014, 23, R9–R16. [Google Scholar] [CrossRef] [Green Version]
- Xue, C.; Rosen, R.B.; Jordan, A.; Hu, D.-N. Management of Ocular Diseases Using Lutein and Zeaxanthin: What Have We Learned from Experimental Animal Studies? J. Ophthalmol. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Strettoi, E.; Gargini, C.; Novelli, E.; Sala, G.; Piano, I.; Gasco, P.; Ghidoni, R. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2010, 107, 18706–18711. [Google Scholar] [CrossRef] [Green Version]
- Lavail, M.M.; Nishikawa, S.; Steinberg, R.H.; Naash, M.I.; Duncan, J.L.; Trautmann, N.; Matthes, M.T.; Yasumura, D.; Lau-Villacorta, C.; Chen, J.; et al. Phenotypic characterization of P23H and S334ter rhodopsin transgenic rat models of inherited retinal degeneration. Exp. Eye Res. 2018, 167, 56–90. [Google Scholar] [CrossRef]
- Takahashi, M.; Miyoshi, H.; Verma, I.M.; Gage, F.H. Rescue from Photoreceptor Degeneration in therd Mouse by Human Immunodeficiency Virus Vector-Mediated Gene Transfer. J. Virol. 1999, 73, 7812–7816. [Google Scholar] [CrossRef] [Green Version]
- Ali, R.R.; Sarra, G.-M.; Stephens, C.; De Alwis, M.; Bainbridge, J.W.; Munro, P.M.; Fauser, S.; Reichel, M.B.; Kinnon, C.; Hunt, D.M.; et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat. Genet. 2000, 25, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Vollrath, D.; Feng, W.; Duncan, J.L.; Yasumura, D.; D’Cruz, P.M.; Chappelow, A.; Matthes, M.T.; Kay, M.A.; Lavail, M.M. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc. Natl. Acad. Sci. USA 2001, 98, 12584–12589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acland, G.M.; Aguirre, G.D.; Ray, J.; Zhang, Q.; Aleman, T.S.; Cideciyan, A.V.; Pearce-Kelling, S.E.; Anand, V.; Zeng, Y.; Maguire, A.M.; et al. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 2001, 28, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Arnal, E.; Ahuja, S.; Alvarez-Nölting, R.; López-Pedrajas, R.; Ekström, P.; Bosch-Morell, F.; Van Veen, T.; Romero, F.J. Antioxidants rescue photoreceptors in rd1 mice: Relationship with thiol metabolism. Free. Radic. Biol. Med. 2010, 48, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Komeima, K.; Rogers, B.S.; Lu, L.; Campochiaro, P.A. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. USA 2006, 103, 11300–11305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komeima, K.; Usui, S.; Shen, J.; Rogers, B.S.; Campochiaro, P.A. Blockade of neuronal nitric oxide synthase reduces cone cell death in a model of retinitis pigmentosa. Free Radic. Biol. Med. 2008, 45, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Sanz, M.; Johnson, L.; Ahuja, S.P.; Ekström, P.; Romero, J.; Van Veen, T. Significant photoreceptor rescue by treatment with a combination of antioxidants in an animal model for retinal degeneration. Neuroscience 2007, 145, 1120–1129. [Google Scholar] [CrossRef]
- Fernández-Sánchez, L.; Lax, P.; Campello, L.; Pinilla, I.; Cuenca, N. Astrocytes and Müller Cell Alterations during Retinal Degeneration in a Transgenic Rat Model of Retinitis Pigmentosa. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Sánchez, L.; Esquiva, G.; Pinilla, I.; Lax, P.; Cuenca, N. Retinal Vascular Degeneration in the Transgenic P23H Rat Model of Retinitis Pigmentosa. Front. Neuroanat. 2018, 12. [Google Scholar] [CrossRef]
- Frasson, M.; Picaud, S.; Léveillard, T.; Simonutti, M.; Mohand-Said, S.; Dreyfus, H.; Hicks, D.; Sabel, J. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2724–2734. [Google Scholar]
- Bush, R.A.; Lei, B.; Tao, W.; Raz, D.; Chan, C.-C.; Cox, T.A.; Santos-Muffley, M.; Sieving, P.A. Encapsulated cell-based intraocular delivery of ciliary neurotrophic factor in normal rabbit: Dose-dependent effects on ERG and retinal histology. Investig. Opthalmol. Vis. Sci. 2004, 45, 2420–2430. [Google Scholar] [CrossRef] [Green Version]
- Uteza, Y.; Rouillot, J.-S.; Kobetz, A.; Marchant, D.; Pecqueur, S.; Arnaud, E.; Prats, H.; Honiger, J.; Dufier, J.-L.; Abitbol, M.; et al. Intravitreous transplantation of encapsulated fibroblasts secreting the human fibroblast growth factor 2 delays photoreceptor cell degeneration in Royal College of Surgeons rats. Proc. Natl. Acad. Sci. USA 1999, 96, 3126–3131. [Google Scholar] [CrossRef] [Green Version]
- Frasson, M.; Sahel, J.-A.; Fabre, M.; Simonutti, M.; Dreyfus, H.; Picaud, S. Retinitis pigmentosa: Rod photoreceptor rescue by a calcium-channel blocker in the rd mouse. Nat. Med. 1999, 5, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.E. Bone marrow–derived stem cells preserve cone vision in retinitis pigmentosa. J. Clin. Investig. 2004, 114, 755–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samardzija, M.; Todorova, V.; Gougoulakis, L.; Barben, M.; Nötzli, S.; Klee, K.; Storti, F.; Gubler, A.; Imsand, C.; Grimm, C. Light stress affects cones and horizontal cells via rhodopsin-mediated mechanisms. Exp. Eye Res. 2019, 186, 107719. [Google Scholar] [CrossRef] [PubMed]
- Rohowetz, L.J.; Kraus, J.G.; Koulen, P. Reactive Oxygen Species-Mediated Damage of Retinal Neurons: Drug Development Targets for Therapies of Chronic Neurodegeneration of the Retina. Int. J. Mol. Sci. 2018, 19, 3362. [Google Scholar] [CrossRef] [Green Version]
- Domènech, E.B.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedić, O.; Rattan, S.I.; Grune, T.; Trougakos, I.P. Molecular effects of advanced glycation end products on cell signalling pathways, ageing and pathophysiology. Free Radic. Res. 2013, 47, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Kajdanek, J.; Morawiec, J.; Pawlowska, E.; Blasiak, J. PGC-1α Protects RPE Cells of the Aging Retina against Oxidative Stress-Induced Degeneration through the Regulation of Senescence and Mitochondrial Quality Control. The Significance for AMD Pathogenesis. Int. J. Mol. Sci. 2018, 19, 2317. [Google Scholar] [CrossRef] [Green Version]
- Honda, S.; Hjelmeland, L.M.; Handa, J.T. Oxidative stress—Induced single-strand breaks in chromosomal telomeres of human retinal pigment epithelial cells in vitro. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2139–2144. [Google Scholar]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Koskela, A.; Felszeghy, S.; Kivinen, N.; Salminen, A.; Kauppinen, A. Fatty acids and oxidized lipoproteins contribute to autophagy and innate immunity responses upon the degeneration of retinal pigment epithelium and development of age-related macular degeneration. Biochimie 2019, 159, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogasawara, M.; Zhang, H. Redox Regulation and Its Emerging Roles in Stem Cells and Stem-Like Cancer Cells. Antioxid. Redox Signal. 2009, 11, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Benhar, M. Oxidants, Antioxidants and Thiol Redox Switches in the Control of Regulated Cell Death Pathways. Antioxidants 2020, 9, 309. [Google Scholar] [CrossRef] [Green Version]
- Saccà, S.S.; Roszkowska, A.M.; Izzotti, A. Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases. Mutat. Res. Mutat. Res. 2013, 752, 153–171. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Nicocia, G.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. Discovery of GLO1 New Related Genes and Pathways by RNA-Seq on A2E-Stressed Retinal Epithelial Cells Could Improve Knowledge on Retinitis Pigmentosa. Antioxidants 2020, 9, 416. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta BBA Bioenerg. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, J.J.; Yu, Q.; Wang, M.; Zhang, S.X. Endoplasmic reticulum stress is implicated in retinal inflammation and diabetic retinopathy. FEBS Lett. 2009, 583, 1521–1527. [Google Scholar] [CrossRef] [Green Version]
- Anderson, P.J.; Kedersha, N. Stress granules. Curr. Biol. 2009, 19, R397–R398. [Google Scholar] [CrossRef] [Green Version]
- Kunchithapautham, K.; Rohrer, B. Apoptosis and Autophagy in Photoreceptors Exposed to Oxidative Stress. Autophagy 2007, 3, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-J.; Kuang, H.Y. Oxidative stress induces autophagy in response to multiple noxious stimuli in retinal ganglion cells. Autophagy 2014, 10, 1692–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Fuhrmann, S.; Zou, C.; Levine, E.M. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp. Eye Res. 2014, 123, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular Stress Responses: Cell Survival and Cell Death. Int. J. Cell Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Berghe, T.V.; Vandenabeele, P.; Kroemer, G. The molecular machinery of regulated cell death. Cell Res. 2019, 29, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Langham, M.E.; Kramer, T. Decreased choroidal blood flow associated with retinitis pigmentosa. Eye 1990, 4, 374–381. [Google Scholar] [CrossRef]
- Murakami, Y.; Ikeda, Y.; Akiyama, M.; Fujiwara, K.; Yoshida, N.; Nakatake, S.; Notomi, S.; Nabeshima, T.; Hisatomi, T.; Enaida, H.; et al. Correlation between macular blood flow and central visual sensitivity in retinitis pigmentosa. Acta Ophthalmol. 2015, 93, e644–e648. [Google Scholar] [CrossRef]
- Marc, R.E.; Jones, B. Retinal Remodeling in Inherited Photoreceptor Degenerations. Mol. Neurobiol. 2003, 28, 139–148. [Google Scholar] [CrossRef]
- Peng, Q.; Zhu, W.; Li, C. A research on the mechanism of pigmentary degeneration of retina belonging to deficiency complicated with blood stasis. Jiangsu Tradit. Chin. Med. 1990, 1, 39–41. [Google Scholar]
- Ayton, L.N.; Guymer, R.; Luu, C.D. Choroidal thickness profiles in retinitis pigmentosa. Clin. Exp. Ophthalmol. 2012, 41. [Google Scholar] [CrossRef] [PubMed]
- Falsini, B.; Anselmi, G.M.; Marangoni, D.; D’Esposito, F.; Fadda, A.; Di Renzo, A.; Campos, E.C.; Riva, C.E. Subfoveal Choroidal Blood Flow and Central Retinal Function in Retinitis Pigmentosa. Investig. Opthalmol. Vis. Sci. 2011, 52, 1064–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bill, A.; Sperber, G.; Ujiie, K. Physiology of the choroidal vascular bed. Int. Ophthalmol. 1983, 6, 101–107. [Google Scholar] [CrossRef]
- Lieberthal, W.; Triaca, V.; Koh, J.S.; Pagano, P.J.; Levine, J.S. Role of superoxide in apoptosis induced by growth factor withdrawal. Am. J. Physiol. Content 1998, 275, F691–F702. [Google Scholar] [CrossRef]
- Yu, D.-Y.; Cringle, S.; Valter, K.; Walsh, N.; Lee, D.; Stone, J. Photoreceptor Death, Trophic Factor Expression, Retinal Oxygen Status, and Photoreceptor Function in the P23H Rat. Investig. Opthalmol. Vis. Sci. 2004, 45, 2013–2019. [Google Scholar] [CrossRef]
- Yu, D.-Y.; Cringle, S.J. Retinal degeneration and local oxygen metabolism. Exp. Eye Res. 2005, 80, 745–751. [Google Scholar] [CrossRef]
- Jain, S.; Thakkar, N.; Chhatai, J.; Bhadra, M.P.; Bhadra, U. Long non-coding RNA: Functional agent for disease traits. RNA Biol. 2016, 14, 522–535. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. Transcriptome Analyses of lncRNAs in A2E-Stressed Retinal Epithelial Cells Unveil Advanced Links between Metabolic Impairments Related to Oxidative Stress and Retinitis Pigmentosa. Antioxidants 2020, 9, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, A.B. Reactive Oxygen Species and Cell Signaling in Lung Ischemia. Cell Signal. Vasc. Inflamm. 2007, 31, 125–135. [Google Scholar] [CrossRef]
- Türksever, C.; Valmaggia, C.; Orgül, S.; Schorderet, D.F.; Flammer, J.; Todorova, M. Retinal vessel oxygen saturation and its correlation with structural changes in retinitis pigmentosa. Acta Ophthalmol. 2014, 92, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Scimone, C.; Nicocia, G.; Denaro, L.; Robledo, R.; Sidoti, A.; D’Angelo, R. GLO1 gene polymorphisms and their association with retinitis pigmentosa: A case–control study in a Sicilian population. Mol. Biol. Rep. 2018, 45, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Cerman, E.; Akkoc, T.; Eraslan, M.; Şahin, Ö.; Ozkara, S.; Aker, F.V.; Subaşı, C.; Karaoz, E.; Akkoç, T. Correction: Retinal Electrophysiological Effects of Intravitreal Bone Marrow Derived Mesenchymal Stem Cells in Streptozotocin Induced Diabetic Rats. PLoS ONE 2016, 11, e0165219. [Google Scholar] [CrossRef] [Green Version]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Zeiss, C.; Johnson, E.A. Proliferation of microglia, but not photoreceptors, in the outer nuclear layer of the rd-1 mouse. Investig. Opthalmol. Vis. Sci. 2004, 45, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Brown, K.E.; Milam, A.H. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp. Eye Res. 2003, 76, 463–471. [Google Scholar] [CrossRef]
- Zeng, H.-Y.; Zhu, X.-A.; Zhang, C.; Yang, L.-P.; Wu, L.-M.; Tso, M.O.M. Identification of Sequential Events and Factors Associated with Microglial Activation, Migration, and Cytotoxicity in Retinal Degeneration inrdMice. Investig. Opthalmol. Vis. Sci. 2005, 46, 2992–2999. [Google Scholar] [CrossRef] [Green Version]
- Detrick, B.; Hooks, J.J. The RPE Cell and the Immune System. In Retinal Pigment Epithelium in Health and Disease; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; pp. 101–114. [Google Scholar]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in Retinal Degeneration. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Banati, R.B.; Gehrmann, J.; Schubert, P.; Kreutzberg, G.W. Cytotoxicity of microglia. Glia 1993, 7, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Boje, K.M.; Arora, P.K. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992, 587, 250–256. [Google Scholar] [CrossRef]
- Zhao, L.; Zabel, M.K.; Wang, X.; Ma, W.; Shah, P.; Fariss, R.N.; Qian, H.; Parkhurst, C.N.; Gan, W.; Wong, W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 2015, 7, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Xiao, J.; Wang, K.; So, K.F.; Tipoe, G.L.; Lin, B. Suppression of Microglial Activation Is Neuroprotective in a Mouse Model of 21. Human Retinitis Pigmentosa. J. Neurosci. 2014, 34, 8139–8150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subirada, P.V.; Paz, M.C.; Ridano, M.E.; Lorenc, V.E.; Vaglienti, M.V.; Barcelona, P.F.; Luna, J.D.; Sánchez, M.C. A journey into the retina: Müller glia commanding survival and death. Eur. J. Neurosci. 2018, 47, 1429–1443. [Google Scholar] [CrossRef]
- Klassen, H. Stem cells in clinical trials for treatment of retinal degeneration. Expert Opin. Biol. Ther. 2015, 16, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Idelson, M.; Alper, R.; Obolensky, A.; Ben-Shushan, E.; Hemo, I.; Yachimovich-Cohen, N.; Khaner, H.; Smith, Y.; Wiser, O.; Gropp, M.; et al. Directed Differentiation of Human Embryonic Stem Cells into Functional Retinal Pigment Epithelium Cells. Cell Stem Cell 2009, 5, 396–408. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Yamanaka, S. Induced pluripotent stem cells in medicine and biology. Development 2013, 140, 2457–2461. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.L.S.; Kumar, S.; Mok, P.L. Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases. Int. J. Mol. Sci. 2017, 18, 1406. [Google Scholar] [CrossRef]
- Huo, D.-M.; Dong, F.-T.; Yu, W.-H.; Gao, F. Differentiation of mesenchymal stem cell in the microenviroment of retinitis pigmentosa. Int. J. Ophthalmol. 2010, 3, 216–219. [Google Scholar]
- Zarbin, M. Cell-Based Therapy for Degenerative Retinal Disease. Trends Mol. Med. 2016, 22, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.C.; Messias, A.; Voltarelli, J.C.; Scott, I.U.; Jorge, R. Intravitreal injection of autologous bone marrow–derived mononuclear cells for hereditary retinal dystrophy. Retina 2011, 31, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.C.; Messias, A.; Messias, K.; Arcieri, R.S.; Ruiz, M.A.; Souza, N.F.; Martins, L.C.; Jorge, R. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell-clinical trial). Stem Cell Res. Ther. 2015, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.; Zawadzki, R.J.; Werner, J.S.; Nolta, J. Intravitreal Autologous Bone Marrow CD34+ Cell Therapy for Ischemic and Degenerative Retinal Disorders: Preliminary Phase 1 Clinical Trial Findings. Investig. Opthalmol. Vis. Sci. 2014, 56, 81–89. [Google Scholar] [CrossRef]
- Jones, M.K.; Lu, B.; Girman, S.; Wang, S. Cell-based therapeutic strategies for replacement and preservation in retinal degenerative diseases. Prog. Retin. Eye Res. 2017, 58, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Romanov, Y.A.; Darevskaya, A.N.; Merzlikina, N.V.; Buravkova, L.B. Mesenchymal Stem Cells from Human Bone Marrow and Adipose Tissue: Isolation, Characterization, and Differentiation Potentialities. Bull. Exp. Biol. Med. 2005, 140, 138–143. [Google Scholar] [CrossRef]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The Potential of Adipose Stem Cells in Regenerative Medicine. Stem Cell Rev. Rep. 2010, 7, 269–291. [Google Scholar] [CrossRef]
- Öner, A.; Sevim, D.G. Complications of stem cell-based therapies in retinal diseases. Stem Cell Res. Open Library 2017, 1, 1–7. [Google Scholar]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bara, J.J.; Richards, R.G.; Alini, M.; Stoddart, M.J. Concise Review: Bone Marrow-Derived Mesenchymal Stem Cells Change Phenotype Following In Vitro Culture: Implications for Basic Research and the Clinic. Stem Cells 2014, 32, 1713–1723. [Google Scholar] [CrossRef]
- Baddour, J.A.; Sousounis, K.; Tsonis, P.A. Organ repair and regeneration: An overview. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Ruster, B.; Gottig, S.; Ludwig, R.J.; Bistrian, R.; Muller, S.; Seifried, E.; Gille, J.; Henschler, R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood 2006, 108, 3938–3944. [Google Scholar] [CrossRef]
- De Becker, A.; Van Hummelen, P.; Bakkus, M.; Broek, I.V.; De Wever, J.; De Waele, M.; Van Riet, I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica 2007, 92, 440–449. [Google Scholar] [CrossRef]
- Luo, S.; Hao, L.; Li, X.; Yu, N.; Diao, Z.; Ren, L.; Xu, H. Adipose tissue-derived stem cells treated with estradiol enhance survival of autologous fat transplants. Tohoku J. Exp. Med. 2013, 231, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.-L.; Li, N.; Wei, X.; Tang, L.; Wang, T.-H.; Chen, X.-M. Neuroprotective effects of BDNF and GDNF in intravitreally transplanted mesenchymal stem cells after optic nerve crush in mice. Int. J. Ophthalmol. 2017, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Mariman, E.; Renes, J.; Keijer, J. The secretory function of adipocytes in the physiology of white adipose tissue. J. Cell. Physiol. 2008, 216, 3–13. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Nakagami, H.; Morishita, R.; Maeda, K.; Kikuchi, Y.; Ogihara, T.; Kaneda, Y. Adipose Tissue-Derived Stromal Cells as a Novel Option for Regenerative Cell Therapy. J. Atheroscler. Thromb. 2006, 13, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Schäffler, A.; Buchler, C.H. Concise Review: Adipose Tissue-Derived Stromal Cells-Basic and Clinical Implications for Novel Cell-Based Therapies. Stem Cells 2007, 25, 818–827. [Google Scholar] [CrossRef]
- Jurk, K.; Kehrel, B.E. Platelets: Physiology and Biochemistry. Semin. Thromb. Hemost. 2005, 31, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Velotta, J.; Brinton, T.J.; Wang, X.; Chang, S.; Palmer, O.; Sheikh, A.; Chung, J.; Yang, P.C.-M.; Robbins, R.; et al. RevaTen platelet-rich plasma improves cardiac function after myocardial injury. Cardiovasc. Revascularization Med. 2011, 12, 158–163. [Google Scholar] [CrossRef]
- Qureshi, A.H.; Chaoji, V.; Maiguel, D.; Faridi, M.H.; Barth, C.J.; Salem, S.M.; Singhal, M.; Stoub, D.; Krastins, B.; Ogihara, M.; et al. Proteomic and Phospho-Proteomic Profile of Human Platelets in Basal, Resting State: Insights into Integrin Signaling. PLoS ONE 2009, 4, e7627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborne, A.; Sanderson, J.; Martin, K.R. Neuroprotective Effects of Human Mesenchymal Stem Cells and Platelet-Derived Growth Factor on Human Retinal Ganglion Cells. Stem Cells 2017, 36, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lykov, A.P.; Poveshchenk, O.V.; Surovtseva, M.A.; Stanishevskaya, O.M.; Chernykh, D.V.; Arben’Eva, N.S.; Bratko, V. Autologous Plasma Enriched with Platelet Lysate for the Treatment of Idiopathic Age-Related Macular Degeneration: A Prospective Study. Ann. Russ. Acad. Med. Sci. 2018, 73, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Arslan, U.; Özmert, E.; Demirel, S.; Örnek, F.; Şermet, F. Effects of subtenon-injected autologous platelet-rich plasma on visual functions in eyes with retinitis pigmentosa: Preliminary clinical results. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 893–908. [Google Scholar] [CrossRef]
- Siqueira, R.C.; Messias, A.; Gurgel, V.P.; Simões, B.P.; Scott, I.U.; Jorge, R. Improvement of ischaemic macular oedema after intravitreal injection of autologous bone marrow-derived haematopoietic stem cells. Acta Ophthalmol. 2014, 93, 174–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öner, A.; Gonen, Z.B.; Sinim, N.; Cetin, M.; Ozkul, Y. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: A phase I clinical safety study. Stem Cell Res. Ther. 2016, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Kahraman, N.S.; Öner, A. Umbilical cord derived mesenchymal stem cell implantation in retinitis pigmentosa: A 6-month follow-up results of a phase 3 trial. Int J Ophthalmol. 2020, 13, 1423–1429. [Google Scholar] [CrossRef]
- Limoli, P.G.; Vingolo, E.M.; Morales, M.U.; Nebbioso, M.; Limoli, C. Preliminary Study on Electrophysiological Changes after Cellular Autograft in Age-Related Macular Degeneration. Medicine 2014, 93, e355. [Google Scholar] [CrossRef]
- Limoli, P.G.; Vingolo, E.M.; Limoli, C.; Nebbioso, M. Stem Cell Surgery and Growth Factors in Retinitis Pigmentosa Patients: Pilot Study after Literature Review. Biomedicines 2019, 7, 94. [Google Scholar] [CrossRef] [Green Version]
- Limoli, P.G.; Limoli, C.S.S.; Morales, M.U.; Vingolo, E.M. Mesenchymal stem cell surgery, rescue and regeneration in retinitis pigmentosa: Clinical and rehabilitative prognostic aspects. Restor. Neurol. Neurosci. 2020, 38, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limoli, P.G.; Limoli, C.; Vingolo, E.M.; Scalinci, S.Z.; Nebbioso, M. Cell surgery and growth factors in dry age-related macular degeneration: Visual prognosis and morphological study. Oncotarget 2016, 7, 46913–46923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limoli, P.G.; Vingolo, E.M.; Limoli, C.; Scalinci, S.Z.; Nebbioso, M. Regenerative Therapy by Suprachoroidal Cell Autograft in Dry Age-related Macular Degeneration: Preliminary In Vivo Report. J. Vis. Exp. 2018, 12, e56469. [Google Scholar] [CrossRef] [PubMed]
- Öner, A. Stem Cell Treatment in Retinal Diseases: Recent Developments. Turk. J. Ophthalmol. 2018, 48, 33–38. [Google Scholar] [CrossRef]
- Mok, P.L.; Leong, C.F.; Cheong, S.-K. Cellular mechanisms of emerging applications of mesenchymal stem cells. Malays. J. Pathol. 2013, 35, 17–32. [Google Scholar]
- Kim, K.-S.; Park, J.-M.; Kong, T.; Kim, C.; Bae, S.-H.; Kim, H.W.; Moon, J. Retinal Angiogenesis Effects of TGF-β1 and Paracrine Factors Secreted from Human Placental Stem Cells in Response to a Pathological Environment. Cell Transplant. 2016, 25, 1145–1157. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.-T.; Zhang, L.-J.; Shao, H.; Bai, L.-L.; Yu, B.; Su, C.; Dong, L.-J.; Liu, X.; Li, X.; Zhang, X. Therapeutic effects of mesenchymal stem cells administered at later phase of recurrent experimental autoimmune uveitis. Int. J. Ophthalmol. 2016, 9, 1381–1389. [Google Scholar] [CrossRef]
- Salehi, H.; Amirpour, N.; Razavi, S.; Esfandiari, E.; Zavar, R. Overview of retinal differentiation potential of mesenchymal stem cells: A promising approach for retinal cell therapy. Ann. Anat. Anat. Anz. 2017, 210, 52–63. [Google Scholar] [CrossRef]
- Moraes, L.; Vasconcelos-Dos-Santos, A.; Santana, F.C.; Godoy, M.A.; Rosado-De-Castro, P.H.; Jasmin; Azevedo-Pereira, R.L.; Cintra, W.M.; Gasparetto, E.L.; Santiago, M.F.; et al. Neuroprotective effects and magnetic resonance imaging of mesenchymal stem cells labeled with SPION in a rat model of Huntington’s disease. Stem Cell Res. 2012, 9, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Kyurkchiev, D. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef]
- Johnson, T.V.; DeKorver, N.W.; Levasseur, V.A.; Osborne, A.; Tassoni, A.; Lorber, B.; Heller, J.P.; Villasmil, R.; Bull, N.D.; Martin, K.R.; et al. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain 2013, 137, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Paracrine-Mediated Neuroprotection and Neuritogenesis of Axotomised Retinal Ganglion Cells by Human Dental Pulp Stem Cells: Comparison with Human Bone Marrow and Adipose-Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e109305. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siniscalco, D.; Giordano, C.; Galderisi, U.; Luongo, L.; Alessio, N.; Di Bernardo, G.; De Novellis, V.; Rossi, F.; Maione, S. Intra-brain microinjection of human mesenchymal stem cells decreases allodynia in neuropathic mice. Cell. Mol. Life Sci. 2009, 67, 655–669. [Google Scholar] [CrossRef]
- Rezanejad, H.; Soheili, Z.-S.; Haddad, F.; Matin, M.M.; Samiei, S.; Manafi, A.; Ahmadieh, H. In vitro differentiation of adipose-tissue-derived mesenchymal stem cells into neural retinal cells through expression of human PAX6 (5a) gene. Cell Tissue Res. 2014, 356, 65–75. [Google Scholar] [CrossRef]
- Emre, E.; Yüksel, N.; Duruksu, G.; Pirhan, D.; Subaşi, C.; Erman, G.; Karaöz, E. Neuroprotective effects of intravitreally transplanted adipose tissue and bone marrow–derived mesenchymal stem cells in an experimental ocular hypertension model. Cytotherapy 2015, 17, 543–559. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef] [Green Version]
- Garcia, T.B.; Hollborn, M.; Bringmann, A. Expression and signaling of NGF in the healthy and injured retina. Cytokine Growth Factor Rev. 2017, 34, 43–57. [Google Scholar] [CrossRef]
- Sluch, V.M.; Zack, D.J. Stem cells, retinal ganglion cells and glaucoma. Dev. Ophthalmol. 2014, 53, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Mesentier-Louro, L.A.; Zaverucha-Do-Valle, C.; Rosado-De-Castro, P.H.; Silva-Junior, A.J.; Pimentel-Coelho, P.M.; Mendez-Otero, R.; Santiago, M.F. Bone Marrow-Derived Cells as a Therapeutic Approach to Optic Nerve Diseases. Stem Cells Int. 2016, 2016, 5078619. [Google Scholar] [CrossRef] [Green Version]
- Lai, R.C.; Yeo, R.W.Y.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyse, R.D.; Dunbar, G.L.; Rossignol, J. Use of Genetically Modified Mesenchymal Stem Cells to Treat Neurodegenerative Diseases. Int. J. Mol. Sci. 2014, 15, 1719–1745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, srep34562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.-F.; Lian, Q. Paracrine Mechanisms of Mesenchymal Stem Cell-Based Therapy: Current Status and Perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef] [Green Version]
- Ezquer, M.; Urzua, C.A.; Montecino, S.; Leal, K.; Conget, P.A.; Ezquer, F. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res. Ther. 2016, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Kinnaird, T.; Stabile, E.; Burnett, J.C.; Shou, M.; Lee, C.; Barr, S.; Fuchs, S.; Epstein, S. Local Delivery of Marrow-Derived Stromal Cells Augments Collateral Perfusion Through Paracrine Mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar] [CrossRef]
- Gao, F.; Hou, H.; Liang, H.; Weinreb, R.N.; Wang, H.; Wang, Y. Bone marrow-derived cells in ocular neovascularization: Contribution and mechanisms. Angiogenesis 2016, 19, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Sheibani, N.; Sorenson, C.M.; Cornelius, L.A.; Frazier, W.A. Thrombospondin-1, a Natural Inhibitor of Angiogenesis, Is Present in Vitreous and Aqueous Humor and Is Modulated by Hyperglycemia. Biochem. Biophys. Res. Commun. 2000, 267, 257–261. [Google Scholar] [CrossRef] [Green Version]
- Carron, J.A.; Hiscott, P.; Hagan, S.; Sheridan, C.M.; Magee, R.; Gallagher, J.A. Cultured human retinal pigment epithelial cells differentially express thrombospondin-1, -2, -3, and -4. Int. J. Biochem. Cell Biol. 2000, 32, 1137–1142. [Google Scholar] [CrossRef]
- Chu, L.-Y.; Ramakrishnan, D.P.; Silverstein, R.L. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood 2013, 122, 1822–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhanot, S.; Alex, J.C. Current Applications of Platelet Gels in Facial Plastic Surgery. Facial Plast. Surg. 2002, 18, 027–034. [Google Scholar] [CrossRef]
- Mammoto, T.; Jiang, A.; Jiang, E.; Mammoto, A. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc. Res. 2013, 89, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Rajashekhar, G.; Ramadan, A.; Abburi, C.; Callaghan, B.; Traktuev, D.O.; Evans-Molina, C.; Maturi, R.; Harris, A.; Kern, T.S.; March, K.L. Regenerative Therapeutic Potential of Adipose Stromal Cells in Early Stage Diabetic Retinopathy. PLoS ONE 2014, 9, e84671. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, N.; Xu, W.; Xu, G. Mesenchymal stem cells attenuate hydrogen peroxide-induced oxidative stress and enhance neuroprotective effects in retinal ganglion cells. Vitr. Cell. Dev. Biol. Anim. 2016, 53, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.L.S.; Subbiah, S.K.; Khan, M.S.A.; Farhana, A.; Mok, P.L. Empowering Mesenchymal Stem Cells for Ocular Degenerative Disorders. Int. J. Mol. Sci. 2019, 20, 1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whone, A.L.; Kemp, K.; Sun, M.; Wilkins, A.; Scolding, N.J. Human bone marrow mesenchymal stem cells protect catecholaminergic and serotonergic neuronal perikarya and transporter function from oxidative stress by the secretion of glial-derived neurotrophic factor. Brain Res. 2012, 1431, 86–96. [Google Scholar] [CrossRef]
- Yamada, H.; Yamada, E.; Ando, A.; Esumi, N.; Bora, N.; Saikia, J.; Sung, C.-H.; Zack, D.J.; Campochiaro, P.A. Fibroblast Growth Factor-2 Decreases Hyperoxia-Induced Photoreceptor Cell Death in Mice. Am. J. Pathol. 2001, 159, 1113–1120. [Google Scholar] [CrossRef] [Green Version]
- Hauck, S.M.; Kinkl, N.; Deeg, C.A.; Lange, M.S.-D.; Schöffmann, S.; Ueffing, M. GDNF Family Ligands Trigger Indirect Neuroprotective Signaling in Retinal Glial Cells. Mol. Cell. Biol. 2006, 26, 2746–2757. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Mohand-Said, S.; Danan, A.; Simonutti, M.; Fontaine, V.; Clérin, E.; Picaud, S.; Léveillard, T.; Sahel, J.-A. Functional Cone Rescue by RdCVF Protein in a Dominant Model of Retinitis Pigmentosa. Mol. Ther. 2009, 17, 787–795. [Google Scholar] [CrossRef]
- Byrne, L.C.; Dalkara, D.; Luna, G.; Fisher, S.K.; Clérin, E.; Sahel, J.-A.; Léveillard, T.; Flannery, J.G. Viral-mediated RdCVF and RdCVFL expression protects cone and rod photoreceptors in retinal degeneration. J. Clin. Investig. 2014, 125, 105–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeira, M.H.; Boia, R.; Santos, P.F.; Ambrósio, A.F.; Santiago, A.R. Contribution of Microglia-Mediated Neuroinflammation to Retinal Degenerative Diseases. Mediat. Inflamm. 2015, 2015, 673090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holan, V.; Hermankova, B.; Krulova, M.; Zajicova, A. Cytokine interplay among the stem cell-based therapy. World J. Stem Cells 2019, 11, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Kosaka, N.; Takeshita, F.; Ochiya, T. The therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Proteom. 2013, 13, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Poston, J.N.; Dreixler, J.C.; Torres, L.; Lopez, J.; Zelkha, R.; Balyasnikova, I.; Lesniak, M.S.; Roth, S. Bone-marrow mesenchymal stem-cell administration significantly improves outcome after retinal ischemia in rats. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1581–1592. [Google Scholar] [CrossRef]
- Hooks, J.J.; Nagineni, C.N.; Hooper, L.C.; Hayashi, K.; Detrick, B. IFN-beta provides immuno-protection in the retina by inhibiting ICAM-1 and CXCL9 in retinal pigment epithelial cells. J. Immunol. 2008, 180, 3789–3796. [Google Scholar] [CrossRef] [Green Version]
- Nemunaitis, J. Macrophage function activating cytokines: Potential clinical application. Crit. Rev. Oncol. 1993, 14, 153–171. [Google Scholar] [CrossRef]
- Schneider, A.; Krüger, C.; Steigleder, T.; Weber, D.; Pitzer, C.; Laage, R.; Aronowski, J.; Maurer, M.H.; Gassler, N.; Mier, W.; et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Investig. 2005, 115, 2083–2098. [Google Scholar] [CrossRef] [Green Version]
- Lavail, M.M.; Unoki, K.; Yasumura, D.; Matthes, M.T.; Yancopoulos, G.D.; Steinberg, R.H. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc. Natl. Acad. Sci. USA 1992, 89, 11249–11253. [Google Scholar] [CrossRef] [Green Version]
- Nagineni, C.N.; Samuel, W.; Nagineni, S.; Pardhasaradhi, K.; Wiggert, B.; Detrick, B.; Hooks, J.J. Transforming growth factor-β induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: Involvement of mitogen-activated protein kinases. J. Cell. Physiol. 2003, 197, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Nagineni, C.N.; Kutty, V.; Detrick, B.; Hooks, J.J. Expression of PDGF and their receptors in human retinal pigment e Bpithelial cells and fibroblasts: Regulation by TGF-beta. J. Cell. Phys. 2005, 203, 35–43. [Google Scholar] [CrossRef]
- Wang, S.K.; Xue, Y.; Cepko, C.L. Microglia modulation by TGF-β1 protects cones in mouse models of retinal degeneration. J. Clin. Investig. 2020, 130, 4360–4369. [Google Scholar] [CrossRef] [PubMed]
- Valentijn, A.; Zouq, N.; Gilmore, A.P. Anoikis. Biochem. Soc. Trans. 2004, 32, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Calkins, D.J.; Benowitz, L.; Crowston, J.; Huberman, A.; Johnson, E.; Lu, R.; Pekny, M.; Sappington, R.M.; Zack, D.; Calkins, D.J.; et al. The challenge of regenerative therapies for the optic nerve in glaucoma. Exp. Eye Res. 2017, 157, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okazaki, T.; Magaki, T.; Takeda, M.; Kajiwara, Y.; Hanaya, R.; Sugiyama, K.; Arita, K.; Nishimura, M.; Kato, Y.; Kurisu, K. Intravenous administration of bone marrow stromal cells increases survivin and Bcl-2 protein expression and improves sensorimotor function following ischemia in rats. Neurosci. Lett. 2008, 430, 109–114. [Google Scholar] [CrossRef]
- Oltval, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 1993, 74, 609–619. [Google Scholar] [CrossRef]
- Reed, J.C. Mitochondria and Apoptosis. Science 1998, 281, 1309–1312. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Q.; Qin, X.; Shen, L.; Cheng, L.; Su, Y.; Phillips, M.I. Paracrine Action Enhances the Effects of Autologous Mesenchymal Stem Cell Transplantation on Vascular Regeneration in Rat Model of Myocardial Infarction. Ann. Thorac. Surg. 2005, 80, 229–237. [Google Scholar] [CrossRef]
- Gerber, H.-P.; Dixit, V.; Ferrara, N. Vascular Endothelial Growth Factor Induces Expression of the Antiapoptotic Proteins Bcl-2 and A1 in Vascular Endothelial Cells. J. Biol. Chem. 1998, 273, 13313–13316. [Google Scholar] [CrossRef] [Green Version]
- Ilić, D.; Almeida, E.A.; Schlaepfer, D.D.; Dazin, P.; Aizawa, S.; Damsky, C.H. Extracellular Matrix Survival Signals Transduced by Focal Adhesion Kinase Suppress p53-mediated Apoptosis. J. Cell Biol. 1998, 143, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Ganju, R.K.; Wang, J.F.; Schweitzer, K.; Weksler, B.; Avraham, S.; Groopman, J.E. Characterization of signal transduction pathways in human bone marrow endothelial cells. Blood J. Am. Soc. Hematol. 1997, 90, 2253–2259. [Google Scholar]
- Lobo, M.; Zachary, I. Nuclear Localization and Apoptotic Regulation of an Amino-Terminal Domain Focal Adhesion Kinase Fragment in Endothelial Cells. Biochem. Biophys. Res. Commun. 2000, 276, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia–reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc. Res. 2014, 92, 10–18. [Google Scholar] [CrossRef]
- Sternfeld, M.D.; Robertson, J.E.; Shipley, G.D.; Tsai, J.; Rosenbaum, J.T. Cultured human retinal pigment epithelial cells express basic fibroblast growth factor and its receptor. Curr. Eye Res. 1989, 8, 1029–1037. [Google Scholar] [CrossRef]
- Tanihara, H.; Yoshida, M.; Matsumoto, M.; Yoshimura, N. Identification of transforming growth factor-beta expressed in cultured human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1993, 34, 413–419. [Google Scholar]
- Adamis, A.; Shima, D.; Yeo, K.; Yeo, T.; Brown, L.; Berse, B.; D’Amore, P.A.; Folkman, J. Synthesis and Secretion of Vascular Permeability Factor/Vascular Endothelial Growth Factor by Human Retinal Pigment Epithelial Cells. Biochem. Biophys. Res. Commun. 1993, 193, 631–638. [Google Scholar] [CrossRef]
- Wahlin, K.J.; Campochiaro, P.A.; Zack, D.J.; Adler, R. Neurotrophic factors cause activation of intracellular signaling pathways in Müller cells and other cells of the inner retina, but not photoreceptors. Investig. Ophthalmol. Vis. Sci. 2000, 41, 927–936. [Google Scholar]
- Bringmann, A. Role of Muller cells in retinal degenerations. Front. Biosci. 2001, 6, e77–e92. [Google Scholar] [CrossRef] [Green Version]
- Kolomeyer, A.M.; Zarbin, M.A. Trophic factors in the pathogenesis and therapy for retinal degenerative diseases. Surv. Ophthalmol. 2014, 59, 134–165. [Google Scholar] [CrossRef]
- Ortín-Martínez, A.; Valiente-Soriano, F.J.; García-Ayuso, D.; Alarcon-Martinez, L.; Jiménez-López, M.; Bernal-Garro, J.M.; Nieto-López, L.; Nadal-Nicolás, F.M.; Villegas-Pérez, M.P.; Wheeler, L.A.; et al. A Novel In Vivo Model of Focal Light Emitting Diode-Induced Cone-Photoreceptor Phototoxicity: Neuroprotection Afforded by Brimonidine, BDNF, PEDF or bFGF. PLoS ONE 2014, 9, e113798. [Google Scholar] [CrossRef] [PubMed]
- Othberg, A.; Odin, P.; Ballagi, A.; Funa, K.; Lindvall, O. Specific effects of platelet derived growth factor (PDGF) on fetal rat and human dopaminergic neurons in vitro. Exp. Brain Res. 1995, 105, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Mocanu, C.; Mcleod, D.S.; Bhutto, I.A.; Merges, C.; Eid, M.; Tong, P.; Lutty, G.A. Expression of pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in sickle cell retina and choroid. Exp. Eye Res. 2003, 77, 433–445. [Google Scholar] [CrossRef]
- Tsang, C.K.; Qi, H.; Liu, L.F.; Zheng, X.F.S. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov. Today 2007, 12, 112–124. [Google Scholar] [CrossRef]
- Chung, S.; Rho, S.; Kim, G.; Kim, S.-R.; Baek, K.-H.; Kang, M.; Lew, H. Human umbilical cord blood mononuclear cells and chorionic plate-derived mesenchymal stem cells promote axon survival in a rat model of optic nerve crush injury. Int. J. Mol. Med. 2016, 37, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Zack, D.J. Neurotrophic Rescue of Photoreceptors. Neuron 2000, 26, 285–286. [Google Scholar] [CrossRef] [Green Version]
- Slomiany, M.G.; Rosenzweig, S.A. Autocrine effects of IGF-I-induced VEGF and IGFBP-3 secretion in retinal pigment epithelial cell line ARPE-19. Am. J. Physiol. Physiol. 2004, 287, C746–C753. [Google Scholar] [CrossRef] [Green Version]
- Gasperi, M.; Castellano, A.E. Growth hormone/insulin-like growth factor I axis in neurodegenerative diseases. J. Endocrinol. Investig. 2010, 33, 587–591. [Google Scholar] [CrossRef]
- Na, L.; Xiao-Rong, L.; Jia-Qin, Y.; Li, N.; Li, X.; Yuan, J.-Q. Effects of bone-marrow mesenchymal stem cells transplanted into vitreous cavity of rat injured by ischemia/reperfusion. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 247, 503–514. [Google Scholar] [CrossRef]
- Mead, B.; Hill, L.J.; Blanch, R.J.; Ward, K.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Mesenchymal stromal cell–mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 2016, 18, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drago, D.; Cossetti, C.; Iraci, N.; Gaude, E.; Musco, G.; Bachi, A.; Pluchino, S. The stem cell secretome and its role in brain repair. Biochimie 2013, 95, 2271–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Liu, X.; Shi, Y.; Ocansey, D.K.W.; Hu, Y.; Li, X.; Zhang, C.; Xu, W.; Qian, H. Therapeutic Advances of Stem Cell-Derived Extracellular Vesicles in Regenerative Medicine. Cells 2020, 9, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Disease | Cell Source | Delivery | WHO Identifier | References |
|---|---|---|---|---|
| AMD (GA), RP and ischaemic retinopathy | Autologous BMHSC | Intravitreal injection | NCT01560715 NCT01518127 NCT01518842 | [103,104] |
| AMD (GA), RP, RVO and DR | Autologous BMHSC | Intravitreal injection | NCT01736059 | [105] |
| RP | Autologous ADMSC | Subretinal application | Not registered | [128] |
| AMD (GA), RP, OA | Autologous ADMSC And PRP | Suprachoroidal application | Not registered | [131,132,133,134] |
| RP | Autologous PRP | Subtenon injection | Not registered | [126] |
| RP | Eterologous UC-MSCs | Suprachoroidal application | Ministry of Health 56733164/203 | [129] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limoli, P.G.; Vingolo, E.M.; Limoli, C.; Nebbioso, M. Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa. Antioxidants 2020, 9, 983. https://doi.org/10.3390/antiox9100983
Limoli PG, Vingolo EM, Limoli C, Nebbioso M. Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa. Antioxidants. 2020; 9(10):983. https://doi.org/10.3390/antiox9100983
Chicago/Turabian StyleLimoli, Paolo Giuseppe, Enzo Maria Vingolo, Celeste Limoli, and Marcella Nebbioso. 2020. "Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa" Antioxidants 9, no. 10: 983. https://doi.org/10.3390/antiox9100983
APA StyleLimoli, P. G., Vingolo, E. M., Limoli, C., & Nebbioso, M. (2020). Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa. Antioxidants, 9(10), 983. https://doi.org/10.3390/antiox9100983






