Cytotoxic and DNA-Damaging Effects of Aronia melanocarpa, Cornus mas, and Chaenomeles superba Leaf Extracts on the Human Colon Adenocarcinoma Cell Line Caco-2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Chemicals, Reagents, and Culture Vessels
2.3. Phytochemical Analysis
2.3.1. Test for Saponins
2.3.2. Test for Alkaloids
2.3.3. Test for Steroids (Salkowski Test)
2.3.4. Test for Glycosides (Keller- Killani Test)
2.3.5. Test for Carbohydrates (Fehling Test)
2.3.6. Test for Proteins
2.3.7. Test for Quinones
2.3.8. Test for Coumarin
2.4. Caco-2 Cell Culture
2.5. MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) Assay
2.6. Microscopic Observations of Morphological Changes
2.7. Genotoxicity Testing (Comet Assay)
2.8. DNA Repair
2.9. Statistical Analysis
3. Results
3.1. Phytochemical Characteristic of Leaf Extracts
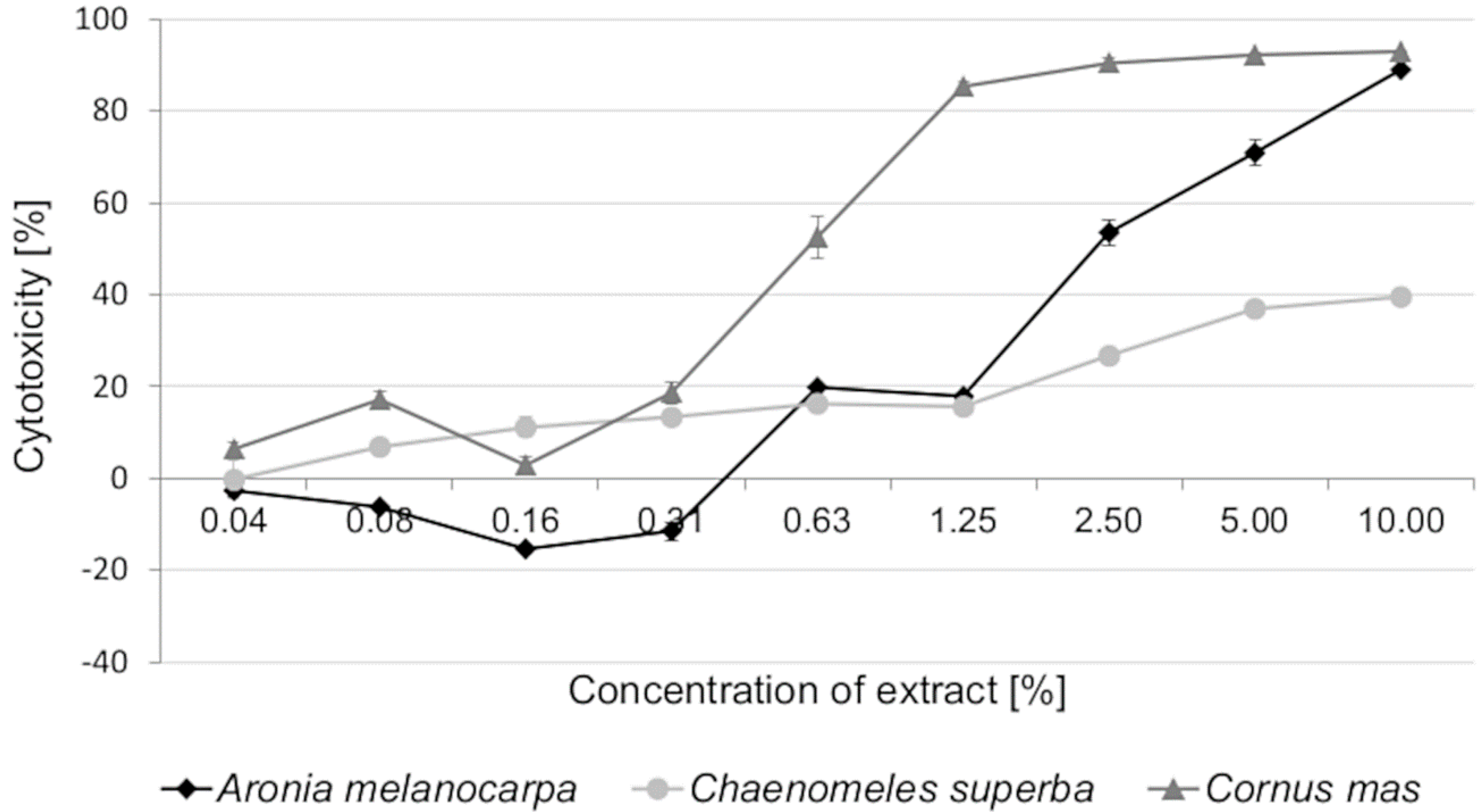
3.2. Cytotoxic Activity of Leaf Extracts and Estimation of Half Maximal Inhibitory Concentration (IC50)
3.3. Changes in the Morphology of Caco-2 Cells
3.4. Basal and Endogenous DNA Damage Induced by Leaf Extracts
3.5. DNA Repair in Caco-2 Cells Induced by Leaf Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gallego, R.; Bueno, M.; Herrero, M. Sub-and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae–An update. Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cancer in World Health Organization. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 12 September 2018).
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer efficacy of polyphenols and their combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A.; Menichini, F. Biological and pharmacological activities of iridoids: Recent developments. Mini Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2020, 1–30. [Google Scholar] [CrossRef]
- Vagiri, M.; Ekholm, A.; Andersson, S.C.; Johansson, E.; Rumpunen, K. An optimized method for analysis of phenolic compounds in buds, leaves, and fruits of black currant (Ribes nigrum L.). J. Agric. Food Chem. 2012, 60, 10501–10510. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Kabir, M.M.; Noman, M.A.A.; Islam, M.R.; Dash, B.K.; Akhter, S.; Uddin, M.J.; Rahman, A. Mikania cordata leaves extract promotes activity against pathogenic bacteria and anticancer activity in EAC cell-bearing swiss albino mice. J. Appl. Pharm. Sci. 2020, 10, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Akhtar, J. Evaluation of anticancer activity of Cordia dichotoma leaves against a human prostate carcinoma cell line, PC3. J. Tradit. Complement. Med. 2017, 7, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Gavamukulya, Y.; Abou-Elella, F.; Wamunyokoli, F.; AEl-Shemy, H. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac. J. Trop. Med. 2014, 7, S355–S363. [Google Scholar] [CrossRef] [Green Version]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A.; Kucharska, A.Z.; Fecka, I. Composition and antibacterial activity of Aronia melanocarpa (Michx.) Elliot, Cornus mas L. and Chaenomeles superba Lindl. leaf extracts. Molecules 2020, 25, 2011. [Google Scholar] [CrossRef] [PubMed]
- Kerem, Z.; Chetrit, D.; Shoseyov, O.; Regev-Shoshani, G. Protection of lipids from oxidation by epicatechin, trans-resveratrol, and gallic and caffeic acids in intestinal model systems. J. Agric. Food Chem. 2006, 54, 10288–10293. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Walver of In Vivo Bioavailability and Bioequivalence Studies for 518 Immediate-Release Solid Oral Dosages Forms Based on a Biopharmaceutics Classification 519 System; US Food and Drug Administration, Center for Drug Evaluation and Research: Washington, DC, USA, 2000. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/waiver-vivo-bioavailability-and-bioequivalence-studies-immediate-release-solid-oral-dosage-forms (accessed on 3 February 2018).
- Soni, A.; Sosa, S. Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J. Pharmacogn. Phytochem. 2013, 2, 22–29. [Google Scholar]
- Vimalkumar, C.S.; Hosagaudar, V.B.; Suja, S.R.; Vilash, V.; Krishnakumar, N.M.; Latha, P.G. Comparative preliminary phytochemical analysis of ethanolic extracts of leaves of Olea dioica Roxb., infected with the rust fungus Zaghouania oleae (EJ Butler) Cummins and non-infected plants. J. Pharmacogn. Phytochem. 2014, 3, 69–72. [Google Scholar]
- Kumar Bargah, R. Preliminary test of phytochemical screening of crude ethanolic and aqueous extract of Moringa pterygosperma Gaertn. J. Pharmacogn. Phytochem. 2015, 4. [Google Scholar]
- OECD Guideline for the Testing of Chemicals In Vitro Skin Sensitisation: ARE-Nrf2 Luciferase Test Method 2015. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd-tg442d-508.pdf (accessed on 27 January 2018).
- Błasiak, J.; Synowiec, E.; Tarnawska, J.; Czarny, P.; Popławski, T.; Reiter, R.J. Dental methacrylates may exert genotoxic effects via the oxidative induction of DNA strand breaks and the inhibition of their repair. Mol. Biol. Rep. 2012, 39, 7487–7496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michałowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health-promoting factors-An overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)–A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [Green Version]
- Szczepaniak, O.M.; Kobus-Cisowska, J.; Kusek, W.; Przeor, M. Functional properties of Cornelian cherry (Cornus mas L.): A comprehensive review. Eur. Food Res. Technol. 2019, 245, 2071–2087. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Li, S.; Zhu, Z.; He, J. Recent advances in valorization of Chaenomeles fruit: A review of botanical profile, phytochemistry, advanced extraction technologies and bioactivities. Trends Food Sci. Technol. 2019, 91, 467–482. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Barreiro, E.J. Recent Advances in development of polyphenols as anticancer agents. Mini Rev. Med. Chem. 2018, 18, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; Green, I.R.; Saleem, M.; Raza, M.L.; Nazir, M. Therapeutic potential of iridoid derivatives: Patent review. Inventions 2019, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Achi, N.K.; Onyeabo, C.; Ekeleme-Egedigwe, C.A.; Onyeanula, J.C. Phytochemical, proximate analysis, vitamin and mineral composition of aqueous extract of Ficus capensis leaves in South Eastern Nigeria. J Appl. Pharm. Sci. 2017, 7, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Shokoohinia, Y.; Sajjadi, S.E.; Gholamzadeh, S.; Fattahi, A.; Behbahani, M. Antiviral and cytotoxic evaluation of coumarins from Prangos ferulacea. Pharm. Biol. 2014, 52, 1543–1549. [Google Scholar] [CrossRef] [Green Version]
- Alam, F.; us Saqib, Q.N.; Waheed, A. Cytotoxic activity of extracts and crude saponins from Zanthoxylum armatum DC. against human breast (MCF-7, MDA-MB-468) and colorectal (Caco-2) cancer cell lines. BMC Complement. Altern. Med. 2017, 17, 368. [Google Scholar] [CrossRef]
- Biradi, M.; Hullatti, K. Bioactivity guided isolation of cytotoxic terpenoids and steroids from Premna serratifolia. Pharm. Biol. 2017, 55, 1375–1379. [Google Scholar] [CrossRef]
- Eghbaliferiz, S.; Emami, S.A.; Tayarani-Najaran, Z.; Iranshahi, M.; Shakeri, A.; Hohmann, J.; Asili, J. Cytotoxic diterpene quinones from Salvia tebesana Bunge. Fitoterapia 2018, 128, 97–101. [Google Scholar] [CrossRef]
- Hosseini, M.; Taherkhani, M.; Ghorbani Nohooji, M. Introduction of Adonis aestivalis as a new source of effective cytotoxic cardiac glycoside. Nat. Prod. Res. 2019, 33, 915–920. [Google Scholar] [CrossRef]
- Van, N.T.H.; Tuyen, T.T.; Quan, P.M.; Long, P.Q.; Nhiem, N.X.; Tai, B.H.; Inh, C.T.; Hoang, V.D.; Kiem, P.V.; Minh, C.V.; et al. Alkaloid glycosides and their cytotoxic constituents from Zanthoxylum nitidum. Phytochem. Lett. 2019, 32, 47–51. [Google Scholar] [CrossRef]
- Yar Khan, H.; Zubair, H.; Fahad Ullah, M.; Ahmad, A.; Mumtaz Hadi, S. A prooxidant mechanism for the anticancer and chemopreventive properties of plant polyphenols. Curr. Drug Targets 2012, 13, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Šavikin, K.; Zdunić, G.; Janković, T.; Stanojković, T.; Juranić, Z.; Menković, N. In vitro cytotoxic and antioxidative activity of Cornus mas and Cotinus coggygria. Nat. Prod. Res. 2009, 23, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- Forman, V.; Haladová, M.; Grančai, D.; Ficková, M. Antiproliferative activities of water infusions from leaves of five Cornus L. species. Molecules 2015, 20, 22546–22552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousefi, B.; Abasi, M.; Abbasi, M.M.; Jahanban-Esfahlan, R. Anti-proliferative properties of Cornus mas fruit in different human cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 5727–5731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiptiri-Kourpeti, A.; Fitsiou, E.; Spyridopoulou, K.; Vasileiadis, S.; Iliopoulos, C.; Galanis, A.; Vekiari, S.; Pappa, A.; Chlichlia, K. Evaluation of antioxidant and antiproliferative properties of Cornus mas L. fruit juice. Antioxidants 2019, 8, 377. [Google Scholar] [CrossRef] [Green Version]
- Radbeh, Z.; Asefi, N.; Hamishehkar, H.; Roufegarinejad, L.; Pezeshki, A. Novel carriers ensuring enhanced anti-cancer activity of Cornus mas (cornelian cherry) bioactive compounds. Biomed. Pharmacother. 2020, 125, 1–8. [Google Scholar] [CrossRef]
- Skupień, K.; Kostrzewa-Nowak, D.; Oszmiański, J.; Tarasiuk, J. In vitro antileukaemic activity of extracts from chokeberry (Aronia melanocarpa [Michx] Elliott) and mulberry (Morus alba L.) leaves against sensitive and multidrug resistant HL60 cells. Phytother. Res. 2008, 22, 689–694. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Mašković, P.; Đurović, S.; Zengin, G.; Delerue-Matos, C.; Lozano-Sánchez, J.; Jakšić, A. Chemical and biological insights on aronia stems extracts obtained by different extraction techniques: From wastes to functional products. J. Supercrit. Fluids 2017, 128, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Cvetanović, A.; Zengin, G.; Zeković, Z.; Švarc-Gajić, J.; Ražić, S.; Damjanović, A.; Mašković, P.; Mitić, M. Comparative in vitro studies of the biological potential and chemical composition of stems, leaves and berries Aronia melanocarpa’s extracts obtained by subcritical water extraction. Food Chem. Toxicol. 2018, 121, 458–466. [Google Scholar] [CrossRef]
- Gao, N.; Wang, Y.; Jiao, X.; Chou, S.; Li, E.; Li, B. Preparative purification of polyphenols from Aronia melanocarpa (Chokeberry) with cellular antioxidant and antiproliferative activity. Molecules 2018, 23, 139. [Google Scholar] [CrossRef] [Green Version]
- Thani, N.A.A.; Keshavarz, S.; Lwaleed, B.A.; Cooper, A.J.; Rooprai, H.K. Cytotoxicity of gemcitabine enhanced by polyphenolics from Aronia melanocarpa in pancreatic cancer cell line AsPC-1. J. Clin. Pathol. 2014, 67, 949–954. [Google Scholar] [CrossRef]
- Chun, J.M.; Nho, K.J.; Lee, A.Y.; Moon, B.C.; Park, J.Y.; Kim, H.K. A methanol fraction from Chaenomeles sinensis inhibits hepatocellular carcinoma growth in vitro and in vivo. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 335–341. [Google Scholar] [CrossRef]
- Kikowska, M.A.; Chmielewska, M.; Włodarczyk, A.; Studzińska-Sroka, E.; Żuchowski, J.; Stochmal, A.; Kotwicka, M.; Thiem, B. Effect of pentacyclic triterpenoids-rich callus extract of Chaenomeles japonica (Thunb.) Lindl. ex Spach on viability, morphology, and proliferation of normal human skin fibroblasts. Molecules 2018, 23, 3009. [Google Scholar] [CrossRef] [Green Version]
- Nowak, A.; Sόjka, M.; Klewicka, E.; Lipińska, L.; Klewicki, R.; Kołodziejczyk, K. Ellagitannins from Rubus idaeus L. exert geno-and cytotoxic effects against human colon adenocarcinoma cell line Caco-2. J. Agric. Food Chem. 2017, 65, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Jena, N.R. DNA damage by reactive species: Mechanisms, mutation and repair. J. Biosci. 2012, 37, 503–517. [Google Scholar] [CrossRef]
- Prasad, R.; Katiyar, S.K. Polyphenols from green tea inhibit the growth of melanoma cells through inhibition of class I histone deacetylases and induction of DNA damage. Genes Cancer 2015, 6, 49–61. [Google Scholar] [CrossRef]
- Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Reynoso-Camacho, R.; Ramos-Gómez, M.; Garcia-Gasca, T.; Rodríguez-Muñoz, M.E.; Guzmán-Maldonado, S.H.; Medina-Torresf, L.; Lujan-García, B.A. Antioxidant activity and genotoxic effect on HeLa cells of phenolic compounds from infusions of Quercus resinosa leaves. Food Chem. 2009, 115, 1320–1325. [Google Scholar] [CrossRef]
- Verschaeve, L.; Edziri, H.; Anthonissen, R.; Boujnah, D.; Skhiri, F.; Aouni, M.; Mastouri, M. In vitro toxicity and genotoxic activity of aqueous leaf and fruit extracts of Ruscus hypophyllum L. Acta Physiol. Plant. 2017, 39, 1–10. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus fruit phenolic compounds as cytoprotective agents able to decrease free fatty acids and glucose uptake by Caco-2 cells. Antioxidants 2019, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Zhao, C.; Pan, J.; Shi, Y.; Liu, G.; Zhou, B.; Zheng, R. In vivo non-enzymatic repair of DNA oxidative damage by polyphenols. Cell Biol. Int. 2009, 33, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Silva, S.C.; Soares, J.P.; Martins-Gomes, C.; Teixeira, J.P.; Leal, F.; Gaivão, I. Ginkgo biloba L. leaf extract protects HepG2 cells against paraquat-induced oxidative DNA damage. Plants 2019, 8, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sitarek, P.; Skała, E.; Wysokińska, H.; Wielanek, M.; Szemraj, J.; Toma, M.; Śliwiński, T. The effect of Leonurus sibiricus plant extracts on stimulating repair and protective activity against oxidative DNA damage in CHO cells and content of phenolic compounds. Oxid. Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellion, P.; Digle, J.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Polyphenolic apple extracts: Effects of raw material and production method on antioxidant effectiveness and reduction of DNA damage in Caco-2 cells. J. Agric. Food Chem. 2010, 58, 6636–6642. [Google Scholar] [CrossRef]
- Bellion, P.; Hofmann, T.; Pool-Zobel, B.L.; Will, F.; Dietrich, H.; Knaup, B.; Richling, E.; Baum, M.; Eisenbrand, G.; Janzowski, C. Antioxidant effectiveness of phenolic apple juice extracts and their gut fermentation products in the human colon carcinoma cell line Caco-2. J. Agric. Food Chem. 2008, 56, 6310–6317. [Google Scholar] [CrossRef]





| Group of Compounds | Aronia melanocarpa | Chaenomeles superba | Cornus mas |
|---|---|---|---|
| Phenolic acids | neochlorogenic acid, chlorogenic acid, caffeoyl-deoxyhexose, p-coumaroylquinic acid | neochlorogenic acid, caffeic acid dimer/caffeoyl hexoside, chlorogenic acid, p-coumaroylhexoside, p-coumaroylquinic acid isomers | gallic acid, caftaric acid isomers, p-coumaroylhexoside |
| Concentration | 114.66 ± 6.50 µg/mL | 644.16 ± 35.33 µg/mL | 145.1 ± 10.01 µg/mL |
| Flavonols | quercetin-3-O-rhamnoside, quercetin-pentoside-deoxydihexoside, quercetin-3-O-dihexoside, quercetin-3-O-dirhamnosylhexoside, quercetin-3-O-vicianoside, quercetin-3-O-robinobioside, quercetin-3-O-rutinoside, quercetin-3-O-glucoside, isorhamnetin-3-O-rutinoside, isorhamnetin-hexoside-pentoside, kaempferol-3-O-rutinoside | dihydroquercetin-hexoside, quercetin-3-O-rutinoside, quercetin-3-O-galactoside, quercetin-3-O-glucoside, kaempferol-3-O-hexoside, kaempferol-3-O-rutinoside, kaempferol-hexosidedeoxyhexoside | quercetin-3-O glucuronylpentoside, quercetin-3-O-rutinoside, quercetin-3-O-glucuronide, quercetin-3-O- glucoside, kaempferol-3-O-glucuronide |
| Concentration | 93.40 ± 5.66 µg/mL | 252.17 ± 13.27 µg/mL | 111.66 ± 7.19 µg/mL |
| Flavones | luteolin-3-O-rutinoside, luteolindihexoside | ||
| Concentration | - | 17.28 ± 0.86 µg/mL | - |
| Flavanones | naringenin-7-O-hexoside | ||
| Concentration | - | 227.30 ± 11.37 µg/mL | - |
| Ellagitannins | camptothin A isomers, cornusiin F 1 and 2, cornusiin A 1 and 2 | ||
| Concentration | - | - | 151.63 ± 8.77 µg/mL |
| Ellagic acid | - | - | ellagic acid |
| Concentration | - | - | 2.56 ± 0.27 µg/mL |
| Substituted phenols | hydroxytyrosol | hydroxytyrosol | |
| Concentration | 7.92 ± 0.40 µg/mL | 75.00 ± 5.25 µg/mL | - |
| Iridoids | - | - | loganic acid isomers, secoxyloganin, cornuside |
| Concentration | - | - | 33.9 ± 1.85 µg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efenberger-Szmechtyk, M.; Nowak, A.; Nowak, A. Cytotoxic and DNA-Damaging Effects of Aronia melanocarpa, Cornus mas, and Chaenomeles superba Leaf Extracts on the Human Colon Adenocarcinoma Cell Line Caco-2. Antioxidants 2020, 9, 1030. https://doi.org/10.3390/antiox9111030
Efenberger-Szmechtyk M, Nowak A, Nowak A. Cytotoxic and DNA-Damaging Effects of Aronia melanocarpa, Cornus mas, and Chaenomeles superba Leaf Extracts on the Human Colon Adenocarcinoma Cell Line Caco-2. Antioxidants. 2020; 9(11):1030. https://doi.org/10.3390/antiox9111030
Chicago/Turabian StyleEfenberger-Szmechtyk, Magdalena, Adriana Nowak, and Agnieszka Nowak. 2020. "Cytotoxic and DNA-Damaging Effects of Aronia melanocarpa, Cornus mas, and Chaenomeles superba Leaf Extracts on the Human Colon Adenocarcinoma Cell Line Caco-2" Antioxidants 9, no. 11: 1030. https://doi.org/10.3390/antiox9111030
APA StyleEfenberger-Szmechtyk, M., Nowak, A., & Nowak, A. (2020). Cytotoxic and DNA-Damaging Effects of Aronia melanocarpa, Cornus mas, and Chaenomeles superba Leaf Extracts on the Human Colon Adenocarcinoma Cell Line Caco-2. Antioxidants, 9(11), 1030. https://doi.org/10.3390/antiox9111030






