Printing-Based Assay and Therapy of Antioxidants
Abstract
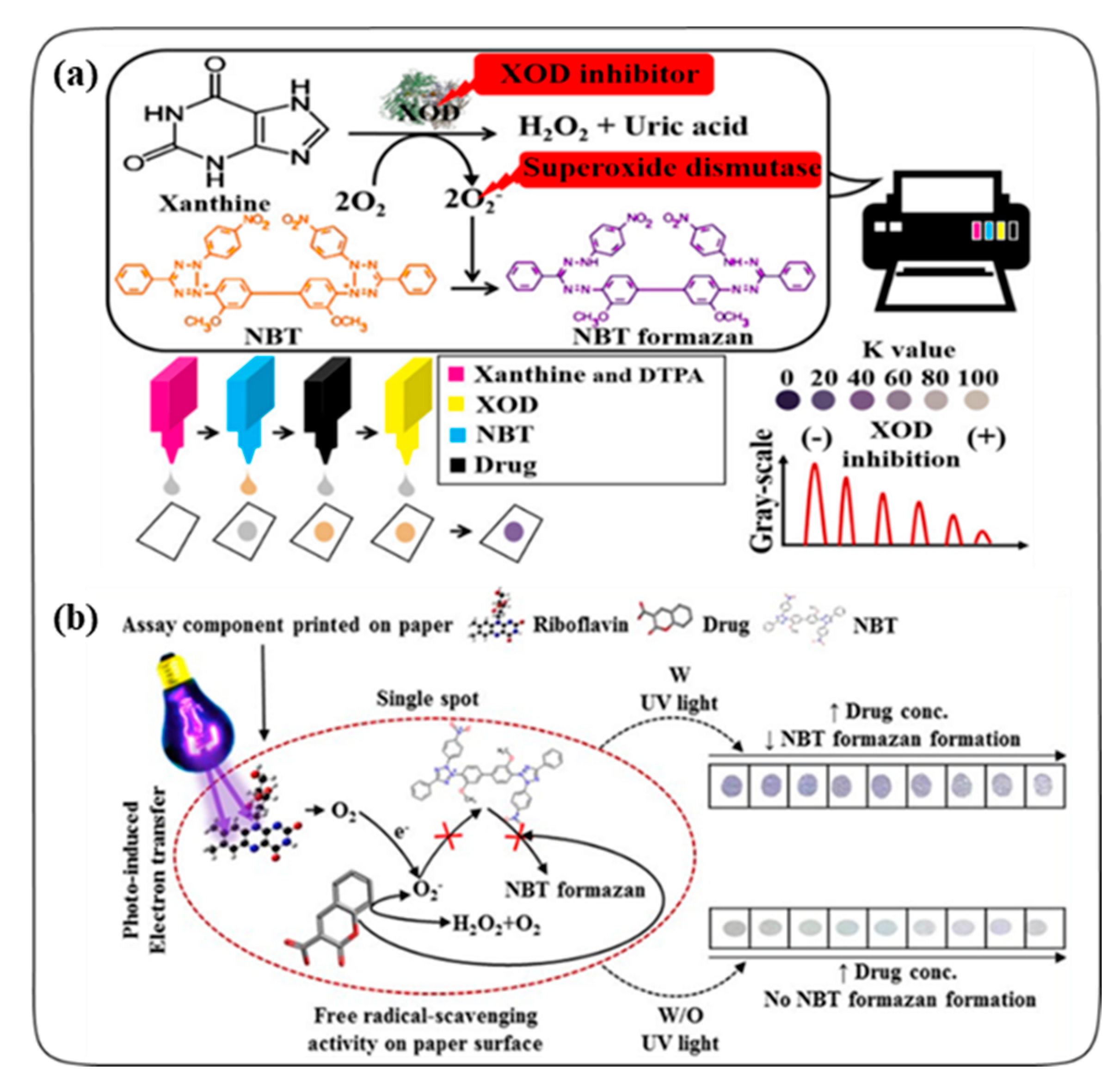
:1. Introduction
2. Printing-Based Antioxidant Assays
2.1. Colorimetric Assays
2.2. Electrochemical Assays
2.2.1. Nanomaterials
Carbon Nanotubes
Metal or Metal Oxide Nanoparticles
Cutting-Edge Nanomaterials
2.2.2. Enzymes
2.2.3. Polymers
2.2.4. Alternative Screen Printing Techniques
3. Printing-Based Antioxidant Therapies
3.1. Skin Therapeutics
3.2. Tissue Mimetic 3D Cultures
3.3. Bone Tissue Engineering
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bagchi, K.; Puri, S. Free radicals and antioxidants in health and disease: A review. East Mediterr. Health J. 1998, 4, 350–360. [Google Scholar]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.R. Coronary artery disease-free radical damage, antioxidant protection and the role of homocysteine. Basic Res. Cardiol. 2000, 95 (Suppl. 1), I65–I71. [Google Scholar] [CrossRef]
- Shah, A.M.; Channon, K.M. Free radicals and redox signalling in cardiovascular disease. Heart 2004, 90, 486–487. [Google Scholar] [CrossRef] [Green Version]
- Losada-Barreiro, S.; Bravo-Díaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Halliwell, B. Role of Free Radicals in the Neurodegenerative Diseases. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef]
- Singh, R.; Devi, S.; Gollen, R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: Larger-than-life. Diabetes-Metab. Res. 2015, 31, 113–126. [Google Scholar] [CrossRef]
- Owen, S.; Pearson, D.; Suarez-Mendez, V.; O’Driscoll, R.; Woodcock, A. Evidence of Free-Radical Activity in Asthma. N. Engl. J. Med. 1991, 325, 586–587. [Google Scholar] [PubMed]
- Moussa, Z.; Judeh, Z.M.; Ahmed, S.A. Nonenzymatic Exogenous and Endogenous Antioxidants. In Free Radical Biology and Medicine; IntechOpen: London, UK, 2019; Chapter 6. [Google Scholar]
- Palozza, P.; Krinsky, N.I. Antioxidant effects of carotenoids in Vivo and in Vitro: An overview. In Methods Enzymology; Academic Press: Cambridge, MA, USA, 1992; Volume 213, pp. 403–420. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell. Longev. 2016, 2016, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Khandel, P.; Shahi, S.K.; Soni, D.K.; Yadaw, R.K.; Kanwar, L. Alpinia calcarata: Potential source for the fabrication of bioactive silver nanoparticles. Nano Converg. 2018, 5, 37. [Google Scholar] [CrossRef]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mohammadi, S.Z.; Safaei, M.; Tajik, S. Applications of electrochemical sensors and biosensors based on modified screen-printed electrodes: A review. Anal. Methods 2020, 12, 1547–1560. [Google Scholar] [CrossRef]
- Lesch, A.; Cortés-Salazar, F.; Amstutz, V.; Tacchini, P.; Girault, H.H. Inkjet Printed Nanohydrogel Coated Carbon Nanotubes Electrodes For Matrix Independent Sensing. Anal. Chem. 2015, 87, 1026–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csiffáry, G.; Futo, P.; Adányi, N.; Kiss, A. Ascorbate oxidase-based amperometric biosensor for L-ascorbic acid determination in beverages. Food Technol. Biotech. 2016, 54, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, F.J.; Paschoal, C.W.A.; Arias, A.C. Printed and flexible biosensor for antioxidants using interdigitated ink-jetted electrodes and gravure-deposited active layer. Biosens. Bioelectron. 2015, 67, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Manavalan, S.; Rajaji, U.; Govindasamy, M.; Chen, T.W.; Ajmal Ali, M.; Alnakhli, A.K.; Al-Hemaid, F.M.A.; Elshikh, M.S. Determination of the antioxidant propyl gallate in meat by using a screen-printed electrode modified with CoSe2 nanoparticles and reduced graphene oxide. Microchim. Acta 2018, 185, 520. [Google Scholar] [CrossRef]
- Brainina, K.; Tarasov, A.; Khamzina, E.; Kazakov, Y.; Stozhko, N. Disposable Potentiometric Sensory System for Skin Antioxidant Activity Evaluation. Sensors 2019, 19, 2586. [Google Scholar] [CrossRef] [Green Version]
- Della Pelle, F.; Compagnone, D. Nanomaterial-Based Sensing and Biosensing of Phenolic Compounds and Related Antioxidant Capacity in Food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Pelle, F.; Rojas, D.; Scroccarello, A.; Del Carlo, M.; Ferraro, G.; Di Mattia, C.; Martuscelli, M.; Escarpa, A.; Compagnone, D. High-performance carbon black/molybdenum disulfide nanohybrid sensor for cocoa catechins determination using an extraction-free approach. Sensor Actuat B Chem. 2019, 296, 126651. [Google Scholar] [CrossRef]
- Pinyou, P.; Blay, V.; Muresan, L.M.; Noguer, T. Enzyme-modified electrodes for biosensors and biofuel cells. Mater. Horiz. 2019, 6, 1336–1358. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Campos, A.M.; Prado, T.M.; Furini, L.N.; Boas, N.V.; Calegaro, M.L.; Machado, S.A.S. Synergy between Printex nano-carbons and silver nanoparticles for sensitive estimation of antioxidant activity. Anal. Chim. Acta 2016, 926, 88–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talarico, D.; Arduini, F.; Constantino, A.; Del Carlo, M.; Compagnone, D.; Moscone, D.; Palleschi, G. Carbon black as successful screen-printed electrode modifier for phenolic compound detection. Electrochem. Commun. 2015, 60, 78–82. [Google Scholar] [CrossRef]
- Sanati, A.; Jalali, M.; Raeissi, K.; Karimzadeh, F.; Kharaziha, M.; Mahshid, S.S.; Mahshid, S. A review on recent advancements in electrochemical biosensing using carbonaceous nanomaterials. Microchim. Acta 2019, 186, 773. [Google Scholar] [CrossRef] [PubMed]
- Seman, R.N.A.R.; Azam, M.A.; Ani, M.H. Graphene/transition metal dichalcogenides hybrid supercapacitor electrode: Status, challenges, and perspectives. Nanotechnology 2018, 29, 502001. [Google Scholar] [CrossRef]
- Rojas, D.; Della Pelle, F.; Del Carlo, M.; Compagnone, D.; Escarpa, A. Group VI transition metal dichalcogenides as antifouling transducers for electrochemical oxidation of catechol-containing structures. Electrochem. Commun. 2020, 115, 106718. [Google Scholar] [CrossRef]
- Lee, J.; Samson, A.A.S.; Song, J.M. Inkjet-Printing Enzyme Inhibitory Assay Based on Determination of Ejection Volume. Anal. Chem. 2017, 89, 2009–2016. [Google Scholar] [CrossRef]
- Samson, A.A.S.; Lee, J.; Song, J.M. Inkjet printing-based photo-induced electron transfer reaction on parchment paper using riboflavin as a photosensitizer. Anal. Chim. Acta 2018, 1012, 49–59. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet Printing for Materials and Devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Delaney, J.T.; Smith, P.J.; Schubert, U.S. Inkjet printing of proteins. Soft Matter 2009, 5, 4866–4877. [Google Scholar] [CrossRef]
- Hasenbank, M.S.; Edwards, T.; Fu, E.; Garzon, R.; Kosar, T.F.; Look, M.; Mashadi-Hossein, A.; Yager, P. Demonstration of multi-analyte patterning using piezoelectric inkjet printing of multiple layers. Anal. Chim. Acta 2008, 611, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Attaran, M. The rise of 3-D printing: The advantages of additive manufacturing over traditional manufacturing. Bus. Horiz. 2017, 60, 677–688. [Google Scholar] [CrossRef]
- Tanaka, T.; Ishikawa, A. Towards three-dimensional optical metamaterials. Nano Converg. 2017, 4, 34. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Sarkar, N.; Banerjee, D. Effects of PCL, PEG and PLGA polymers on curcumin release from calcium phosphate matrix for in vitro and in vivo bone regeneration. Mater. Today Chem. 2018, 8, 110–120. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Rial-Hermida, M.I.; Donnelly, R.F.; Larrañeta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Rijal, G.; Kim, B.S.; Pati, F.; Ha, D.-H.; Kim, S.W.; Cho, D.-W. Robust tissue growth and angiogenesis in large-sized scaffold by reducing H2O2-mediated oxidative stress. Biofabrication 2017, 9, 015013. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192. [Google Scholar] [CrossRef]
- Zhang, X.; Morits, M.; Jonkergouw, C.; Ora, A.; Valle-Delgado, J.J.; Farooq, M.; Ajdary, R.; Huan, S.; Linder, M.; Rojas, O.; et al. Three-Dimensional Printed Cell Culture Model Based on Spherical Colloidal Lignin Particles and Cellulose Nanofibril-Alginate Hydrogel. Biomacromolecules 2020, 21, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Liaw, C.-Y.; Guvendiren, M.J.B. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef]
- Tiboni, M.; Benedetti, S.; Skouras, A.; Curzi, G.; Perinelli, D.R.; Palmieri, G.F.; Casettari, L. 3D-printed microfluidic chip for the preparation of glycyrrhetinic acid-loaded ethanolic liposomes. Int. J. Pharm. 2020, 584, 119436. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Moreno, C. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Nn, A.J.M.; Plants, A. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 1–6. [Google Scholar]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet Printing—Process and Its Applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef]
- Sochor, J.; Dobes, J.; Krystofova, O.; Ruttkay-Nedecky, B.; Babula, P.; Pohanka, M.; Jurikova, T.; Zitka, O.; Adam, V.; Klejdus, B. Electrochemistry as a tool for studying antioxidant properties. Int. J. Electrochem. Sci 2013, 8, 8464–8489. [Google Scholar]
- Zrinski, I.; Pungjunun, K.; Martinez, S.; Zavašnik, J.; Stanković, D.; Kalcher, K.; Mehmeti, E. Evaluation of phenolic antioxidant capacity in beverages based on laccase immobilized on screen-printed carbon electrode modified with graphene nanoplatelets and gold nanoparticles. Microchem. J. 2020, 152, 104282. [Google Scholar] [CrossRef]
- Bardpho, C.; Rattanarat, P.; Siangproh, W.; Chailapakul, O. Ultra-high performance liquid chromatographic determination of antioxidants in teas using inkjet-printed graphene–polyaniline electrode. Talanta 2016, 148, 673–679. [Google Scholar] [CrossRef]
- Braik, M.; Barsan, M.M.; Dridi, C.; Ben Ali, M.; Brett, C.M.A. Highly sensitive amperometric enzyme biosensor for detection of superoxide based on conducting polymer/CNT modified electrodes and superoxide dismutase. Sens. Actuators B Chem. 2016, 236, 574–582. [Google Scholar] [CrossRef]
- Chae, S.H.; Lee, Y.H. Carbon nanotubes and graphene towards soft electronics. Nano Converg. 2014, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Murph, S.E.H.; Coopersmith, K.J.; Larsen, G.K. Anisotropic and Shape-Selective Nanomaterials: Structure-Property Relationships; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Bounegru, A.V.; Apetrei, C. Carbonaceous Nanomaterials Employed in the Development of Electrochemical Sensors Based on Screen-Printing Technique—A Review. Catalysts 2020, 10, 680. [Google Scholar] [CrossRef]
- Trojanowicz, M. Impact of nanotechnology on design of advanced screen-printed electrodes for different analytical applications. TrAC Trends Anal. Chem. 2016, 84, 22–47. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Kozma, G.; Kónya, Z. Multi-Walled Carbon Nanotubes. In Springer Handbook of Nanomaterials; Vajtai, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 147–188. [Google Scholar]
- Ma, X.; Yang, H.; Xiong, H.; Li, X.; Gao, J.; Gao, Y. Electrochemical behavior and determination of chlorogenic acid based on multi-walled carbon nanotubes modified screen-printed electrode. Sensors 2016, 16, 1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newair, E.F.; Kilmartin, P.A.; Garcia, F. Square wave voltammetric analysis of polyphenol content and antioxidant capacity of red wines using glassy carbon and disposable carbon nanotubes modified screen-printed electrodes. Eur. Food Res. Technol. 2018, 244, 1225–1237. [Google Scholar] [CrossRef]
- Araújo, D.A.G.; Camargo, J.R.; Pradela-Filho, L.A.; Lima, A.P.; Muñoz, R.A.A.; Takeuchi, R.M.; Janegitz, B.C.; Santos, A.L. A lab-made screen-printed electrode as a platform to study the effect of the size and functionalization of carbon nanotubes on the voltammetric determination of caffeic acid. Microchem. J. 2020, 158, 105297. [Google Scholar] [CrossRef]
- Wang, J.; Xu, D.; Kawde, A.-N.; Polsky, R. Metal Nanoparticle-Based Electrochemical Stripping Potentiometric Detection of DNA Hybridization. Anal. Chem. 2001, 73, 5576–5581. [Google Scholar] [CrossRef] [PubMed]
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P.J.B. Screen-Printed Electrodes Modified with Metal Nanoparticles for Small Molecule Sensing. Biosensors 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Singh, N.; Tomar, V.; Chandra, R. A review on electrochemical detection of serotonin based on surface modified electrodes. Biosens. Bioelectron. 2018, 107, 76–93. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; González-Cortés, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Nurul Karim, M.; Lee, H.J. Amperometric phenol biosensor based on covalent immobilization of tyrosinase on Au nanoparticle modified screen printed carbon electrodes. Talanta 2013, 116, 991–996. [Google Scholar] [CrossRef]
- Puangjan, A.; Chaiyasith, S. An efficient ZrO2/Co3O4/reduced graphene oxide nanocomposite electrochemical sensor for simultaneous determination of gallic acid, caffeic acid and protocatechuic acid natural antioxidants. Electrochim. Acta 2016, 211, 273–288. [Google Scholar] [CrossRef]
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello-Filho, O. Electrochemical Biosensors Based on Nanostructured Carbon Black: A Review. J. Nanomater. 2017, 2017, 4571614. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Kirsch, J.; Fergus, J.; Simonian, A. Modeling analysis of electrode fouling during electrolysis of phenolic compounds. Electrochim. Acta 2013, 94, 259–268. [Google Scholar] [CrossRef]
- Hanssen, B.L.; Siraj, S.; Wong, D.K.Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef]
- Rodríguez-Sevilla, E.; Ramírez-Silva, M.-T.; Romero-Romo, M.; Ibarra-Escutia, P.; Palomar-Pardavé, M. Electrochemical Quantification of the Antioxidant Capacity of Medicinal Plants Using Biosensors. Sensors 2014, 14, 14423–14439. [Google Scholar] [CrossRef]
- Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Matos, P.; Arcos-Martínez, M.J. Electrochemical determination of levetiracetam by screen-printed based biosensors. Bioelectrochemistry 2009, 74, 306–309. [Google Scholar] [CrossRef]
- Couto, R.A.S.; Lima, J.L.F.C.; Quinaz, M.B. Recent developments, characteristics and potential applications of screen-printed electrodes in pharmaceutical and biological analysis. Talanta 2016, 146, 801–814. [Google Scholar] [CrossRef]
- Mello, L.D.; Sotomayor, M.D.P.T.; Kubota, L.T. HRP-based amperometric biosensor for the polyphenols determination in vegetables extract. Sens. Actuat. B-Chem. 2003, 96, 636–645. [Google Scholar] [CrossRef]
- Bollella, P.; Katz, E. Enzyme-Based Biosensors: Tackling Electron Transfer Issues. Sensors 2020, 20, 3517. [Google Scholar] [CrossRef]
- Sinha, A.; Kalambate, P.K.; Mugo, S.M.; Kamau, P.; Chen, J.; Jain, R. Polymer hydrogel interfaces in electrochemical sensing strategies: A review. Trends Analyt. Chem. 2019, 118, 488–501. [Google Scholar] [CrossRef]
- Gouveia-Caridade, C.; Brett, C. Strategies, development and applications of polymer-modified electrodes for stripping analysis. Curr. Anal. Chem. 2008, 4, 206–214. [Google Scholar] [CrossRef]
- Cinti, S. Polymeric Materials for Printed-Based Electroanalytical (Bio)Applications. Chemosensors 2017, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.J.; Yoon, J.H.; Lee, K.G.; Choi, B.G. Potentiometric performance of flexible pH sensor based on polyaniline nanofiber arrays. Nano Converg. 2019, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Rodríguez, J.F.; Rojas, D.; Escarpa, A. Rapid and cost-effective benchtop microfabrication of disposable carbon-based electrochemical microfluidic devices. Sens. Actuat. B Chem. 2020, 324, 128679. [Google Scholar] [CrossRef]
- Martínez-López, J.I.; Mojica, M.; Rodríguez, C.A.; Siller, H.R. Xurography as a Rapid Fabrication Alternative for Point-of-Care Devices: Assessment of Passive Micromixers. Sensors 2016, 16, 705. [Google Scholar] [CrossRef] [Green Version]
- Bandodkar, A.J.; Jia, W.; Ramírez, J.; Wang, J. Biocompatible Enzymatic Roller Pens for Direct Writing of Biocatalytic Materials: “Do-it-Yourself”. Electrochem. Biosens. 2015, 4, 1215–1224. [Google Scholar]
- Ghosale, A.; Shankar, R.; Ganesan, V.; Shrivas, K. Direct-Writing of Paper Based Conductive Track using Silver Nano-ink for Electroanalytical Application. Electrochim. Acta 2016, 209, 511–520. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Bandodkar, A.J.; Parkhomovsky, S.; Wang, J.J.A. Stamp transfer electrodes for electrochemical sensing on non-planar and oversized surfaces. Analyst 2012, 137, 1570–1575. [Google Scholar] [CrossRef]
- Nayak, P.; Kurra, N.; Xia, C.; Alshareef, H.N. Highly Efficient Laser Scribed Graphene Electrodes for On-Chip Electrochemical Sensing Applications. Adv. Electron. Mater. 2016, 2, 1600185. [Google Scholar] [CrossRef]
- Eom, T.; Kim, E.; Kim, J.-S. In Vitro Antioxidant, Antiinflammation, and Anticancer Activities and Anthraquinone Content from Rumex crispus Root Extract and Fractions. Antioxidants 2020, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Fuente, B.d.l.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Antiproliferative Effect of Bioaccessible Fractions of Four Brassicaceae Microgreens on Human Colon Cancer Cells Linked to Their Phytochemical Composition. Antioxidants 2020, 9, 368. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Y.-X.; Liu, A.-L.; Wang, H.-D.; Wang, Y.-L.; Du, G.-H. Antioxidant, Anti-Inflammatory and Anti-Influenza Properties of Components from Chaenomeles speciosa. Molecules 2010, 15, 8507–8517. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Z.; Chen, H.; Chen, Z.; Tian, Y. Antioxidants: Potential antiviral agents for Japanese encephalitis virus infection. Int. J. Infect. Dis. 2014, 24, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Barai, A.C.; Paul, K.; Dey, A.; Manna, S.; Roy, S.; Bag, B.G.; Mukhopadhyay, C. Green synthesis of Nerium oleander-conjugated gold nanoparticles and study of its in vitro anticancer activity on MCF-7 cell lines and catalytic activity. Nano Converg. 2018, 5, 10. [Google Scholar] [CrossRef]
- Haghiashtiani, G.; Qiu, K.; Zhingre Sanchez, J.D.; Fuenning, Z.J.; Nair, P.; Ahlberg, S.E.; Iaizzo, P.A.; McAlpine, M.C. 3D printed patient-specific aortic root models with internal sensors for minimally invasive applications. Sci. Adv. 2020, 6, eabb4641. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Parameswaran, C.; Gupta, D. Large area flexible pressure/strain sensors and arrays using nanomaterials and printing techniques. Nano Converg. 2019, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Dudek, P. FDM 3D Printing Technology in Manufacturing Composite Elements. Arch. Metall. Mater. 2013, 58, 1416. [Google Scholar] [CrossRef]
- Giordano, R.A.; Wu, B.M.; Borland, S.W.; Cima, L.G.; Sachs, E.M.; Cima, M.J. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. J. Biomater. Sci. Polym. Ed. 1997, 8, 63–75. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Pineda, E.T.; Nerem, R.M.; Ahsan, T. Differentiation Patterns of Embryonic Stem Cells in Two- versus Three-Dimensional Culture. Cells Tissues Organs 2013, 197, 399–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitcholtan, K.; Asselin, E.; Parent, S.; Sykes, P.H.; Evans, J.J. Differences in growth properties of endometrial cancer in three dimensional (3D) culture and 2D cell monolayer. Exp. Cell Res. 2013, 319, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.P.; Vaquette, C.; Shabab, T.; Pérez, R.A.; Yang, Y.; Dargaville, T.R.; Shafiee, A.; Tran, P.A. Porous 3D Printed Scaffolds For Guided Bone Regeneration In a Rat Calvarial Defect Model. Appl. Mater. Today 2020, 20, 100706. [Google Scholar] [CrossRef]
- Vidal, L.; Kampleitner, C.; Brennan, M.Á.; Hoornaert, A.; Layrolle, P. Reconstruction of Large Skeletal Defects: Current Clinical Therapeutic Strategies and Future Directions Using 3D Printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [Green Version]
- Dang, W.; Ma, B.; Huan, Z.; Lin, R.; Wang, X.; Li, T.; Wu, J.; Ma, N.; Zhu, H.; Chang, J.; et al. LaB6 surface chemistry-reinforced scaffolds for treating bone tumors and bone defects. Appl. Mater. Today 2019, 16, 42–55. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Purushothaman, B.; Song, J.M. Printing-Based Assay and Therapy of Antioxidants. Antioxidants 2020, 9, 1052. https://doi.org/10.3390/antiox9111052
Hong S, Purushothaman B, Song JM. Printing-Based Assay and Therapy of Antioxidants. Antioxidants. 2020; 9(11):1052. https://doi.org/10.3390/antiox9111052
Chicago/Turabian StyleHong, Sera, Baskaran Purushothaman, and Joon Myong Song. 2020. "Printing-Based Assay and Therapy of Antioxidants" Antioxidants 9, no. 11: 1052. https://doi.org/10.3390/antiox9111052




