Antioxidants in Plants: A Valorization Potential Emphasizing the Need for the Conservation of Plant Biodiversity in Cuba
Abstract
1. Introduction
2. Cuba and Its Endemic Potentials
3. Antioxidants in Plant Protection
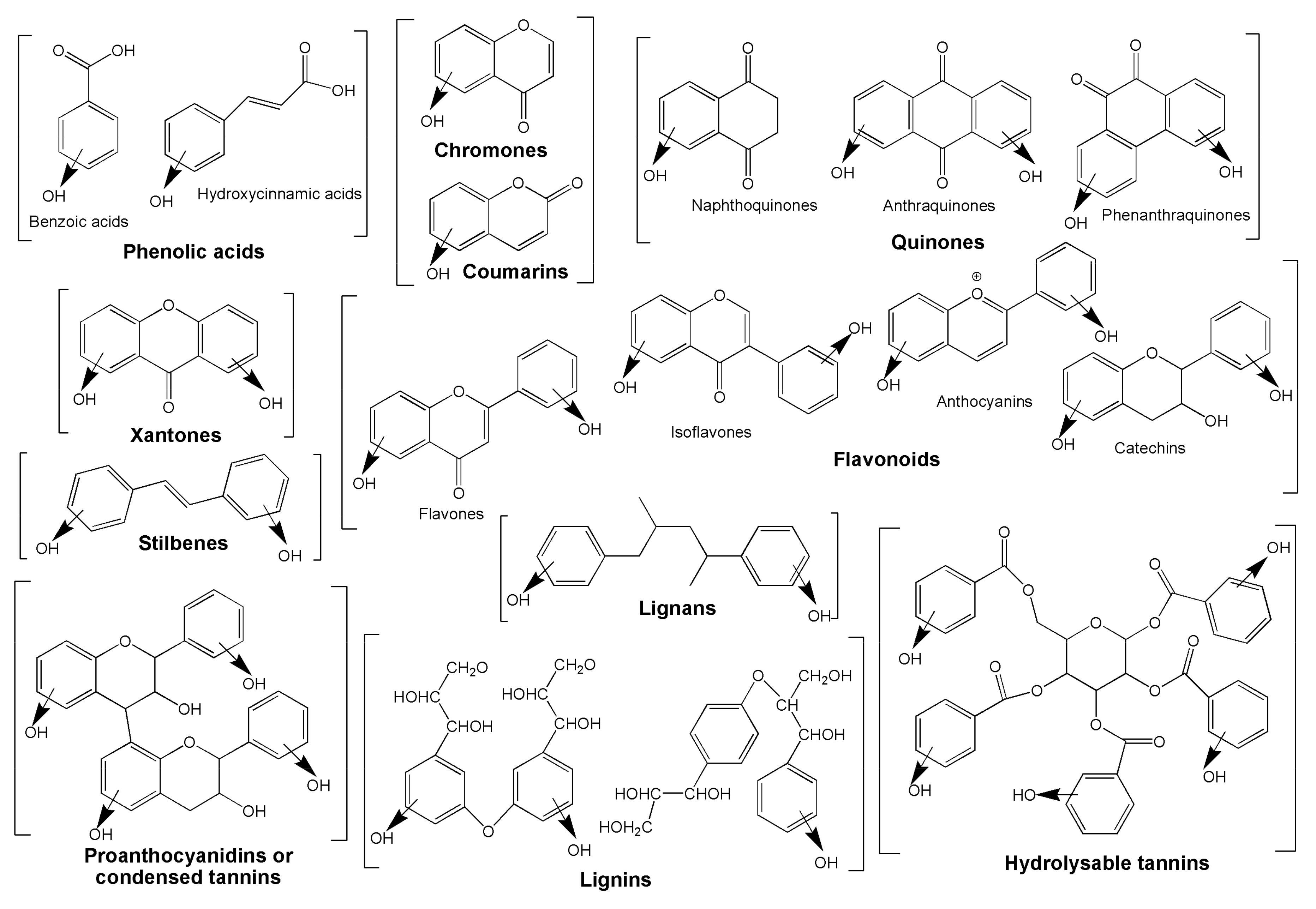
3.1. Non-Enzymatic Antioxidants in Plants
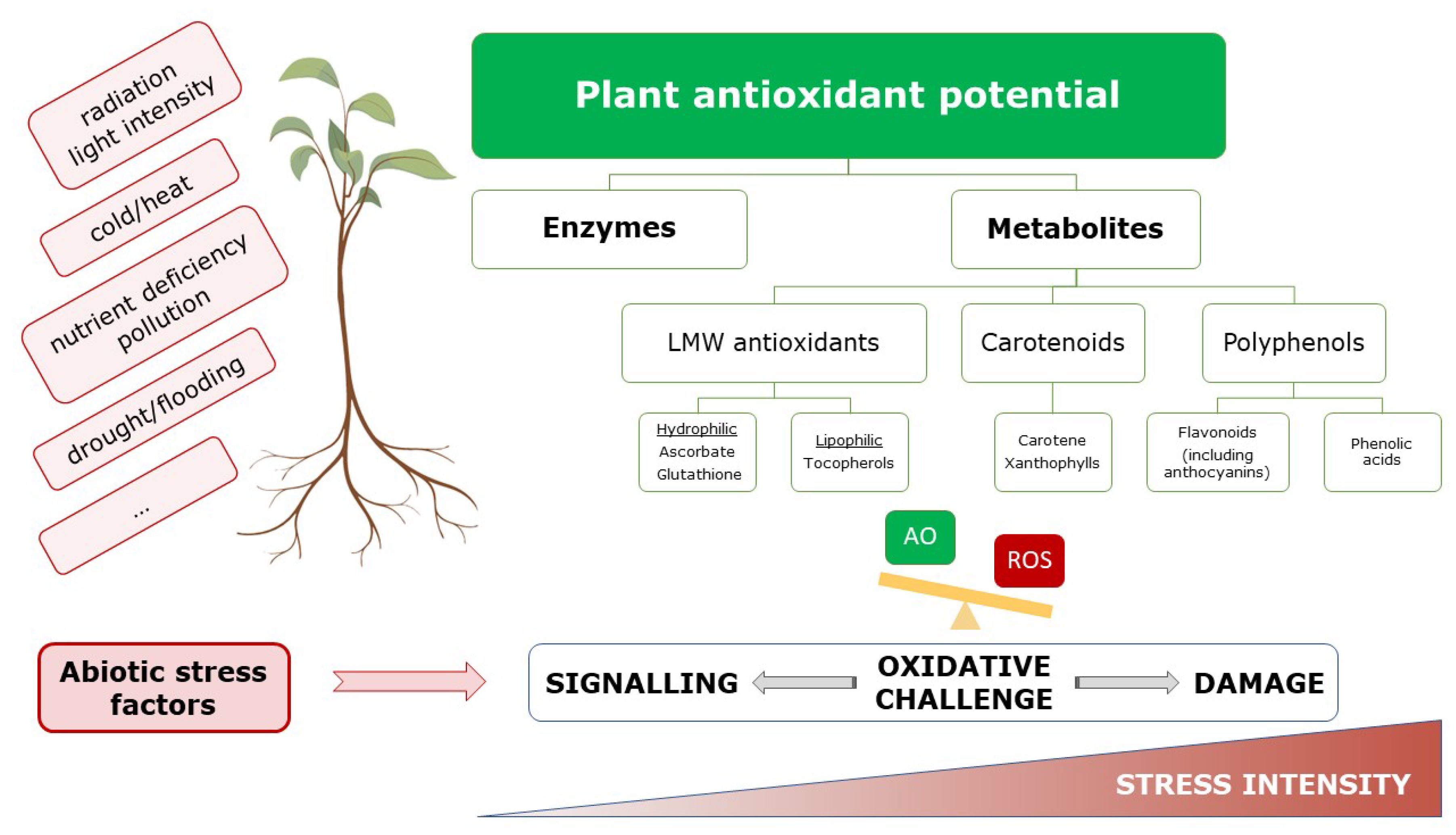
3.2. Antioxidants in Plants under Abiotic Stress Conditions
3.3. Plants under Harsh Environmental Conditions as a Source of Antioxidants
4. Plant Nutritional Value for Human Health
4.1. Plant Secondary Metabolites with Antioxidant Activity: In Vitro versus In Vivo
4.2. Plant Medicinal and Nutritional Value of Antioxidant Candidates
4.2.1. Cuban Plant Antioxidants and Their Therapeutic Potential
4.3. Advances in Natural Antioxidant Formulations: A Potential to Valorize Cuban Nutraceuticals and Cosmeceuticals
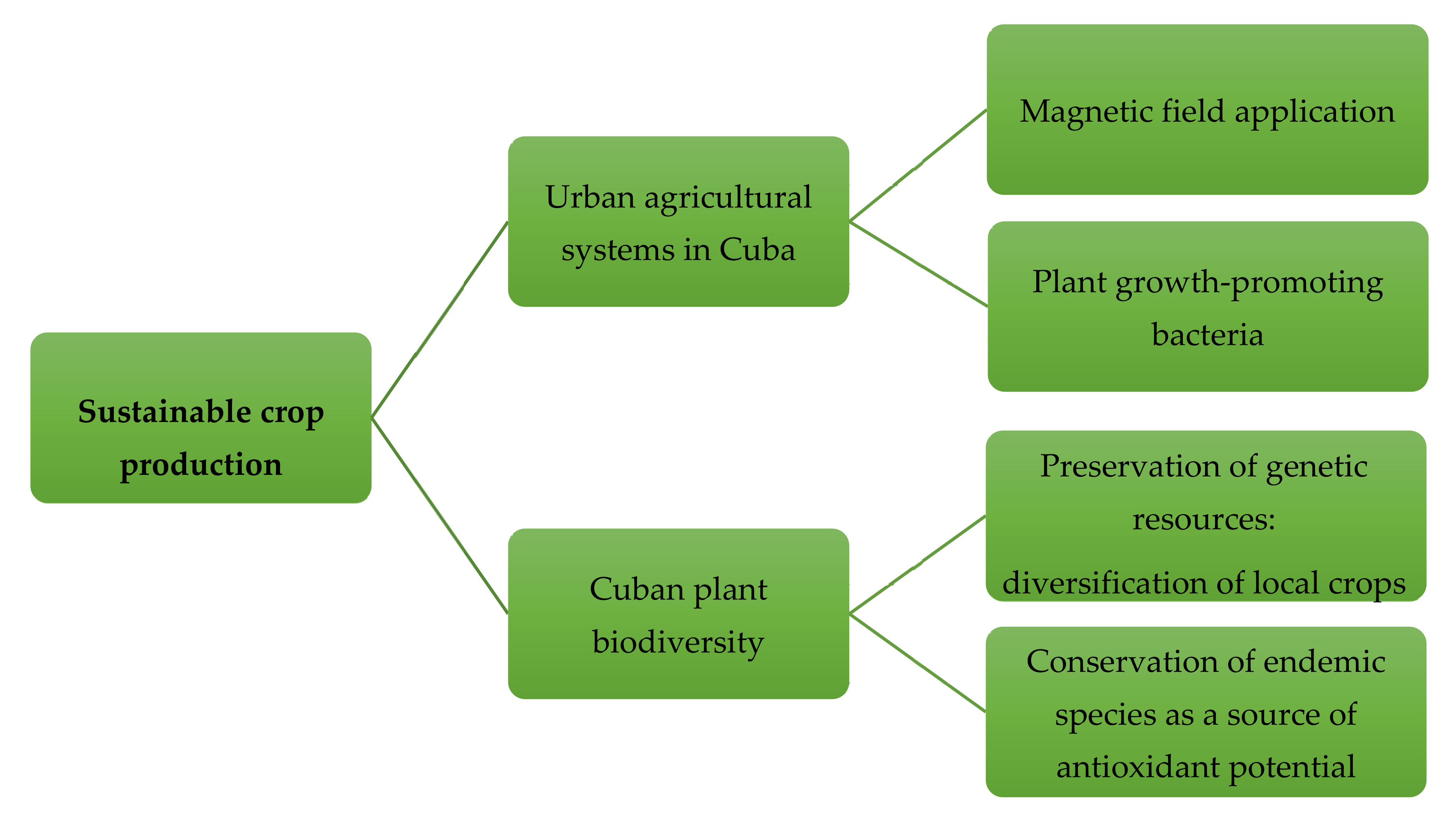
5. Sustainable Production of Plants/Crops with Improved Antioxidant Potential
5.1. Urban Agricultural Systems in Cuba: An Unexplored Niche of Sustainable Agricultural Practices to Improve Nutritional Quality of Cultivable Crops
5.1.1. Application of Magnetic Field to Improve Seedling Performance and Crop Yield and Quality
5.1.2. Plant Growth-Promoting Bacteria Stimulate Plant Growth Performance in Economically Valuable Crops
5.2. Cuban Plant Biodiversity as a Source of Antioxidant Potential
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
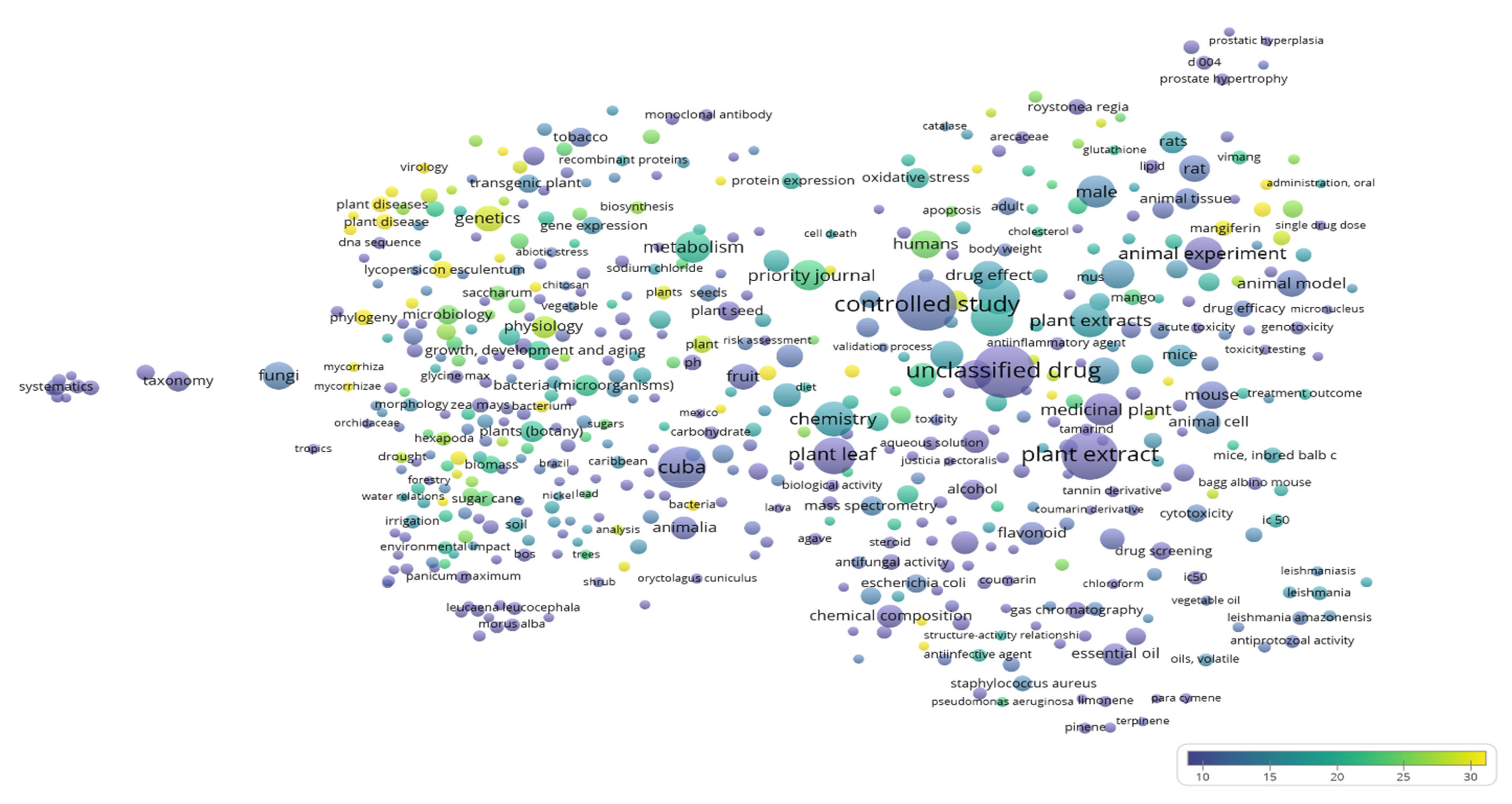
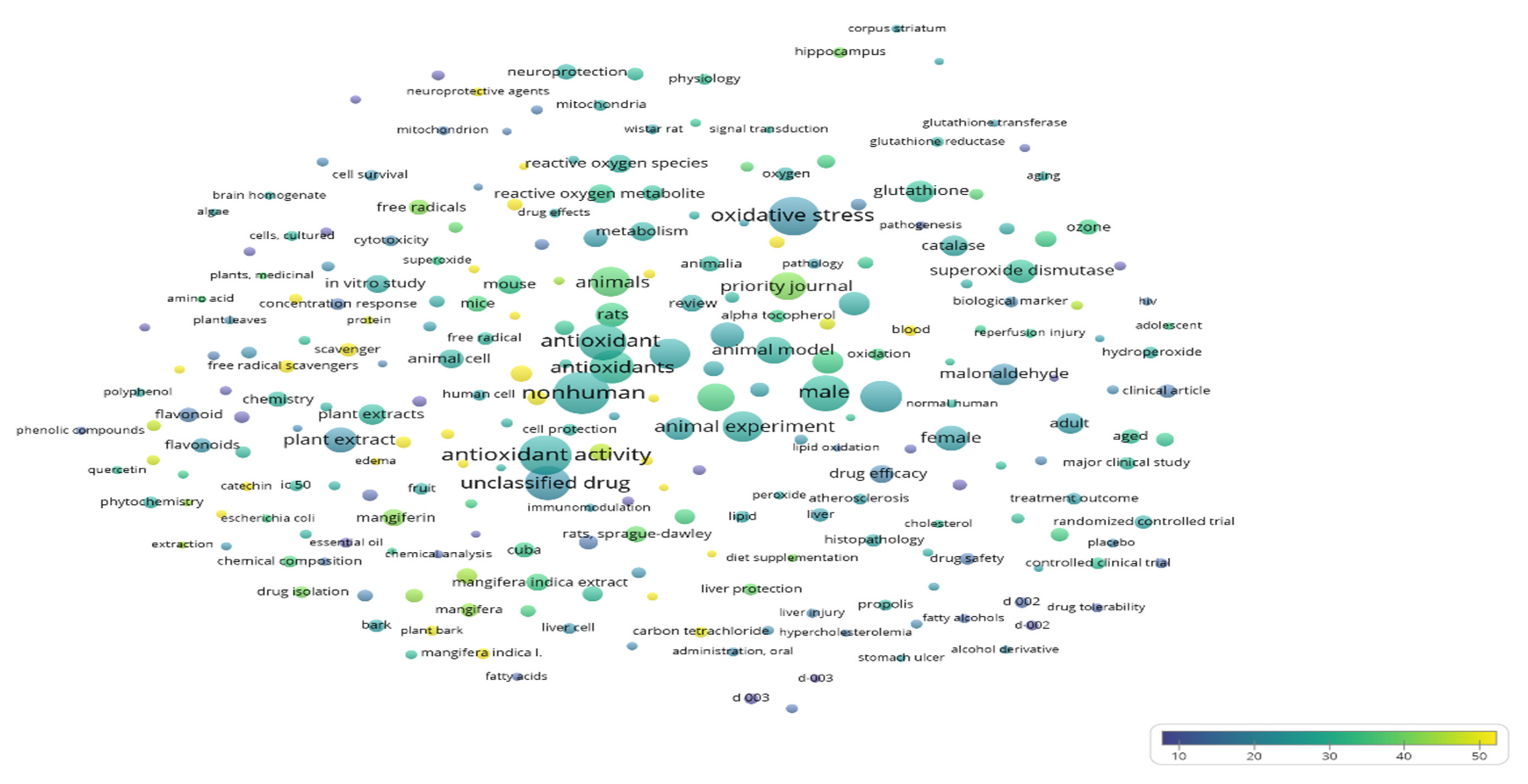
References
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, F.; Simón-Grao, S.; Martínez-Nicolás, J.J.; Alfosea-Simón, M.; Liu, C.; Chatzissavvidis, C.; Pérez-Pérez, J.G.; Cámara-Zapata, J.M. Multiple stresses occurring with boron toxicity and deficiency in plants. J. Hazard. Mater. 2020, 397, 122713. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.; Zhuo, C.; Lu, S.; Guo, Z. A Universal Stress Protein from Medicago falcata (MfUSP1) confers multiple stress tolerance by regulating antioxidant defense and proline accumulation. Environ. Exp. Bot. 2020, 178, 104168. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Cuypers, A.; Hendrix, S.; dos Reis, R.A.; De Smet, S.; Deckers, J.; Gielen, H.; Jozefczak, M.; Loix, C.; Vercampt, H.; Vangronsveld, J.; et al. Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front. Plant Sci. 2016, 7, 1–25. [Google Scholar] [CrossRef]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. Biochem. 2016, 171, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-H.; Wang, P.-Q.; Zhang, P.-P.; Nie, X.-M.; Li, B.-B.; Tai, L.; Liu, W.-T.; Li, W.-Q.; Chen, K.-M. NADPH oxidases: The vital performers and center hubs during plant growth and signaling. Cells 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Kreuzwieser, J.; Winkler, J.B.; Ghirardo, A.; Schnitzler, J.-P.; Ache, P.; Alfarraj, S.; Hedrich, R.; White, P.; Rennenberg, H. Physiological responses of date palm (Phoenix dactylifera) seedlings to acute ozone exposure at high temperature. Environ. Pollut. 2018, 242, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Hashim, A.M.; Alharbi, B.M.; Abdulmajeed, A.M.; Elkelish, A.; Hozzein, W.N.; Hassan, H.M. Oxidative stress responses of some endemic plants to high altitudes by intensifying antioxidants and secondary metabolites content. Plants 2020, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.F.; Coley, P.D.; Younkin, G.C.; Forrister, D.L.; Mills, A.G.; Kursar, T.A. Phenolics lie at the centre of functional versatility in the responses of two phytochemically diverse tropical trees to canopy thinning. J. Exp. Bot. 2019, 70, 5853–5864. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Osbourn, A.; Ma, P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef]
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their Importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Scarano, A.; Chieppa, M.; Santino, A. Looking at flavonoid biodiversity in horticultural crops: A colored mine with nutritional benefits. Plants 2018, 7, 98. [Google Scholar] [CrossRef]
- Sindhi, V.; Gupta, V.; Sharma, K.; Bhatnagar, S.; Kumari, R.; Dhaka, N. Potential applications of antioxidants—A review. J. Pharm. Res. 2013, 7, 828–835. [Google Scholar] [CrossRef]
- Singh, R.L.; Sharma, S.; Singh, P. Antioxidants: Their Health Benefits and Plant Sources. In Phytochemicals of Nutraceutical Importance; Prakash, D., Sharma, G., Eds.; CAB International: Wallingford, UK, 2014. [Google Scholar]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxid. Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D. Antioxidant activity of polyphenolic plant extracts. Antioxidants 2020, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Gong, X.; Li, X.; Wang, C.; Li, M. Advanced research on the antioxidant activity and mechanism of polyphenols from Hippophae species—A review. Molecules 2020, 25, 917. [Google Scholar] [CrossRef]
- Soto, M.L.; Parada, M.; Falqué, E.; Domínguez, H. Personal-care products formulated with natural antioxidant extracts. Cosmetics 2018, 5, 13. [Google Scholar] [CrossRef]
- Jaramillo Flores, M.E. Cocoa flavanols: Natural agents with attenuating effects on metabolic syndrome risk factors. Nutrients 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical polyphenols: New analytical challenges and opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Dorni, A.I.C.; Amalraj, A.; Gopi, S.; Varma, K.; Anjana, S.N. Novel cosmeceuticals from plants—An industry guided review. J. Appl. Res. Med. Aromat. Plants 2017, 7, 1–26. [Google Scholar] [CrossRef]
- Berni, R.; Cantini, C.; Romi, M.; Hausman, J.F.; Guerriero, G.; Cai, G. Agrobiotechnology goes wild: Ancient local varieties as sources of bioactives. Int. J. Mol. Sci. 2018, 19, 2248. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Baenas, N.; García-Viguera, C. New insights in (poly)phenolic compounds: From dietary sources to health evidence. Foods 2020, 9, 543. [Google Scholar] [CrossRef]
- Baião, D.D.S.; De Freitas, C.S.; Gomes, L.P.; Da Silva, D.; Correa, A.C.N.T.F.; Pereira, P.R.; Aguila, E.M.D.; Paschoalin, V.M.F. Polyphenols from root, tubercles and grains cropped in Brazil: Chemical and nutritional characterization and their effects on human health and diseases. Nutrients 2017, 9, 1044. [Google Scholar] [CrossRef]
- Peisino, M.C.O.; Zouain, M.S.; de Christo Scherer, M.M.; Schmitt, E.F.P.; Toledo e Silva, M.V.; Barth, T.; Endringer, D.C.; Scherer, R.; Fronza, M. Health-promoting properties of Brazilian unconventional food plants. Waste Biomass Valori. 2020, 11, 4691–4700. [Google Scholar] [CrossRef]
- Bravo, K.; Quintero, C.; Agudelo, C.; García, S.; Bríñez, A.; Osorio, E. CosIng database analysis and experimental studies to promote Latin American plant biodiversity for cosmetic use. Ind. Crops Prod. 2020, 144, 112007. [Google Scholar] [CrossRef]
- Letelier, L.; Gaete-Eastman, C.; Peñailillo, P.; Moya-León, M.A.; Herrera, R. Southern species from the biodiversity hotspot of central Chile: A source of color, aroma, and metabolites for global agriculture and food industry in a scenario of climate change. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Noriega, P.; Ballesteros, J.; De la Cruz, A.; Veloz, T. Chemical composition and preliminary antimicrobial activity of the hydroxylated sesquiterpenes in the essential oil from Piper barbatum Kunth leaves. Plants 2020, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Giam, X.; Bradshaw, C.J.A.; Tan, H.T.W.; Sodhi, N.S. Future habitat loss and the conservation of plant biodiversity. Biol. Conserv. 2010, 143, 1594–1602. [Google Scholar] [CrossRef]
- Capote López, R.P.; Mitrani Arenal, I.; Suárez, A.G. Conservacíon de la biodiversidad Cubana y cambio climático an el archipiélago Cubano. Anal. Acad. Cienc. Cuba 2011, 1, 1–24. [Google Scholar]
- González-Torres, L.R.; Palmarola, A.; Bécquer, E.R.; Berazaín, R.; Barrios, D.; Gómez, J. Top 50—Las 50 plantas más amenazadas de Cuba. Bissea 2013, 7. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography. Ecology, Evolution, and Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Kier, G.; Kreft, H.; Lee, T.M.; Jetz, W.; Ibisch, P.L.; Nowicki, C.; Mutke, J.; Barthlott, W. A global assessment of endemism and species richness across island and mainland regions. Proc. Natl. Acad. Sci. USA 2009, 106, 9322. [Google Scholar] [CrossRef]
- González-Torres, L.R.; Palmarola, A.; González-Oliva, L.; Bécquer, E.R.; Testé, E.; Barrios, D. Lista Roja de Flora Cuba. Bissea 2016, 10. [Google Scholar] [CrossRef]
- Balslev, H.; Borhidi, A. 1991. Phytogeography and vegetation ecology of Cuba. Nord. J. Bot. 1992, 12, 470. [Google Scholar] [CrossRef]
- Borhidi, A. Phytogreaphic survey of Cuba. Acta Bot. Hung. 1985, 31, 3–34. [Google Scholar]
- Oviedo, R.; Faife-Cabrera, M.; Noa-Monzón, A.; Arroyo, J.; Valiente-Banuet, A.; Verdú, M. Facilitation allows plant coexistence in Cuban serpentine soils. Plant Biol. 2014, 16, 711–716. [Google Scholar] [CrossRef]
- Cruz, A.; Ramos, Z. Characterization of the pine forest on quartz sands of the Ecological Reserve “Los Pretiles”, Pinar del Río, Cuba. Rev. Cuba. Cienc. For. 2019, 7, 125–144. [Google Scholar]
- Geekiyanage, N.; Goodale, U.M.; Cao, K.; Kitajima, K. Plant ecology of tropical and subtropical karst ecosystems. Biotropica 2019, 51, 626–640. [Google Scholar] [CrossRef]
- Planos Gutiérrez, E.O.; Gutiérrez Pérez, T.L.; Capote López, R.; Barranco Rodríguez, G.; Salabarría Fernández, D.; Vales García, M. (Eds.) Aportes 2013–2018 del Programa Nacional de Ciencia Cambio Climático en Cuba: Impactos, Adaptación y Mitigación; Editorial Agencia de Medio Ambiente (AMA): La Habana, Cuba, 2018. [Google Scholar]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S.H. An Introduction to Antioxidants and Their Roles in Plant Stress Tolerance. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Khan, M.I.R., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 1–23. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Zechmann, B. Compartment-specific importance of ascorbate during environmental stress in plants. Antiox. Redox Signal. 2018, 29, 1488–1501. [Google Scholar] [CrossRef]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the balance right: ROS homeostasis and redox signalling in fruit. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Asthir, B.; Kaur, G.; Kaur, B. Convergence of pathways towards ascorbate–glutathione for stress mitigation. J. Plant Biol. 2020, 63, 243–257. [Google Scholar] [CrossRef]
- Bilska, K.; Wojciechowska, N.; Alipour, S.; Kalemba, E.M. Ascorbic acid-The little-known antioxidant in woody plants. Antioxidants 2019, 8, 645. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Hendrix, S.; Schröder, P.; Keunen, E.; Huber, C.; Cuypers, A. Chapter Six - Molecular and Cellular Aspects of Contaminant Toxicity in Plants: The Importance of Sulphur and Associated Signalling Pathways. Adv. Bot. Res. 2017, 83, 223–276. [Google Scholar] [CrossRef]
- Ma, J.; Qiu, D.; Pang, Y.; Gao, H.; Wang, X.; Qin, Y. Diverse roles of tocopherols in response to abiotic and biotic stresses and strategies for genetic biofortification in plants. Mol. Breed. 2020, 40, 18. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid metabolism in plants: The role of plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Dharshini, S.; Hoang, N.V.; Mahadevaiah, C.; Sarath Padmanabhan, T.S.; Alagarasan, G.; Suresha, G.S.; Kumar, R.; Meena, M.R.; Ram, B.; Appunu, C. Root transcriptome analysis of Saccharum spontaneum uncovers key genes and pathways in response to low-temperature stress. Environ. Exp. Bot. 2020, 171, 103935. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Li, Y.-R.; Liao, J.-X. Involvement of anthocyanins in the resistance to chilling-induced oxidative stress in Saccharum officinarum L. leaves. Plant Physiol. Biochem. 2013, 73, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.-C.; Wei, L.-P.; Huang, C.-M.; Wei, Y.-W.; Cao, H.-Q.; Xu, L.; Luo, H.-B.; Jiang, S.-L.; Deng, Z.-N.; Li, Y.-R. Transcriptome reveals differentially expressed genes in Saccharum spontaneum GX83-10 leaf under drought stress. Sugar Tech 2018, 20, 756–764. [Google Scholar] [CrossRef]
- Passamani, L.Z.; Barbosa, R.R.; Reis, R.S.; Heringer, A.S.; Rangel, P.L.; Santa-Catarina, C.; Grativol, C.; Veiga, C.F.M.; Souza-Filho, G.A.; Silveira, V. Salt stress induces changes in the proteomic profile of micropropagated sugarcane shoots. PLoS ONE 2017, 12, e0176076. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, A.S.; Lidon, F.C.; Batista-Santos, P.; Leitão, A.E.; Pais, I.P.; Ribeiro, A.I.; Ramalho, J.C. Biochemical and molecular characterization of the antioxidative system of Coffea sp. under cold conditions in genotypes with contrasting tolerance. J. Plant Physiol. 2010, 167, 333–342. [Google Scholar] [CrossRef]
- Guedes, F.A.d.F.; Nobres, P.; Rodrigues Ferreira, D.C.; Menezes-Silva, P.E.; Ribeiro-Alves, M.; Correa, R.L.; DaMatta, F.M.; Alves-Ferreira, M. Transcriptional memory contributes to drought tolerance in coffee (Coffea canephora) plants. Environ. Exp. Bot. 2018, 147, 220–233. [Google Scholar] [CrossRef]
- Kashyap, S.P.; Kumari, N.; Mishra, P.; Prasad Moharana, D.; Aamir, M.; Singh, B.; Prasanna, H.C. Transcriptional regulation-mediating ROS homeostasis and physio-biochemical changes in wild tomato (Solanum chilense) and cultivated tomato (Solanum lycopersicum) under high salinity. Saudi. J. Biol. Sci. 2020, 27, 1999–2009. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Moreno, D.A.; Ferreres, F.; del Mar Rubio-Wilhelmi, M.; Ruiz, J.M. Differential responses of five cherry tomato varieties to water stress: changes on phenolic metabolites and related enzymes. Phytochemistry 2011, 72, 723–729. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Kofroňová, M.; Hrdinová, A.; Mašková, P.; Tremlová, J.; Soudek, P.; Petrová, Š.; Pinkas, D.; Lipavská, H. Multi-component antioxidative system and robust carbohydrate status, the essence of plant arsenic tolerance. Antioxidants 2020, 9, 283. [Google Scholar] [CrossRef]
- Athar, H.-u.-R.; Khan, A.; Ashraf, M. Inducing salt tolerance in wheat by exogenously applied ascorbic acid through different modes. J. Plant Nutr. 2009, 32, 1799–1817. [Google Scholar] [CrossRef]
- Penella, C.; Calatayud, Á.; Melgar, J.C. Ascorbic acid alleviates water stress in young peach trees and improves their performance after rewatering. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, A.; Namdjoyan, S.; Soorki, A.A. Effects of exogenous melatonin and glutathione on zinc toxicity in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicol. Environ. Saf. 2020, 201, 110853. [Google Scholar] [CrossRef]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Polash, M.A.S.; Belal Chowdhury, M.; Afrin, S.; Burritt, D.J.; Murata, Y.; Hossain, M.A.; Afzal Hossain, M. Exogenous glutathione-mediated drought stress tolerance in Rice (Oryza sativa L.) is associated with lower oxidative damage and favorable ionic homeostasis. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 955–971. [Google Scholar] [CrossRef]
- Broad, R.C.; Bonneau, J.P.; Hellens, R.P.; Johnson, A.A.T. Manipulation of ascorbate biosynthetic, recycling, and regulatory pathways for improved abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 1790. [Google Scholar] [CrossRef] [PubMed]
- Sahli, R.; Rivière, C.; Neut, C.; Bero, J.; Sahuc, M.E.; Smaoui, A.; Beaufay, C.; Roumy, V.; Hennebelle, T.; Rouillé, Y.; et al. An ecological approach to discover new bioactive extracts and products: the case of extremophile plants. J. Pharm. Pharmacol. 2017, 69, 1041–1055. [Google Scholar] [CrossRef] [PubMed]
- Eshel, G.; Shaked, R.; Kazachkova, Y.; Khan, A.; Eppel, A.; Cisneros, A.; Acuna, T.; Gutterman, Y.; Tel-Zur, N.; Rachmilevitch, S.; et al. Anastatica hierochuntica, an Arabidopsis desert relative, Is tolerant to multiple abiotic stresses and exhibits species-specific and common stress tolerance strategies with its halophytic relative, Eutrema (Thellungiella) salsugineum. Front. Plant Sci. 2016, 7, 1992. [Google Scholar] [CrossRef] [PubMed]
- Wiszniewska, A.; Kamińska, I.; Hanus-Fajerska, E.; Sliwinska, E.; Koźmińska, A. Distinct co-tolerance responses to combined salinity and cadmium exposure in metallicolous and non-metallicolous ecotypes of Silene vulgaris. Ecotoxicol. Environ. Saf. 2020, 201, 110823. [Google Scholar] [CrossRef]
- Rana, A.; Bhangalia, S.; Singh, H.P. A new phenylethanoid glucoside from Jacaranda mimosifolia. Nat. Prod. Res. 2013, 27, 1167–1173. [Google Scholar] [CrossRef]
- Casagrande, J.C.; Macorini, L.F.B.; Antunes, K.A.; Santos, U.P.d.; Campos, J.F.; Dias-Júnior, N.M.; Sangalli, A.; Lima Cardoso, C.A.; do Carmo Vieira, M.; Rabelo, L.A.; et al. Antioxidant and cytotoxic activity of hydroethanolic extract from Jacaranda decurrens Leaves. PLoS ONE 2014, 9, e112748. [Google Scholar] [CrossRef]
- Pino, L.L.; García, T.H.; Delgado-Roche, L.; Rodeiro, I.; Hernández, I.; Vilegas, W.; Spengler, I. Polyphenolic profile by FIA/ESI/IT/MSn and antioxidant capacity of the ethanolic extract from the barks of Maytenus cajalbanica (Borhidi & O. Muñiz) Borhidi & O. Muñiz. Nat. Prod. Res. 2020, 34, 1481–1485. [Google Scholar] [CrossRef]
- Menéndez-Perdomo, I.M.; Wong-Guerra, M.; Fuentes-León, F.; Carrazana, E.; Casadelvalle, I.; Vidal, A.; Sánchez-Lamar, Á. Antioxidant, photoprotective and antimutagenic properties of Phyllanthus spp. from Cuban flora. J. Pharm. Pharmacogn. Res. 2017, 5, 251–261. [Google Scholar]
- Marrero Delange, D.; Morales Rico, C.L.; Canavaciolo, V.G.; Rodríguez Leyes, E.A.; Pérez, R.S. Volatile constituents from leaves of endemic Scutellaria havanensis Jacq. in Cuba. J. Essent. Oil Bear. Plants 2013, 16, 368–371. [Google Scholar] [CrossRef]
- Fernández-Calienes Valdés, A.; Monzote Fidalgo, L.; Sariego Ramos, I.; Marrero Delange, D.; Morales Rico, C.L.; Mendiola Martínez, J.; Cuéllar, A.C. Antiprotozoal screening of the Cuban native plant Scutellaria havanensis. Pharm. Biol. 2016, 54, 3197–3202. [Google Scholar] [CrossRef] [PubMed]
- Marrero Delange, D.; Morales Rico, C.L.; Gutierrez Cuesta, R. Chemical composition of chloroform extract from Scutellaria havanensis Jacq. Biotecnia 2017, 19, 40–44. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Kabani, A.O.; Lotti, C.; Alarcon, A.B.; Cuesta-Rubio, O.; Rastrelli, L. A fast and efficient HPLC-PDA–MS method for detection and identification of pyranochromanone acids in Calophyllum species. J. Pharm. Biomed. Anal. 2013, 76, 157–163. [Google Scholar] [CrossRef]
- Oubada, A.; García, M.; Bello-Alarcon, A.; Cuesta-Rubio, O.; Monzote, L. Antileishmanial activity of leaf extract from Calophyllum rivulare against Leishmania amazonensis Emir. J. Food Agric. 2014, 26, 807–812. [Google Scholar] [CrossRef]
- Perera Córdova, W.H.; Gómez Matos, M.; Tabart, J.; Sipel, A.; Kevers, C.; Dommes, J. In vitro characterization of antioxidant properties of Cuban endemic varieties of Erythroxylum alaternifolium A. Rich. isolation of two flavonol glycosides. J. Chil. Chem. Soc. 2012, 57, 1340–1343. [Google Scholar] [CrossRef]
- Nguyen, N.T.M.; Vicet-Muro, L.; Siverio-Mota, D.; Jorge-Rodriguez, M.E.; Gonzalez-Mosquera, D.M.; Castaneda-Noa, I. Phytochemical screening and evaluation of the central nervous system activity of the ethanolic extract of Eugenia clarensis Britton & P.Wilson. J. Pharm. Pharmacogn. Res. 2016, 4, 39–48. [Google Scholar]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Nediani, C.; Giovannelli, L. Oxidative stress and inflammation as targets for novel preventive and therapeutic approaches in non communicable diseases. Antioxidants 2020, 9, 290. [Google Scholar] [CrossRef]
- Kabera, J. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Cassels, B.K.; Asencio, M.; Conget, P.; Speisky, H.; Videla, L.A.; Lissi, E.A. Structure-antioxidative activity relationships in benzylisoquinoline alkaloids. Pharmacol. Res. 1995, 31, 103–107. [Google Scholar] [CrossRef]
- Rehman, S.; Khan, H. Advances in antioxidant potential of natural alkaloids. Curr. Bioact. Compd. 2017, 13, 101–108. [Google Scholar] [CrossRef]
- Pérez-Rosés, R.; Risco, E.; Vila, R.; Peñalver, P.; Cañigueral, S. Biological and nonbiological antioxidant activity of some essential oils. J. Agric. Food. Chem. 2016, 64, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some Biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.-M.; Enache, T.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food. Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef]
- Shen, T.; Wang, X.N.; Lou, H.X. Natural stilbenes: an overview. Nat. Prod. Rep. 2009, 26, 916–935. [Google Scholar] [CrossRef]
- Panda, S.S.; Chand, M.; Sakhuja, R.; Jain, S.C. Xanthones as potential antioxidants. Curr. Med. Chem. 2013, 20, 4481–4507. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: an updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T.I. BDDCS, the rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Magrone, T.; Villaño Valencia, D.; Chen, C.Y.O.; Palmery, M. Antioxidant, anti-Inflammatory, and microbial-modulating activities of nutraceuticals and functional foods. Oxid. Med. Cell. Longev. 2017, 2017, 7658617. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: a challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Jaganath, I.B.; Mullen, W.; Edwards, C.A.; Crozier, A. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic. Res. 2006, 40, 1035–1046. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Res. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Comet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Salehi, B.; Martorell, M.; Arbiser, J.L.; Sureda, A.; Martins, N.; Maurya, P.K.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or negative actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from plants protect against skin photoaging. Oxid. Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Shetty, A.; Magadum, S.; Managanvi, K. Vegetables as sources of antioxidants. J. Food Nutr. Disord. 2013, 2. [Google Scholar] [CrossRef]
- Anwar, H.; Hussain, G.; Mustafa, I. Antioxidants from Natural Sources. In Antioxidants in Foods and Its Applications; Shalaby, E., Azzam, G.M., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Dembinska-Kiec, A.; Mykkänen, O.; Kiec-Wilk, B.; Mykkänen, H. Antioxidant phytochemicals against type 2 diabetes. Br. J. Nutr. 2008, 99, ES109–ES117. [Google Scholar] [CrossRef]
- Ramsaha, S.; Aumjaud, B.E.; Neergheen-Bhujun, V.S.; Bahorun, T. Polyphenolic rich traditional plants and teas improve lipid stability in food test systems. J. Food Sci. Technol. 2015, 52, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Sardarodiyan, M.; Mohamadi Sani, A. Natural antioxidants: Sources, extraction and application in food systems. Nutr. Food Sci. 2016, 46, 363–373. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi-Kia, F.; Wibowo, J.K.; Elachouri, M.; Masumi, R.; Salehifard-Jouneghani, A.; Abolhassanzadeh, Z.; Lorigooini, Z. Battle between plants as antioxidants with free radicals in human body. J. Herbmed Pharmacol. 2020, 9, 191–199. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Racchi, M.L. Antioxidant defenses in plants with attention to Prunus and Citrus spp. Antioxidants 2013, 2, 340–369. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Can, Z.; Laghari, A.Q.; Şahin, H.; Malkoç, M. Wild edible mushrooms as a natural source of phenolics and antioxidants. J. Food Biochem. 2015, 39, 148–154. [Google Scholar] [CrossRef]
- Wilson, D.W.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The role of food antioxidants, benefits of functional foods, and Influence of feeding habits on the health of the older person: An overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cao, H.; Xiao, J. 2-Polyphenols: Absorption, bioavailability, and metabolomics. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 45–67. [Google Scholar] [CrossRef]
- Morebise, O. Medicinal plants of Dominica—Uses, chemical constituents, bioactivities and prospects. J. Med. Plants Stud. 2015, 3, 144–154. [Google Scholar]
- Alonso-Castro, A.J.; Domínguez, F.; Zapata-Morales, J.R.; Carranza-Álvarez, C. Plants used in the traditional medicine of Mesoamerica (Mexico and Central America) and the Caribbean for the treatment of obesity. J. Ethnopharmacol. 2015, 175, 335–345. [Google Scholar] [CrossRef]
- Clement, Y.N.; Baksh-Comeau, Y.S.; Seaforth, C.E. An ethnobotanical survey of medicinal plants in Trinidad. J. Ethnobiol. Ethnomed. 2015, 11, 67. [Google Scholar] [CrossRef]
- Rivera, D.E.; Ocampo, Y.C.; Castro, J.P.; Barrios, L.; Diaz, F.; Franco, L.A. A screening of plants used in Colombian traditional medicine revealed the anti-inflammatory potential of Physalis angulata calyces. Saudi J. Biol. Sci. 2019, 26, 1758–1766. [Google Scholar] [CrossRef]
- Stafford, L. The rich history, current state, and possible future of natural and traditional medicine in Cuba. HerbalGram 2010, 85, 40–49. [Google Scholar]
- Varona, P.; Herrera, D.; García, R.G.; Bonet, M.; Romero, T.; Venero, S.J. Smoking-attributable mortality in Cuba. MEDICC Rev. 2009, 11, 43–47. [Google Scholar] [PubMed]
- Burroughs Peña, M.S.; Patel, D.; Rodríguez Leyva, D.; Khan, B.V.; Sperling, L. Lifestyle risk factors and cardiovascular disease in Cubans and Cuban Americans. Cardiol. Res. Pract. 2012, 2012, 470705. [Google Scholar] [CrossRef] [PubMed]
- Landrove-Rodríguez, O.; Morejón-Giraldoni, A.; Venero-Fernández, S.; Suárez-Medina, R.; Almaguer-López, M.; Pallarols-Mariño, E.; Ramos-Valle, I.; Varona-Pérez, P.; Pérez-Jiménez, V.; Ordúñez, P. Enfermedades no transmisibles: Factores de riesgo y acciones para su prevención y control en Cuba. Rev. Panam. Salud. Publica 2018, 42, e23. [Google Scholar] [CrossRef] [PubMed]
- Lage, A. Science and challenges for Cuban public health in the 21st century. MEDICC Rev. 2019, 21, 7–14. [Google Scholar] [PubMed]
- Peña-Oyarzun, D.; Bravo-Sagua, R.; Diaz-Vega, A.; Aleman, L.; Chiong, M.; Garcia, L.; Bambs, C.; Troncoso, R.; Cifuentes, M.; Morselli, E.; et al. Autophagy and oxidative stress in non-communicable diseases: A matter of the inflammatory state? Free Radic. Biol. Med. 2018, 124, 61–78. [Google Scholar] [CrossRef]
- Cardona, L.M.F.; Cantillo, F.A.; Ojeda, J.B. Composición florística y estructura horizontal del bosque semideciduo micrófilo de la reserva ecológica Siboney-Juticí, Cuba. Rodriguésia 2017, 68, 315–324. [Google Scholar] [CrossRef]
- Berenguer Rivas, C.; Mas-Ortiz, M.; Batista-Corbal, P.; Costa-Acosta, J.; Escalona-Arranz, J. Chemical composition and in-vitro antioxidant activity of extracts of Adelia ricinella L. Rev. Cuba. Quim. 2018, 30, 191–209. [Google Scholar]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Ginwala, R.; McTish, E.; Raman, C.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.; Sagar, D.; Jain, P.; Khan, Z.K. Apigenin, a natural flavonoid, attenuates EAE severity through the modulation of dendritic cell and other immune cell functions. J. Neuroimmune Pharmacol. 2016, 11, 36–47. [Google Scholar] [CrossRef]
- Ochoa, A.; Fechine, J.; Escalona Arranz, J.; García Díaz, J.; Santos, S.G.; Silva, M. Phytochemical study of nonpolar extracts from Excoecaria lucida Sw. Leaves (Euphorbiaceae). Acta Chromatogr. 2015, 28, 1–9. [Google Scholar] [CrossRef]
- Ochoa-Pacheco, A.; Escalona Arranz, J.C.; Beaven, M.; Peres-Roses, R.; Gámez, Y.M.; Camacho-Pozo, M.I.; Maury, G.L.; de Macedo, M.B.; Cos, P.; Tavares, J.F.; et al. Bioassay-guided in vitro study of the antimicrobial and cytotoxic properties of the leaves from Excoecaria Lucida Sw. Pharmacogn. Res. 2017, 9, 396–400. [Google Scholar] [CrossRef]
- Da Silva, C.; Ochoa Pacheco, A.; Nogueira alves, R.; Tavares, J.; Silva, M.; Escalona Arranz, J. Anti-Trypanosoma cruzi activity in vitro of phases and isolated compounds from Excoecaria lucida leaves. Med. Chem. 2018, 14. [Google Scholar] [CrossRef]
- Kilic, I.; Yeşiloğlu, Y.; Bayrak, Y. Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Prieto, S.; Molina, J.; González, J.A. Contribution to the natural therapy for antiviral treatment. Rev. Latinoam. Quimica 2000, 18, 108. [Google Scholar]
- Díaz, Y.; Escalona Arranz, J.; Ochoa Pacheco, A.; García Díaz, J.; Monzote, L.; Gama, D.; Batista, J.; Silva, C.; Cos, P. Trypanocidal potentialities of skimmianine an alkaloid isolated from Zanthoxylum pistaciifolium griseb leaves. Pharmacogn. Res. 2020, 12, 322–327. [Google Scholar] [CrossRef]
- Huang, A.; Xu, H.; Zhan, R.; Chen, W.; Liu, J.; Chi, Y.; Chen, D.; Ji, X.; Luo, C. Metabolic profile of skimmianine in rats determined by ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. Molecules 2017, 22, 489. [Google Scholar] [CrossRef]
- Kiplimo, J.; Islam, M.; Koorbanally, N. A novel flavonoid and furoquinoline alkaloids from Vepris glomerata and their antioxidant activity. Nat. Prod. Commun. 2011, 6, 1847–1850. [Google Scholar] [CrossRef]
- Roig, J.T. (Ed.) Plantas Medicinales, Aromáticas o Venenosas de Cuba, 2nd ed.; Científico-Técnica: La Habana, Cuba, 2012; pp. 829–830. [Google Scholar]
- García Díaz, J.; Arranz, J.C.E.; Batista, D.; Fidalgo, L.M.; Acosta, J.E.; de Macedo, M.; Cos, P. Antileishmanial potentialities of croton linearis leaf essential oil. Nat. Prod. Commun. 2018, 13, 629–634. [Google Scholar] [CrossRef]
- García Díaz, J.; Tuenter, E.; Escalona Arranz, J.C.; Llauradó Maury, G.; Cos, P.; Pieters, L. Antimicrobial activity of leaf extracts and isolated constituents of Croton linearis. J. Ethnopharmacol. 2019, 236, 250–257. [Google Scholar] [CrossRef]
- Osorio, E.J.; Robledo, S.M.; Bastida, J. Chapter 2 Alkaloids with Antiprotozoal Activity. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2008; Volume 66, pp. 113–190. [Google Scholar]
- Zhao, Q.; Zhao, Y.; Wang, K. Antinociceptive and free radical scavenging activities of alkaloids isolated from Lindera angustifolia Chen. J. Ethnopharmacol. 2006, 106, 408–413. [Google Scholar] [CrossRef]
- Núñez Sellés, A.J.; Vélez Castro, H.T.; Agüero-Agüero, J.; González-González, J.; Naddeo, F.; De Simone, F.; Rastrelli, L. Isolation and quantitative analysis of phenolic antioxidants, free sugars, and polyols from mango (Mangifera indica L.) stem bark aqueous decoction used in Cuba as a nutritional supplement. J. Agric. Food Chem. 2002, 50, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Escalona Arranz, J.; Pérez-Rosés, R.; Urdaneta, I.; Camacho, M.; Rodriguez Amado, J.R.; Licea-Jiménez, I. Antimicrobial activity of extracts from Tamarindus indica L. leaves. Pharmacogn. Mag. 2010, 6, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Escalona Arranz, J.; Rodriguez Amado, J.R.; Pérez-Rosés, R.; Cañizares-Lay, J.; Sierra, G.; Morris, H.; Licea-Jiménez, I. Metabolites extraction optimization in Tamarindus indica L. leaves. Bol. Latinoam. Caribe Plant. Med. Aromat. 2011, 10, 369–378. [Google Scholar]
- Dehesa, M.A.; Jauregui, O.; Cañigueral, S. Estudio por HPLC-MS/MS de compuestos fenólicos presentes en las hojas de Tamarindus indica L. Rev. Fitoter. 2006, 6, 79–82. [Google Scholar]
- Escalona-Arranz, J.C.; Garcia-Diaz, J.; Perez-Rosés, R.; De la Vega, J.; Rodríguez-Amado, J.; Morris-Quevedo, H.J. Effect of Tamarindus indica L. leaves’ fluid extract on human blood cells. Nat. Prod. Res. 2014, 28, 1485–1488. [Google Scholar] [CrossRef]
- Escalona-Arranz, J.C.; Perez-Rosés, R.; Rodríguez-Amado, J.; Morris-Quevedo, H.J.; Mwasi, L.B.; Cabrera-Sotomayor, O.; Machado-García, R.; Fong-Lórez, O.; Alfonso-Castillo, A.; Puente-Zapata, E. Antioxidant and toxicological evaluation of a Tamarindus indica L. leaf fluid extract. Nat. Prod. Res. 2016, 30, 456–459. [Google Scholar] [CrossRef]
- Rodeiro, I.; Donato, M.T.; Jiménez, N.; Garrido, G.; Delgado, R.; Gómez-Lechón, M.J. Effects of Mangifera indica L. aqueous extract (Vimang) on primary culture of rat hepatocytes. Food Chem. Toxicol. 2007, 45, 2506–2512. [Google Scholar] [CrossRef]
- Garrido-Suárez, B.B.; Garrido, G.; Delgado, R.; Bosch, F.; del Rabí, M. A Mangifera indica L. extract could be used to treat neuropathic pain and implication of mangiferin. Molecules 2010, 15, 9035–9045. [Google Scholar] [CrossRef]
- Martínez, G.; Delgado, R.; Pérez, G.; Garrido, G.; Núñez Sellés, A.J.; León, O.S. Evaluation of the in vitro antioxidant activity of Mangifera indica L. extract (Vimang). Phytother. Res. 2000, 14, 424–427. [Google Scholar] [CrossRef]
- Martínez Sánchez, G.; Rodríguez, H.M.A.; Giuliani, A.; Núñez Sellés, A.J.; Pons Rodríguez, N.; Fernández, O.S.L.; Re, L. Protective effect of Mangifera indica L. extract (Vimang®) on the injury associated with hepatic ischaemia reperfusion. Phytother. Res. 2003, 17, 197–201. [Google Scholar] [CrossRef]
- Núñez, A.J.; Páez, E.; Amaro, D.; Acosta, J.; Agüero, J.; Capote, R.; Gárciga, M.R.; Morales, I.G.; García, O.; Garrido, G.; et al. Composiciones Farmacéuticas y Suplementos Nutricionales a Partir de Extractos de Mangifera indica. L. Patent No. 22846, 30 October 2002. Available online: http://databaseinvestigacion.ucn.cl/index.php?option=com_abook&view=book&id=579:composiciones-farmaceuticas-y-suplementos-nutricionales-a-partir-de-extractos-de-mangifera-indica-l&catid=7:patentes&Itemid=341 (accessed on 26 October 2020).
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and functional foods: The foods for the future world. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ novel formulations: The good, the Bad, the unknown and patents involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef]
- Zeisel, S.H. Regulation of “Nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K. Functional nutraceuticals: Past, present, and future. In Neutraceuticals; Grumezescu, A.M., Ed.; Academic Press: Cambridge, UK; Elsevier: London, UK, 2016; Volume 4, pp. 41–78. [Google Scholar]
- Santini, A. Nutraceuticals: Redefining a concept. Ann. Pharmacol. Pharm. 2018, 3, 1147. [Google Scholar]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from food and natural products: Neuroprotection and safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef]
- Saha, R. Cosmeceuticals and herbal drugs: Practical uses. Int. J. Pharm. Sci. Res. 2012, 3, 59–65. [Google Scholar] [CrossRef]
- Yahya, N.A.; Attan, N.; Wahab, R.A. An overview of cosmeceutically relevant plant extracts and strategies for extraction of plant-based bioactive compounds. Food Bioprod. Process. 2018, 112, 69–85. [Google Scholar] [CrossRef]
- Mohammad, I.S.; Naveed, M.; Ijaz, S.; Shumzaid, M.; Hassan, S.; Muhammad, K.S.; Rasool, F.; Akhtar, N.; Ishaq, H.M.; Khan, H.M.S. Phytocosmeceutical formulation development, characterization and its in-vivo investigations. Biomed. Pharmacother. 2018, 107, 806–817. [Google Scholar] [CrossRef]
- Aburjai, T.; Natsheh, F.M. Plants used in cosmetics. Phytother. Res. 2003, 17, 987–1000. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The use of plants in skin-Care products, cosmetics and fragrances: Past and present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Arct, J.; Pytkowska, K. Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Campanella, L.; Crescentini, G.; Avino, P. Chemical composition and nutritional evaluation of some natural and commercial food products based on Spirulina. Analusis 1999, 27, 533–540. [Google Scholar] [CrossRef]
- Pérez, L.O.; Ferrer, B.B.; Suárez, V.M.; Leyva, I.T.; Segura, M.S.; Padrón, Y.C.; Abraham, C.M.; Hernández Ramírez, P.; Santovenia, J.M. In vitro effect of Spirulina on the human lymphocytes of healthy donors and patients with cellular immunodeficiency. Rev. Cuba. Hematol. Inmunol. Hemoter. 2008, 24, 1–7. [Google Scholar]
- Bonal Ruiz, R.; Odio, R.M.R.; Carrión, M.E.B. Moringa oleifera: A healthy option for the well-being. Revista Medisan 2012, 16, 1596–1608. [Google Scholar]
- Baldermann, S.; Blagojević, L.; Frede, K.; Klopsch, R.; Neugart, S.; Neumann, A.; Ngwene, B.; Norkeweit, J.; Schröter, D.; Schröter, A.; et al. Are neglected plants the food for the future? Crit. Rev. Plant Sci. 2016, 35, 106–119. [Google Scholar] [CrossRef]
- D’Amelia, V.; Aversano, R.; Chiaiese, P.; Carputo, D. The antioxidant properties of plant flavonoids: their exploitation by molecular plant breeding. Phytochem. Rev. 2018, 17, 611–625. [Google Scholar] [CrossRef]
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Khan, M.M.A.; Mohi-Ud-Din, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Plant probiotic bacteria Bacillus and Paraburkholderia improve growth, yield and content of antioxidants in strawberry fruit. Sci. Rep. 2018, 8, 2504. [Google Scholar] [CrossRef]
- Roriz, M.; Carvalho, S.M.P.; Castro, P.M.L.; Vasconcelos, M.W. Legume biofortification and the role of plant growth-promoting bacteria in a sustainable agricultural era. Agronomy 2020, 10, 435. [Google Scholar] [CrossRef]
- Sanghera, G.S.; Malhotra, P.K.; Sidhu, G.S.; Sharma, V.K.; Sharma, B.B.; Karan, R. Genetic engineering of crop plants for enhanced antioxidants activity. Int. J. Adv. Res. Technol. 2013, 2, 428–458. [Google Scholar]
- Ku, Y.-S.; Rehman, H.M.; Lam, H.-M. Possible roles of rhizospheric and endophytic microbes to provide a safe and affordable means of crop biofortification. Agronomy 2019, 9, 764. [Google Scholar] [CrossRef]
- Wright, J. Sustainable Agriculture and Food Security in an Era of Oil Scarcity: Lessons from Cuba; Eartscan: London, UK, 2009. [Google Scholar]
- Goulart, F.; Galán, Á.L.; Nelson, E.; Soares-Filho, B. Conservation lessons from Cuba: Connecting science and policy. Biol. Conserv. 2018, 217, 280–288. [Google Scholar] [CrossRef]
- Companioni, N.; Rodriíguez-Nodals, A.; Sardiñas, J. Avances de la agricultura urbana, suburbana y familiar. Agroecología 2017, 12, 91–98. [Google Scholar]
- Altieri, M.A.; Companioni, N.; Cañizares, K.; Murphy, C.; Rosset, P.; Bourque, M.; Nicholls, C.I. The greening of the “barrios”: Urban agriculture for food security in Cuba. Agric. Hum. Values 1999, 16, 131–140. [Google Scholar] [CrossRef]
- Terry-Alfonso, E.; Ruiz-Padrón, J.; Falcón-Rodríguez, A.; Socarrás-Armenteros, Y. Oligosacarinas stimulate the growth and yield on tomato (Solanum lycopersicum L.) under protected conditions. Cult. Trop. 2019, 40, 4. [Google Scholar]
- Poinapen, D.; Brown, D.C.W.; Beeharry, G.K. Seed orientation and magnetic field strength have more influence on tomato seed performance than relative humidity and duration of exposure to non-uniform static magnetic fields. J. Plant Physiol. 2013, 170, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- González Aguilera, J.; Martín, R. Magnetically treated water stimulated of germination and development of Solanum lycopersicum L. seedlings. Rev. Bras. Agropecu. Sustent. 2016, 6, 47–53. [Google Scholar]
- Ferrer Dubois, A.E.; González Aguilera, J.; Fung Boix, Y.; Isaac Aleman, E.; Zuffo, A. Use of GREMAG® technology to improve seed germination and seedling survival. In Ciência em Foco; Zuffo, A.M., González Aguilera, J., Rodrigues de Oliveira, B., Eds.; Pantanal Editoria: Nova Xavantina, Mato Grosso, Brazil, 2019; pp. 138–149. [Google Scholar]
- Isaac Aleman, E.; Jumwa, A.; Fung Boix, Y.; Gonzalez Olmedo, J.; Chalfun-Junior, A. Effects of EMFs on some biological parameters in coffee plants (Coffea arabica L.) obtained by in vitro propagation. Pol. J. Environ. Stud. 2014, 23, 95–101. [Google Scholar]
- Boix, Y.F.; Victório, C.P.; Lage, C.L.S.; Defaveri, A.C.A.; Arruda, R.O.; Sato, A. Efecto de la aplicación de un campo magnético sobre la germinación in vitro de semillas de Rosmarinus officinalis L. Biotecnol. Veg. 2010, 10, 105–111. [Google Scholar]
- Boix, Y.F.; Arruda, R.C.O.; Defaveri, A.C.A.; Sato, A.; Lage, C.L.S.; Victório, C.P. Callus in Rosmarinus officinalis L. (Lamiaceae): A morphoanatomical, histochemical and volatile analysis. Plant Biosyst. 2013, 147, 751–757. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Dobránszki, J. Magnetic fields: How is plant growth and development impacted? Protoplasma 2016, 253, 231–248. [Google Scholar] [CrossRef]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef] [PubMed]
- Dannehl, D. Effects of electricity on plant responses. Sci. Hortic. 2018, 234, 382–392. [Google Scholar] [CrossRef]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic field (MF) applications in plants: An overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.S.; Akhade, O.P.; Bhojane, K.G.; Sadar, M.D.; Folane, P.; Biyani, K.R. A review on recent techniques of extraction and isolation of lycopene from tomato. Int. J. Res. Rev. 2020, 7, 487–490. [Google Scholar]
- Ferrer Dubois, A.E.; Leite, G.O.; Rocha, J.B.T. Irrigation of Solanum lycopersicum L. with magnetically treated water increases antioxidant properties of its tomato fruits. Electromagn. Biol. Med. 2013, 32, 355–362. [Google Scholar] [CrossRef]
- Ferrer Dubois, A.E.; Fung Boix, Y.; Isaac Aleman, E.; Beenaerts, N.; Cuypers, A. Phytochemical determination of Solanum lycopersicum L. fruits irrigated with water treated with static magnetic field. Rev. Cub. Quím. 2018, 30, 232–242. [Google Scholar]
- Boix, Y.F.; Aleman, E.I.; Dubois, A.F.; Botta, A.M. Riego con agua tratada magnéticamente en Rosmarinus officinalis L. (romero) como alternativa en la propagación convencional. Centro Agrícola 2008, 35, 23–27. [Google Scholar]
- Fung Boix, Y.; Isaac Aleman, E.; Molina-Torres, J.; Ramírez-Chávez, E.; Arruda, R.; Hendrix, S.; Beenaerts, N.; Victório, C.; Gómez Luna, L.; Martínez Manrique, C.; et al. Magnetically treated water on phytochemical compounds of Rosmarinus officinalis L. Int. J. Agric. Environ. Biotechnol. 2018, 3, 297–303. [Google Scholar] [CrossRef]
- Fung Boix, Y.; Molina Torres, J.; Ramírez Chávez, E.; Gómez Luna, L.; Quiñones-Galvez, J.; Ferrer Dubois, A.; Isaac Alemán, E.; Cuypers, A. Evaluación cualitativa de monoterpenos en Rosmarinus officinalis cultivados con agua tratada magnéticamente. Cult. Trop. 2016, 37, 136–141. [Google Scholar]
- Rodríguez-Ferreiro, A.O.; Fung-Boix, Y.; Ochoa-Pacheco, A.; Ortiz-Beaton, E.; Díaz-Fernádez, U. Parámetros físicos, físicos-químicos y químicos de extractos de Origanum majorana l. cultivado utilizando agua magnetizada. Rev. Cuba. Quim. 2018, 30, 454–469. [Google Scholar]
- Ninfali, P.; Antonini, E.; Frati, A.; Scarpa, E.S. C-glycosyl flavonoids from Beta vulgaris cicla and Betalains from Beta vulgaris rubra: Antioxidant, anticancer and antiinflammatory activities—A review. Phytother. Res. 2017, 31, 871–884. [Google Scholar] [CrossRef]
- Hozayn, M.; Abd El Monem, A.A.; Abdelraouf, R.E.; Abdalla, M.M. Do magnetic water affect water use efficiency, quality and yield of sugar beet (Beta vulgaris L.) plant under arid region conditions? J. Agron. 2013, 12, 1–10. [Google Scholar] [CrossRef]
- Ospina-Salazar, D.I.; Benavides-Bolaños, J.A.; Zúñiga-Escobar, O.; Muñoz-Perea, C.G. Fotosíntesis y rendimiento de biomasa en ají Tabasco, rábano y maíz sometidos a agua tratada magnéticamente. Cienc. Tecnol. Agropecu. 2018, 19, 291–305. [Google Scholar] [CrossRef]
- Khalid, M.; Hassani, D.; Bilal, M.; Liao, J.; Huang, D. Elevation of secondary metabolites synthesis in Brassica campestris ssp. chinensis L. via exogenous inoculation of Piriformospora indica with appropriate fertilizer. PLoS ONE 2017, 12, e0177185. [Google Scholar] [CrossRef]
- Jiménez Gómez, A.; Celador Lera, L.; Fradejas-Bayón, M.; Rivas, R. Plant probiotic bacteria enhance the quality of fruit and horticultural crops. AIMS Microbiol. 2017, 3, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, A.; García-Fraile, P.; Flores-Félix, J.D.; Rivas, R. Plants Probiotics as a Tool to Produce Highly Functional Fruits. In Bioactive Molecules in Food; Reference Series in Phytochemistry; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–13. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Lee, I.J. Gibberellins producing Bacillus methylotrophicus KE2 supports plant growth and enhances nutritional metabolites and food values of lettuce. Plant Physiol. Biochem. 2016, 109, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Hossain, M.M. Plant Probiotics in Phosphorus Nutrition in Crops, with Special Reference to Rice. In Bacteria in Agrobiology: Plant Probiotics; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 325–363. [Google Scholar] [CrossRef]
- He, Y.; Pantigoso, H.A.; Wu, Z.; Vivanco, J.M. Co-inoculation of Bacillus sp. and Pseudomonas putida at different development stages acts as a biostimulant to promote growth, yield and nutrient uptake of tomato. J. Appl. Microbiol. 2019, 127, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Peña Borrego, M.D.; de Zayas Pérez, M.R.; Rodríguez Fernández, R.M. La producción científica sobre biofertilizantes en Cuba en el período 2008–2012: Un análisis bibliometrico de las revistas cubanas. Cult. Trop. 2015, 36, 44–54. [Google Scholar]
- Ochoa-Velasco, C.E.; Valadez-Blanco, R.; Salas-Coronado, R.; Sustaita-Rivera, F.; Hernández-Carlos, B.; García-Ortega, S.; Santos-Sánchez, N.F. Effect of nitrogen fertilization and Bacillus licheniformis biofertilizer addition on the antioxidants compounds and antioxidant activity of greenhouse cultivated tomato fruits (Solanum lycopersicum L. var. Sheva). Sci. Hortic. 2016, 201, 338–345. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Majumder, S.; Datta, K.; Datta, S.K. Rice biofortification: High iron, zinc, and vitamin-A to fight against “Hidden Hunger”. Agronomy 2019, 9, 803. [Google Scholar] [CrossRef]
- De los Milagros Orberá Ratón, T.; Nápoles Vinent, S.; de Jesús Serrat Díaz, M.; Ortega Delgado, E.; Ramos Barbosa, H. The new rhizospheric bacteria Brevibacillus benefits eggplant and peeper growth and productivity under organoponic system. Agric. Res. 2014, 3, 395–398. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, A.; Rives-Rodríguez, N.; Acebo-Guerrero, Y.; Diaz-de la Osa, A.; Heydrich-Pérez, M.; Divan Baldani, V.L. Potencialidades de las bacterias diazotróficas asociativas en la promoción del crecimiento vegetal y el control de Pyricularia oryzae (Sacc.) en el cultivo del arroz (Oryza sativa L.). Rev. Prot. Veg. 2014, 29, 1–10. [Google Scholar]
- Riahi, L.; Cherif, H.; Miladi, S.; Neifar, M.; Bejaoui, B.; Chouchane, H.; Masmoudi, A.S.; Cherif, A. Use of plant growth promoting bacteria as an efficient biotechnological tool to enhance the biomass and secondary metabolites production of the industrial crop Pelargonium graveolens L’Hér. under semi-controlled conditions. Ind. Crops Prod. 2020, 154, 112721. [Google Scholar] [CrossRef]
- Pandey, C.; Bajpai, V.K.; Negi, Y.K.; Rather, I.A.; Maheshwari, D.K. Effect of plant growth promoting Bacillus spp. on nutritional properties of Amaranthus hypochondriacus grains. Saudi J. Biol. Sci. 2018, 25, 1066–1071. [Google Scholar] [CrossRef]
- Cappellari, L.D.R.; Santoro, M.V.; Schmidt, A.; Gershenzon, J.; Banchio, E. Induction of essential oil production in Mentha x piperita by plant growth promoting bacteria was correlated with an increase in jasmonate and salicylate levels and a higher density of glandular trichomes. Plant Physiol. Biochem. 2019, 141, 142–153. [Google Scholar] [CrossRef]
- Rojas Badía, M.; Bello-González, M.; Ríos-Rocafull, Y.; Lugo-Moya, D.; Sánchez, J. Utilización de cepas de Bacillus como promotores de crecimiento en hortalizas comerciales. Acta Agron. 2020, 69, 54–60. [Google Scholar] [CrossRef]
- Terry, E.; Ángel, L.; Hernández-Rodríguez, A. Microorganismos benéficos como biofertilizantes eficientes para el cultivo del tomate (Lycopersicon esculentum, Mill). Rev. Colomb. Biotecnol. 2005, 7, 47–54. [Google Scholar]
- Nápoles Vinent, S.; Serrat Díaz, M.; Ortega Delgado, E.; Ramos Barbosa, H.; Orberá Ratón, T. Efectos de Brevibacillus bortelensis B65 sobre la germinación y el desarrollo de posturas de hortalizas en fase de semillero. Cult. Trop. 2014, 35, 17–23. [Google Scholar]
- Tellez-Soria, T.; Orberá-Ratón, T. Efecto estimulador del crecimiento de dos biopreparados biotecnológicos en cultivos de remolacha (Beta vulgaris L.). Rev. Cuba. Quím. 2018, 30, 483–494. [Google Scholar]
- Hernández-Rodríguez, A.; Heydrich-Pérez, M.; Diallo, B.; El Jaziri, M.; Vandeputte, O.M. Cell-free culture medium of Burkholderia cepacia improves seed germination and seedling growth in maize (Zea mays) and rice (Oryza sativa). Plant Growth Regul. 2010, 60, 191–197. [Google Scholar] [CrossRef]
- Sánchez, D.; Inguanzo, L.; del Monte-Martínez, A.; Rojas Badía, M. Biological feasibility of the use of Bacilli strains producing indolacetic acid 3 in the in vitro growth of rice crop. Rev. Cuba. Cien. Biol. 2019, 7, 1–10. [Google Scholar]
- Calero Hurtado, A.; Pérez Díaz, Y.; Olivera Viciedo, D.; Quintero Rodríguez, E.; Peña Calzada, K.; Theodore Nedd, L.L.; Jiménez Hernández, J. Effect of different application forms of efficient microorganisms on the agricultural productive of two bean cultivars. Rev. Fac. Nacional Agron. Medellín 2019, 72, 8927–8935. [Google Scholar] [CrossRef]
- Nelissen, H.; Moloney, M.; Inzé, D. Translational research: From pot to plot. Plant Biotechnol. J. 2014, 12, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.S.; Beebe, S.; Crespi, M.; Delbreil, B.; González, E.M.; Gruber, V.; Lejeune-Henaut, I.; Link, W.; Monteros, M.J.; Prats, E.; et al. Abiotic stress responses in legumes: Strategies used to cope with environmental challenges. Crit. Rev. Plant Sci. 2015, 34, 237–280. [Google Scholar] [CrossRef]
- Kohli, A.; Miro, B.; Balié, J.; d’A Hughes, J. Photosynthesis research: a model to bridge fundamental science, translational products, and socio-economic considerations in agriculture. J. Exp. Bot. 2020, 71, 2281–2298. [Google Scholar] [CrossRef]
- Passioura, J.B. Translational research in agriculture. Can we do it better? Crop Pasture Sci. 2020, 71, 517–528, 512. [Google Scholar] [CrossRef]
| Species | Natural Products |
|---|---|
| Capsicum sp. | Cream; Tincture |
| Bixa orellana | Oil extract |
| Allium cepa | Cream; Syrup |
| Zea mays | Syrup |
| Passiflora incarnata | Syrup |
| Mentha piperita | Dry drug; Fluid Extract; Syrup |
| Citrus aurantium | Tincture; Syrup |
| Origanum vulgare | Syrup |
| Pinus caribaea | Cream; Fluid extract |
| Bidens alba | Dry drug; infusion |
| Calendula officinalis | Syrup |
| Allium sativum | Tincture; Syrup; Tablet |
| Zingiber officinale | Tincture |
| Musa paradisiaca | Pediculicidal lotion |
| Cymbopogon citratus | Cream |
| Salvia officinalis | Cream; Fluid extract; Syrup |
| Mangifera indica | Cosmetic cream; Tablet; Aqueous extract |
| Rhizophora mangle | Tincture; Fluid extract |
| Matricaria recutita o Matricaria chamomilla | Mouthwashes |
| Psidium guajava | Dry drug; infusion |
| Moringa oleífera | Dry drug |
| Endemic Species | Antioxidant Compounds | Bioactive Properties | References |
|---|---|---|---|
| Jacaranda arborea | Polyphenols and flavonoids (luteolin, jacarananone, tripterpens, ursolic acid and oleanolic acid) | Anticarcinogenic and sedative | [82,83] |
| Maytenus cajalbanica | Polyphenols | [84] | |
| Phyllanthus orbicularis | Phenols and flavonoids | Photoprotective and antimutagenic | [85] |
| Phyllanthus chamaecristoides | |||
| Phyllanthus microdictyus | |||
| Phyllanthus williamioides | |||
| Scutellaria havanensis | Flavonoids (wogonin) | Anti-inflammatory, anti-allergic, anxiolytic, neuroprotective, anticonvulsant, antithrombotic, anticarcinogenic, anti-arthritic, antisplasmodial, antiviral and antimicrobial | [86,87,88] |
| Calophyllum rivulare | Pyranochromanone acids and amentoflavone | [89,90] | |
| Erythroxylum alaternifolium var. alaternifolium | Flavonols (quercetin-3-O-rutinoside and ombuin-3-O-rutinoside) | [91] | |
| Erythroxylum alaternifolium var. parvifolium | |||
| Eugenia clarensis | Phenols, tannins, triterpenoids, sterols, flavonoids, coumarins, | Sedative | [92] |
| TOPIC | Total of Documents | % of the Total |
|---|---|---|
| Antioxidants | 455,065 | 100 |
| Antioxidants/plant | 114,148 | 25.08 |
| Antioxidant category | % of the Plant Total | |
| Alkaloids | 4937 | 4.33 |
| Sulfur compounds | 1237 | 1.08 |
| Partial scores for group one | 6174 | 5.41 |
| Terpene/terpenoid | 6086 | 5.34 |
| Essential oil | 5931 | 5.20 |
| Carotene/carotenoid | 9689 | 8.49 |
| Partial scores for group two | 21,706 | 19.03 |
| Polyphenol/phenol | 49,267 | 43.16 |
| Flavonoid | 27,058 | 23.70 |
| Tannin | 6191 | 5.42 |
| Coumarin | 1487 | 1.30 |
| Partial scores for group three | 84,003 | 73.58 |
| Crop Species (Scientific Name) | Plant Growth-Promoting Bacteria * | ||
|---|---|---|---|
| PGPB | Plant Growth-Promoting Effects | Reference | |
| Tomato (Solanum lycopersicum) | Bacillus sp. | Seed germination and vigor; root development | [231] |
| Azospirillum brasilense | Crop yield | [232] | |
| Eggplant (Solanum melongena) | Brevibacillus borstelensis * | Seedlings: Germination index; shoot root length, biomass | [233] |
| Adult plants: Number of flowers, leaves and fruits; fruit, crop yield | [226] | ||
| Sugar beet (Beta vulgaris) | Brevibacillus borstelensis * | Seed emergence; plant growth | [234] |
| Pepper (Capsicum annum) | Brevibacillus borstelensis * | Seedlings: seed emergence; biomass, root development | [233] |
| Adult plants: Number of flowers, leaves and fruits; crop yield | [226] | ||
| Maize (Zea mays) | Bacillus sp. | Seed germination and vigor; root development | [231] |
| Burkholderia cepacia | Seed germination, seedling growth | [235] | |
| Rice (Oryza sativa) | Burkholderia cepacia | Seed germination, seedling growth | [235] |
| Bacillus sp. | Root formation and development | [236] | |
| Pseudomonas sp. | Increased plant growth, dry biomass, total N | [227] | |
| Common bean (Phaseolus vulgaris) | Consortium: Bacillus subtilis, Lactobacillus bulgaricus, Saccharomyces cerevisiae | Crop yield | [237] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llauradó Maury, G.; Méndez Rodríguez, D.; Hendrix, S.; Escalona Arranz, J.C.; Fung Boix, Y.; Pacheco, A.O.; García Díaz, J.; Morris-Quevedo, H.J.; Ferrer Dubois, A.; Aleman, E.I.; et al. Antioxidants in Plants: A Valorization Potential Emphasizing the Need for the Conservation of Plant Biodiversity in Cuba. Antioxidants 2020, 9, 1048. https://doi.org/10.3390/antiox9111048
Llauradó Maury G, Méndez Rodríguez D, Hendrix S, Escalona Arranz JC, Fung Boix Y, Pacheco AO, García Díaz J, Morris-Quevedo HJ, Ferrer Dubois A, Aleman EI, et al. Antioxidants in Plants: A Valorization Potential Emphasizing the Need for the Conservation of Plant Biodiversity in Cuba. Antioxidants. 2020; 9(11):1048. https://doi.org/10.3390/antiox9111048
Chicago/Turabian StyleLlauradó Maury, Gabriel, Daniel Méndez Rodríguez, Sophie Hendrix, Julio César Escalona Arranz, Yilan Fung Boix, Ania Ochoa Pacheco, Jesús García Díaz, Humberto J. Morris-Quevedo, Albys Ferrer Dubois, Elizabeth Isaac Aleman, and et al. 2020. "Antioxidants in Plants: A Valorization Potential Emphasizing the Need for the Conservation of Plant Biodiversity in Cuba" Antioxidants 9, no. 11: 1048. https://doi.org/10.3390/antiox9111048
APA StyleLlauradó Maury, G., Méndez Rodríguez, D., Hendrix, S., Escalona Arranz, J. C., Fung Boix, Y., Pacheco, A. O., García Díaz, J., Morris-Quevedo, H. J., Ferrer Dubois, A., Aleman, E. I., Beenaerts, N., Méndez-Santos, I. E., Orberá Ratón, T., Cos, P., & Cuypers, A. (2020). Antioxidants in Plants: A Valorization Potential Emphasizing the Need for the Conservation of Plant Biodiversity in Cuba. Antioxidants, 9(11), 1048. https://doi.org/10.3390/antiox9111048








