A Comprehensive Review on Source, Types, Effects, Nanotechnology, Detection, and Therapeutic Management of Reactive Carbonyl Species Associated with Various Chronic Diseases
Abstract
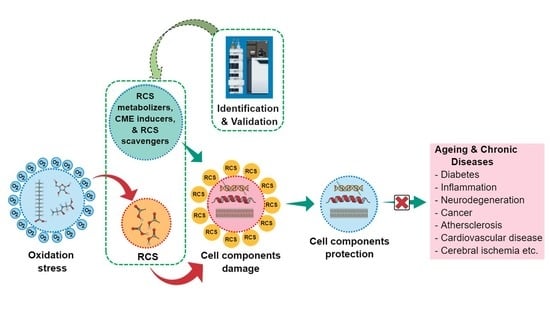
1. Introduction
2. RCS Sources and Types
2.1. RCS Generation via LP
2.2. RCS Generation via Glycoxidation
2.3. Types and Properties of RCS
2.3.1. 4-Hydroxy-2-Nonenal (HNE)
2.3.2. 4-Oxo-2-Nonenal (ONE)
2.3.3. Acrolein
2.3.4. Isolevuglandins (IsoLG)
2.3.5. Hexanal
2.3.6. Malondialdehyde (MDA)
2.3.7. Methylglyoxal (MGO)
3. RCS Actions
3.1. Cytotoxic Effects of RCS
3.2. Beneficial and Detrimental Effects of RCS (Receptor Level)
4. RCS Management
4.1. Endogenous RCS Metabolizers
4.1.1. ALDH
4.1.2. CYP450
4.1.3. Reductases
4.1.4. GST
4.1.5. Glyoxalase
4.2. Carbonyl Metabolizing Enzyme (CME) Inducers
4.2.1. Inducers of ALDH
4.2.2. Inducers of AKR
4.2.3. Inducers of CBR
4.2.4. Inducers of GST
4.2.5. Inducer of Glyoxalase
4.3. RCS Scavengers
4.3.1. Thiol-Based Scavengers
MESNA
Amifostine
4.3.2. Imidazole-Based Scavengers
4.3.3. Aminomethyl Phenols
Pyridoxamine (Pyridorin)
2-Hydroxybenzylamine (HOBA) and 5′-O-pentyl-pyridoxamine (PPM)
4.4. Natural RCS Scavangers
4.4.1. Carnosine (Endogenous)
4.4.2. Plant Products
5. Nanotechnology to Enhance the Bioavailability and Bio-efficacy of RCS Scavengers
6. Detection of RCS
6.1. Untargeted Analysis
6.2. Targeted Analysis
6.3. Mass Spectrometric (MS) Approaches
6.3.1. Label-Free MS
6.3.2. Label-Based MS
6.4. Non-MS Approaches
6.5. Validation Strategies
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwang, S.W.; Lee, Y.-M.; Aldini, G.; Yeum, K.-J. Targeting reactive carbonyl species with natural sequestering agents. Molecules 2016, 21, 280. [Google Scholar] [CrossRef]
- Davies, S.S.; Zhang, L.S. Reactive carbonyl species scavengers—Novel therapeutic approaches for chronic diseases. Curr. Pharmacol. Rep. 2017, 3, 51–67. [Google Scholar] [CrossRef]
- Semchyshyn, H.M. Reactive carbonyl species in vivo: Generation and dual biological effects. Sci. World J. 2014, 2014, 417842. [Google Scholar] [CrossRef] [PubMed]
- Zimniak, P. Relationship of electrophilic stress to aging. Free Radic. Biol. Med. 2011, 51, 1087–1105. [Google Scholar] [CrossRef] [PubMed]
- Voziyan, P.; Brown, K.L.; Chetyrkin, S.; Hudson, B. Site-specific AGE modifications in the extracellular matrix: A role for glyoxal in protein damage in diabetes. Clin. Chem. Lab. Med. 2014, 52, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.; Schmitt, C.P.; Zschocke, J.; Gross, M.-L.; Brismar, K.; Forsberg, E. Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice. Amino Acids 2012, 42, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Sun, G.; Ye, J.; Xu, H.; Wang, H.; Sun, X. Notoginsenoside R1-mediated neuroprotection involves estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways: A novel mechanism of Nrf2/ARE signaling activation. Free Radic. Res. 2014, 48, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Menini, S.; Iacobini, C.; Ricci, C.; Scipioni, A.; Fantauzzi, C.B.; Giaccari, A.; Salomone, E.; Canevotti, R.; Lapolla, A.; Orioli, M. D-carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. Br. J. Pharmacol. 2012, 166, 1344–1356. [Google Scholar] [CrossRef]
- Ellis, E.M. Reactive carbonyls and oxidative stress: Potential for therapeutic intervention. Pharmacol. Ther. 2007, 115, 13–24. [Google Scholar] [CrossRef]
- Talukdar, D.; Chaudhuri, B.S.; Ray, M.; Ray, S. Critical evaluation of toxic versus beneficial effects of methylglyoxal. Biochem. Mosc. 2009, 74, 1059–1069. [Google Scholar] [CrossRef]
- Birlouez-Aragon, I.; Morales, F.; Fogliano, V.; Pain, J.-P. The health and technological implications of a better control of neoformed contaminants by the food industry. Pathol. Biol. 2010, 58, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, M.; Xie, J. Mutagenicity of acrolein and acrolein-induced DNA adducts. Toxicol. Mech. Methods 2010, 20, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-K.; Baek, S.-O. Characterization of carbonyl compounds in the ambient air of an industrial city in Korea. Sensors 2011, 11, 949–963. [Google Scholar] [CrossRef]
- Robert, L.; Robert, A.-M.; Labat-Robert, J. The Maillard reaction–Illicite (bio) chemistry in tissues and food. Pathol. Biol. 2011, 59, 321–328. [Google Scholar] [CrossRef]
- Sticozzi, C.; Belmonte, G.; Pecorelli, A.; Arezzini, B.; Gardi, C.; Maioli, E.; Miracco, C.; Toscano, M.; Forman, H.J.; Valacchi, G. Cigarette smoke affects keratinocytes SRB1 expression and localization via H2O2 production and HNE protein adducts formation. PLoS ONE 2012, 7, 33592. [Google Scholar] [CrossRef]
- Uchida, K. Role of reactive aldehyde in cardiovascular diseases. Free Radic. Biol. Med. 2000, 28, 1685–1696. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Radic. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Metz, T.O.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: A novel therapy for treatment of diabetic complications. Arch. Biochem. Biophys. 2003, 419, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Turk, Z. Glycotoxines, carbonyl stress and relevance to diabetes and its complications. Physiol. Res. 2010, 59, 147–156. [Google Scholar]
- Kalapos, M.P. Where does plasma methylglyoxal originate from? Diabetes Res. Clin. Pract. 2013, 99, 260–271. [Google Scholar] [CrossRef]
- Onyango, A.N. Small reactive carbonyl compounds as tissue lipid oxidation products; and the mechanisms of their formation thereby. Chem. Phys. Lipids 2012, 165, 777–786. [Google Scholar] [CrossRef]
- Yadav, U.; Ramana, K.V. Regulation of NF-B-induced inflammatory signaling by lipid peroxidation-derived aldehydes. Oxidative Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Uchida, K.; Kanematsu, M.; Morimitsu, Y.; Osawa, T.; Noguchi, N.; Niki, E. Acrolein is a product of lipid peroxidation reaction Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J. Biol. Chem. 1998, 273, 16058–16066. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xie, C.; Markesbery, W.R. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging 2001, 22, 187–194. [Google Scholar] [CrossRef]
- Calingasan, N.Y.; Uchida, K.; Gibson, G.E. Protein-bound acrolein: A novel marker of oxidative stress in Alzheimer’s disease. J. Neurochem. 1999, 72, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.I.; Lynn, B.C.; Markesbery, W.R.; Lovell, M.A. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol. Aging 2006, 27, 1094–1099. [Google Scholar] [CrossRef]
- Kawaguchi-Niida, M.; Shibata, N.; Morikawa, S.; Uchida, K.; Yamamoto, T.; Sawada, T.; Kobayashi, M. Crotonaldehyde accumulates in glial cells of Alzheimer’s disease brain. Acta Neuropathol. 2006, 111, 422–429. [Google Scholar] [CrossRef]
- Asselin, C.; Bouchard, B.; Tardif, J.-C.; Rosiers, C.D. Circulating 4-hydroxynonenal–protein thioether adducts assessed by gas chromatography–mass spectrometry are increased with disease progression and aging in spontaneously hypertensive rats. Free Radic. Biol. Med. 2006, 41, 97–105. [Google Scholar] [CrossRef]
- Leonarduzzi, G.; Chiarpotto, E.; Biasi, F.; Poli, G. 4-Hydroxynonenal and cholesterol oxidation products in atherosclerosis. Mol. Nutr. Food Res. 2005, 49, 1044–1049. [Google Scholar] [CrossRef]
- Toyokuni, S.; Yamada, S.; Kashima, M.; Ihara, Y.; Yamada, Y.; Tanaka, T.; Hiai, H.; Seino, Y.; Uchida, K. Serum 4-hydroxy-2-nonenal-modified albumin is elevated in patients with type 2 diabetes mellitus. Antioxid. Redox Signal. 2000, 2, 681–685. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lin, X.; Bu, C.; Zhang, X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr. Metab. 2018, 15, 72. [Google Scholar] [CrossRef]
- Thornalley, P.J. Dicarbonyl intermediates in the Maillard reaction. Ann. N.Y. Acad. Sci. 2005, 1043, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef]
- Ahmed, N.; Ahmed, U.; Thornalley, P.J.; Hager, K.; Fleischer, G.; Münch, G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J. Neurochem. 2005, 92, 255–263. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Glycation research in amino acids: A place to call home. Amino Acids 2012, 42, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A. Energy and fructose from beverages sweetened with sugar or high-fructose corn syrup pose a health risk for some people. Adv. Nutr. 2013, 4, 220–225. [Google Scholar] [CrossRef]
- White, J.S. Challenging the fructose hypothesis: New perspectives on fructose consumption and metabolism. Adv. Nutr. 2013, 4, 246–256. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, K.; Wong, S.M.; O’Brien, P.J. Hepatocyte or serum albumin protein carbonylation by oxidized fructose metabolites: Glyceraldehyde or glycolaldehyde as endogenous toxins? Chem. Biol. Interact. 2010, 188, 31–37. [Google Scholar] [CrossRef]
- Yang, K.; Feng, C.; Lip, H.; Bruce, W.R.; O’Brien, P.J. Cytotoxic molecular mechanisms and cytoprotection by enzymic metabolism or autoxidation for glyceraldehyde, hydroxypyruvate and glycolaldehyde. Chem. Biol. Interact. 2011, 191, 315–321. [Google Scholar] [CrossRef]
- Spasojević, I.; Bajić, A.; Jovanović, K.; Spasić, M.; Andjus, P. Protective role of fructose in the metabolism of astroglial C6 cells exposed to hydrogen peroxide. Carbohydr. Res. 2009, 344, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Semchyshyn, H.M. Fructation in vivo: Detrimental and protective effects of fructose. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M. The polyol pathway as a mechanism for diabetic retinopathy: Attractive, elusive, and resilient. Exp. Diabetes Res. 2007, 2007. [Google Scholar] [CrossRef]
- Chung, S.S.; Ho, E.C.; Lam, K.S.; Chung, S.K. Contribution of polyol pathway to diabetes-induced oxidative stress. J. Am. Soc. Nephrol. 2003, 14, S233–S236. [Google Scholar] [CrossRef]
- Phillips, S.A.; Thornalley, P.J. The formation of methylglyoxal from triose phosphates: Investigation using a specific assay for methylglyoxal. Eur. J. Biochem. 1993, 212, 101–105. [Google Scholar] [CrossRef]
- Anderson, M.M.; Hazen, S.L.; Hsu, F.F.; Heinecke, J.W. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha, beta-unsaturated aldehydes by phagocytes at sites of inflammation. J. Clin. Investig. 1997, 99, 424–432. [Google Scholar]
- Liu, W.; Porter, N.A.; Schneider, C.; Brash, A.R.; Yin, H. Formation of 4-hydroxynonenal from cardiolipin oxidation: Intramolecular peroxyl radical addition and decomposition. Free Radic. Biol. Med. 2011, 50, 166–178. [Google Scholar] [CrossRef]
- Sayre, L.M.; Arora, P.K.; Iyer, R.S.; Salomon, R.G. Pyrrole formation from 4-hydroxynonenal and primary amines. Chem. Res. Toxicol. 1993, 6, 19–22. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Zhu, H.; Xiong, Y.L. Mass spectrometric evidence of malonaldehyde and 4-hydroxynonenal adductions to radical-scavenging soy peptides. J. Agric. Food Chem. 2012, 60, 9727–9736. [Google Scholar] [CrossRef]
- Li, W.; Laird, J.M.; Lu, L.; Roychowdhury, S.; Nagy, L.E.; Zhou, R.; Crabb, J.W.; Salomon, R.G. Isolevuglandins covalently modify phosphatidylethanolamines in vivo: Detection and quantitative analysis of hydroxylactam adducts. Free Radic. Biol. Med. 2009, 47, 1539–1552. [Google Scholar] [CrossRef]
- Guichardant, M.; Taibi-Tronche, P.; Fay, L.B.; Lagarde, M. Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic. Biol. Med. 1998, 25, 1049–1056. [Google Scholar] [CrossRef]
- Linhart, K.-B.; Glassen, K.; Peccerella, T.; Waldherr, R.; Linhart, H.; Bartsch, H.; Seitz, H.K. The generation of carcinogenic etheno-DNA adducts in the liver of patients with nonalcoholic fatty liver disease. Hepatobiliary Surg. Nutr. 2015, 4, 117. [Google Scholar]
- Itakura, K.; Osawa, T.; Uchida, K. Structure of a fluorescent compound formed from 4-hydroxy-2-nonenal and N α-hippuryllysine: A model for fluorophores derived from protein modifications by lipid peroxidation. J. Org. Chem. 1998, 63, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-H.; Liu, J.; Xu, G.; Yuan, Q.; Sayre, L.M. Model studies on protein side chain modification by 4-oxo-2-nonenal. Chem. Res. Toxicol. 2003, 16, 512–523. [Google Scholar] [CrossRef]
- Galligan, J.J.; Rose, K.L.; Beavers, W.N.; Hill, S.; Tallman, K.A.; Tansey, W.P.; Marnett, L.J. Stable histone adduction by 4-oxo-2-nonenal: A potential link between oxidative stress and epigenetics. J. Am. Chem. Soc. 2014, 136, 11864–11866. [Google Scholar] [CrossRef]
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.; Trent, M.B.; He, N.; Lick, S.D.; Zimniak, P.; Awasthi, Y.C.; Boor, P.J. Glutathione-S-transferase A4-4 modulates oxidative stress in endothelium: Possible role in human atherosclerosis. Atherosclerosis 2004, 173, 211–221. [Google Scholar] [CrossRef]
- Kehrer, J.P.; Biswal, S.S. The molecular effects of acrolein. Toxicol. Sci. 2000, 57, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Zemski Berry, K.A.; Murphy, R.C. Characterization of acrolein-glycerophosphoethanolamine lipid adducts using electrospray mass spectrometry. Chem. Res. Toxicol. 2007, 20, 1342–1351. [Google Scholar] [CrossRef]
- Uchida, K.; Kanematsu, M.; Sakai, K.; Matsuda, T.; Hattori, N.; Mizuno, Y.; Suzuki, D.; Miyata, T.; Noguchi, N.; Niki, E. Protein-bound acrolein: Potential markers for oxidative stress. Proc. Natl. Acad. Sci. USA 1998, 95, 4882–4887. [Google Scholar] [CrossRef]
- McCall, M.R.; Tang, J.Y.; Bielicki, J.K.; Forte, T.M. Inhibition of lecithin-cholesterol acyltransferase and modification of HDL apolipoproteins by aldehydes. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1599–1606. [Google Scholar] [CrossRef]
- Brame, C.J.; Salomon, R.G.; Morrow, J.D.; Roberts, L.J. Identification of extremely reactive γ-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J. Biol. Chem. 1999, 274, 13139–13146. [Google Scholar] [CrossRef]
- Zagol-Ikapitte, I.; Bernoud-Hubac, N.; Amarnath, V.; Roberts, L.J.; Boutaud, O.; Oates, J.A. Characterization of bis (levuglandinyl) urea derivatives as products of the reaction between prostaglandin H2 and arginine. Biochemistry 2004, 43, 5503–5510. [Google Scholar] [CrossRef]
- Carrier, E.J.; Amarnath, V.; Oates, J.A.; Boutaud, O. Characterization of covalent adducts of nucleosides and DNA formed by reaction with levuglandin. Biochemistry 2009, 48, 10775–10781. [Google Scholar] [CrossRef]
- DiFranco, E.; Subbanagounder, G.; Kim, S.; Murthi, K.; Taneda, S.; Monnier, V.M.; Salomon, R.G. Formation and stability of pyrrole adducts in the reaction of levuglandin E2 with proteins. Chem. Res. Toxicol. 1995, 8, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Jang, G.-F.; Zhang, L.; Crabb, J.W.; Laird, J.; Linetsky, M.; Salomon, R.G. Molecular structures of isolevuglandin-protein cross-links. Chem. Res. Toxicol. 2016, 29, 1628–1640. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Z.; Cox, B.E.; Amarnath, V.; Epand, R.F.; Epand, R.M.; Davies, S.S. Phosphatidylethanolamines modified by γ-ketoaldehyde (γKA) induce endoplasmic reticulum stress and endothelial activation. J. Biol. Chem. 2011, 286, 18170–18180. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, Z.; Amarnath, V.; Yancey, P.G.; Van Lenten, B.J.; Savage, J.R.; Fazio, S.; Linton, M.F.; Davies, S.S. Isolevuglandin-type lipid aldehydes induce the inflammatory response of macrophages by modifying phosphatidylethanolamines and activating the receptor for advanced glycation endproducts. Antioxid. Redox Signal. 2015, 22, 1633–1645. [Google Scholar] [CrossRef]
- Kirabo, A.; Fontana, V.; De Faria, A.P.; Loperena, R.; Galindo, C.L.; Wu, J.; Bikineyeva, A.T.; Dikalov, S.; Xiao, L.; Chen, W. DC isoketal-modified proteins activate T cells and promote hypertension. J. Clin. Investig. 2014, 124, 4642–4656. [Google Scholar] [CrossRef]
- Wu, J.; Saleh, M.A.; Kirabo, A.; Itani, H.A.; Montaniel, K.R.C.; Xiao, L.; Chen, W.; Mernaugh, R.L.; Cai, H.; Bernstein, K.E. Immune activation caused by vascular oxidation promotes fibrosis and hypertension. J. Clin. Investig. 2016, 126, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Davies, S.S.; Nakajima, T.; Ong, B.-H.; Kupershmidt, S.; Fessel, J.; Amarnath, V.; Anderson, M.E.; Boyden, P.A.; Viswanathan, P.C. Oxidative mediated lipid peroxidation recapitulates proarrhythmic effects on cardiac sodium channels. Circ. Res. 2005, 97, 1262–1269. [Google Scholar] [CrossRef]
- Sidorova, T.N.; Yermalitskaya, L.V.; Mace, L.C.; Wells, K.S.; Boutaud, O.; Prinsen, J.K.; Davies, S.S.; Roberts, L.J., II; Dikalov, S.I.; Glabe, C.G. Reactive γ-ketoaldehydes promote protein misfolding and preamyloid oligomer formation in rapidly-activated atrial cells. J. Mol. Cell. Cardiol. 2015, 79, 295–302. [Google Scholar] [CrossRef]
- Davies, S.S.; Amarnath, V.; Montine, K.S.; Bernoud-Hubac, N.; Boutaud, O.; Montine, T.J.; Roberts, L.J. Effects of reactive γ-ketoaldehydes formed by the isoprostane pathway (isoketals) and cyclooxygenase pathway (levuglandins) on proteasome function. FASEB J. 2002, 16, 715–717. [Google Scholar] [CrossRef]
- Jalali, M.; Sakhvid, M.J.Z.; Bahrami, A.; Berijani, N.; Mahjub, H. Oxidative stress biomarkers in exhaled breath of workers exposed to crystalline silica dust by SPME-GC-MS. J. Res. Health Sci. 2016, 16, 153. [Google Scholar]
- Alhamdani, M.-S.S.; Al-Kassir, A.-H.A.; Jaleel, N.A.; Hmood, A.M.; Ali, H.M. Elevated levels of alkanals, alkenals and 4-HO-alkenals in plasma of hemodialysis patients. Am. J. Nephrol. 2006, 26, 299–303. [Google Scholar] [CrossRef]
- Zhou, S.; Decker, E.A. Ability of amino acids, dipeptides, polyamines, and sulfhydryls to quench hexanal, a saturated aldehydic lipid oxidation product. J. Agric. Food Chem. 1999, 47, 1932–1936. [Google Scholar] [CrossRef]
- Pryor, W.A.; Stanley, J.P. Suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymic production of prostaglandin endoperoxides during autoxidation. J. Org. Chem. 1975, 40, 3615–3617. [Google Scholar] [CrossRef]
- Chio, K.; Tappel, A.L. Synthesis and characterization of the fluorescent products derived from malonaldehyde and amino acids. Biochemistry 1969, 8, 2821–2827. [Google Scholar] [CrossRef]
- Marnett, L.J. Chemistry and biology of DNA damage by malondialdehyde. IARC Sci. Publ. 1999, 17–27. [Google Scholar]
- Shuck, S.C.; Wauchope, O.R.; Rose, K.L.; Kingsley, P.J.; Rouzer, C.A.; Shell, S.M.; Sugitani, N.; Chazin, W.J.; Zagol-Ikapitte, I.; Boutaud, O. Protein modification by adenine propenal. Chem. Res. Toxicol. 2014, 27, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.W.; Westwood, M.E.; McLellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar]
- Ahmed, M.U.; Frye, E.B.; Degenhardt, T.P.; Thorpe, S.R.; Baynes, J.W. N ε-(carboxyethyl) lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 1997, 324, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, E.; Wells-Knecht, K.J.; Thorpe, S.R.; Baynes, J.W. Characterization of an imidazolium compound formed by reaction of methylglyoxal and N α-hippuryllysine. J. Chem. Soc. Perkin 1 1995, 2817–2818. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj. J. 2016, 33, 513–525. [Google Scholar] [CrossRef]
- Bierhaus, A.; Schiekofer, S.; Schwaninger, M.; Andrassy, M.; Humpert, P.M.; Chen, J.; Hong, M.; Luther, T.; Henle, T.; Klöting, I. Diabetes-associated sustained activation of the transcription factor nuclear factor-κB. Diabetes 2001, 50, 2792–2808. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P. RAGE mediates a novel proinflammatory axis: A central cell surface receptor for S100/calgranulin polypeptides. Cell 1999, 97, 889–901. [Google Scholar] [CrossRef]
- Coughlan, M.T.; Thorburn, D.R.; Penfold, S.A.; Laskowski, A.; Harcourt, B.E.; Sourris, K.C.; Tan, A.L.; Fukami, K.; Thallas-Bonke, V.; Nawroth, P.P. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J. Am. Soc. Nephrol. 2009, 20, 742–752. [Google Scholar] [CrossRef]
- Ye, Y.; Klenerman, D.; Finley, D. N-terminal ubiquitination of amyloidogenic proteins triggers removal of their oligomers by the proteasome holoenzyme. J. Mol. Biol. 2020, 432, 585–596. [Google Scholar] [CrossRef]
- Mano, J.; Biswas, M.; Sugimoto, K. Reactive carbonyl species: A missing link in ROS signaling. Plants 2019, 8, 391. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Aldini, G.; Carini, M.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2006, 10, 389–406. [Google Scholar] [CrossRef]
- Renke, J.; Popadiuk, S.; Korzon, M.; Bugajczyk, B.; Woźniak, M. Protein carbonyl groups’ content as a useful clinical marker of antioxidant barrier impairment in plasma of children with juvenile chronic arthritis. Free Radic. Biol. Med. 2000, 29, 101–104. [Google Scholar] [CrossRef]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.-A.; Zarkovic, N. Short overview of ROS as cell function regulators and their implications in therapy concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Mano, J. Reactive carbonyl species: Their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol. Biochem. 2012, 59, 90–97. [Google Scholar] [CrossRef]
- Buss, I.H.; Darlow, B.A.; Winterbourn, C.C. Elevated protein carbonyls and lipid peroxidation products correlating with myeloperoxidase in tracheal aspirates from premature infants. Pediatr. Res. 2000, 47, 640–645. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Bonham, M.J.; Buss, H.; Abu-Zidan, F.M.; Windsor, J.A. Elevated protein carbonyls as plasma markers of oxidative stress in acute pancreatitis. Pancreatology 2003, 3, 375–382. [Google Scholar] [CrossRef]
- Keller, J.N.; Schmitt, F.A.; Scheff, S.W.; Ding, Q.; Chen, Q.; Butterfield, D.A.; Markesbery, W.R. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 2005, 64, 1152–1156. [Google Scholar] [CrossRef]
- Forman, H.J.; Fukuto, J.M.; Miller, T.; Zhang, H.; Rinna, A.; Levy, S. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch. Biochem. Biophys. 2008, 477, 183–195. [Google Scholar] [CrossRef]
- Kalapos, M.P. The tandem of free radicals and methylglyoxal. Chem. Biol. Interact. 2008, 171, 251–271. [Google Scholar] [CrossRef]
- Huang, S.-X.; Yun, B.-S.; Ma, M.; Basu, H.S.; Church, D.R.; Ingenhorst, G.; Huang, Y.; Yang, D.; Lohman, J.R.; Tang, G.-L. Leinamycin E1 acting as an anticancer prodrug activated by reactive oxygen species. Proc. Natl. Acad. Sci. USA 2015, 112, 8278–8283. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, B.; Zhong, P.; Rajamanickam, V.; Dai, X.; Karvannan, K.; Zhou, H.; Zhang, X.; Liang, G. Increased Intracellular Reactive Oxygen Species Mediates the Anti-Cancer Effects of WZ35 via Activating Mitochondrial Apoptosis Pathway in Prostate Cancer Cells. Prostate 2017, 77, 489–504. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Harvey, K.; Stillwell, W. Anticancer properties of oxidation products of docosahexaenoic acid. Chem. Phys. Lipids 2008, 153, 47–56. [Google Scholar] [CrossRef]
- Dharmaraja, A.T. Role of reactive oxygen species (ROS) in therapeutics and drug resistance in cancer and bacteria. J. Med. Chem. 2017, 60, 3221–3240. [Google Scholar] [CrossRef]
- Si, Y.; Zhang, Z.; Wu, W.; Fu, Q.; Huang, K.; Nitin, N.; Ding, B.; Sun, G. Daylight-driven rechargeable antibacterial and antiviral nanofibrous membranes for bioprotective applications. Sci. Adv. 2018, 4, 5931. [Google Scholar] [CrossRef]
- Semba, T.; Sammons, R.; Wang, X.; Xie, X.; Dalby, K.N.; Ueno, N.T. JNK Signaling in Stem Cell Self-Renewal and Differentiation. Int. J. Mol. Sci. 2020, 21, 2613. [Google Scholar] [CrossRef]
- Luczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Dinkova-Kostova, A.T.; Iavicoli, I.; Di Paola, R.; Koverech, A.; Cuzzocrea, S.; Rizzarelli, E.; Calabrese, E.J. Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2012, 1822, 753–783. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzym. Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Jaiswal, A.K. Antioxidant-induced phosphorylation of tyrosine 486 leads to rapid nuclear export of Bach1 that allows Nrf2 to bind to the antioxidant response element and activate defensive gene expression. J. Biol. Chem. 2010, 285, 153–162. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020. [Google Scholar] [CrossRef]
- Prasad, K. AGE–RAGE stress: A changing landscape in pathology and treatment of Alzheimer’s disease. Mol. Cell. Biochem. 2019, 459, 95–112. [Google Scholar] [CrossRef]
- Rojas, A.; Morales, M.; Gonzalez, I.; Araya, P. Inhibition of RAGE axis signaling: A pharmacological challenge. Curr. Drug Targets 2019, 20, 340–346. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, J.; Chen, H.; Zhuge, Y.; Chen, H.; Qian, F.; Zhou, K.; Niu, C.; Wang, F.; Qiu, H. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Choudhury, D. Insulin Promotes Wound Healing by Inactivating NFkβP50/P65 and Activating Protein and Lipid Biosynthesis and alternating Pro/Anti-inflammatory Cytokines Dynamics. Biomol. Concepts 2019, 10, 11–24. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Nishino, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Glucagon-like peptide–1 suppresses advanced glycation end product–induced monocyte chemoattractant protein–1 expression in mesangial cells by reducing advanced glycation end product receptor level. Metabolism 2011, 60, 1271–1277. [Google Scholar] [CrossRef]
- Chen, X.; Mori, T.; Guo, Q.; Hu, C.; Ohsaki, Y.; Yoneki, Y.; Zhu, W.; Jiang, Y.; Endo, S.; Nakayama, K. Carbonyl stress induces hypertension and cardio–renal vascular injury in Dahl salt-sensitive rats. Hypertens. Res. 2013, 36, 361–367. [Google Scholar] [CrossRef]
- Sil, R.; Ray, D.; Chakraborti, A.S. Glycyrrhizin ameliorates metabolic syndrome-induced liver damage in experimental rat model. Mol. Cell. Biochem. 2015, 409, 177–189. [Google Scholar] [CrossRef]
- Lu, J.; Wu, D.; Zheng, Z.; Zheng, Y.; Hu, B.; Zhang, Z. Troxerutin protects against high cholesterol-induced cognitive deficits in mice. Brain 2011, 134, 783–797. [Google Scholar] [CrossRef]
- Bettiga, A.; Fiorio, F.; Di Marco, F.; Trevisani, F.; Romani, A.; Porrini, E.; Salonia, A.; Montorsi, F.; Vago, R. The modern western diet rich in advanced glycation end-products (AGEs): An overview of its impact on obesity and early progression of renal pathology. Nutrients 2019, 11, 1748. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Hu, F.B.; Schulze, M.B. Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013, 1, 152–162. [Google Scholar] [CrossRef]
- Bellier, J.; Nokin, M.-J.; Lardé, E.; Karoyan, P.; Peulen, O.; Castronovo, V.; Bellahcène, A. Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res. Clin. Pract. 2019, 148, 200–211. [Google Scholar] [CrossRef]
- Taguchi, A.; Blood, D.C.; del Toro, G.; Canet, A.; Lee, D.C.; Qu, W.; Tanji, N.; Lu, Y.; Lalla, E.; Fu, C. Blockade of RAGE–amphoterin signalling suppresses tumour growth and metastases. Nature 2000, 405, 354–360. [Google Scholar] [CrossRef]
- Siems, W.; Grune, T. Intracellular metabolism of 4-hydroxynonenal. Mol. Aspects Med. 2003, 24, 167–175. [Google Scholar] [CrossRef]
- Vasiliou, V.; Pappa, A.; Estey, T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab. Rev. 2004, 36, 279–299. [Google Scholar] [CrossRef]
- Townsend, A.J.; Leone-Kabler, S.; Haynes, R.L.; Wu, Y.; Szweda, L.; Bunting, K.D. Selective protection by stably transfected human ALDH3A1 (but not human ALDH1A1) against toxicity of aliphatic aldehydes in V79 cells. Chem. Biol. Interact. 2001, 130, 261–273. [Google Scholar] [CrossRef]
- Estey, T.; Piatigorsky, J.; Lassen, N.; Vasiliou, V. ALDH3A1: A corneal crystallin with diverse functions. Exp. Eye Res. 2007, 84, 3–12. [Google Scholar] [CrossRef]
- Amunom, T.; Stephens, L.J.; Conklin, D.J.; Srivastava, S.; Bhatnagar, A.; Prough, R.A. Several cytochromes P450 are aldehyde monooxygenases. Enzymol. Mol. Biol. Carbonyl Metab. 2005, 12, 118–123. [Google Scholar]
- Shoeb, M.H.; Ansari, N.K.; Srivastava, S.V.; Ramana, K. 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr. Med. Chem. 2014, 21, 230–237. [Google Scholar] [CrossRef]
- Short, D.M.; Lyon, R.; Watson, D.G.; Barski, O.A.; McGarvie, G.; Ellis, E.M. Metabolism of trans, trans-muconaldehyde, a cytotoxic metabolite of benzene, in mouse liver by alcohol dehydrogenase Adh1 and aldehyde reductase AKR1A4. Toxicol. Appl. Pharmacol. 2006, 210, 163–170. [Google Scholar] [CrossRef]
- Jin, Y.; Penning, T.M. Aldo-keto reductases and bioactivation/detoxication. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Glyoxalase I–structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 2003, 31, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, K.; Fujii-Kuriyama, Y. Ah receptor, a novel ligand-activated transcription factor. J. Biochem. 1997, 122, 1075–1079. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev. Pharmacol Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Sládek, N.E. Transient induction of increased aldehyde dehydrogenase 3A1 levels in cultured human breast (adeno) carcinoma cell lines via 5′-upstream xenobiotic, and electrophile, responsive elements is, respectively, estrogen receptor-dependent and-independent. Chem. Biol. Interact. 2003, 143, 63–74. [Google Scholar] [CrossRef]
- Liu, Y.; Kurita, A.; Nakashima, S.; Zhu, B.; Munemasa, S.; Nakamura, T.; Murata, Y.; Nakamura, Y. 3, 4-Dihydroxyphenylacetic acid is a potential aldehyde dehydrogenase inducer in murine hepatoma Hepa1c1c7 cells. Biosci. Biotech. Biochem. 2017, 81, 1978–1983. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Z.; Zhu, H.; Trush, M.A. Differential roles of 3H-1, 2-dithiole-3-thione-induced glutathione, glutathione S-transferase and aldose reductase in protecting against 4-hydroxy-2-nonenal toxicity in cultured cardiomyocytes. Arch. Biochem. Biophys. 2005, 439, 80–90. [Google Scholar] [CrossRef]
- Barski, O.A.; Tipparaju, S.M.; Bhatnagar, A. The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metabol. Rev. 2008, 40, 553–624. [Google Scholar] [CrossRef]
- Balcz, B.; Kirchner, L.; Cairns, N.; Fountoulakis, M.; Lubec, G. Increased brain protein levels of carbonyl reductase and alcohol dehydrogenase in Down syndrome and Alzheimer’s disease. In Protein Expression in Down Syndrome Brain; Springer: Vienna, Austria, 2001; pp. 193–201. [Google Scholar]
- Hu, R.; Xu, C.; Shen, G.; Jain, M.R.; Khor, T.O.; Gopalkrishnan, A.; Lin, W.; Reddy, B.; Chan, J.Y.; Kong, A.-N.T. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006, 79, 1944–1955. [Google Scholar] [CrossRef]
- Knight, T.R.; Choudhuri, S.; Klaassen, C.D. Induction of hepatic glutathione S-transferases in male mice by prototypes of various classes of microsomal enzyme inducers. Toxicol. Sci. 2008, 106, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Alfarano, M.; Pastore, D.; Fogliano, V.; Schalkwijk, C.G.; Oliviero, T. The effect of sulforaphane on glyoxalase I expression and activity in peripheral blood mononuclear cells. Nutrients 2018, 10, 1773. [Google Scholar] [CrossRef]
- Boutaud, O.; Brame, C.J.; Salomon, R.G.; Roberts, L.J.; Oates, J.A. Characterization of the lysyl adducts formed from prostaglandin H2 via the levuglandin pathway. Biochemistry 1999, 38, 9389–9396. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, L.; De Courten, B.; Garzon, D.; Altomare, A.; Marinello, C.; Jakubova, M.; Vallova, S.; Krumpolec, P.; Carini, M.; Ukropec, J. A carnosine intervention study in overweight human volunteers: Bioavailability and reactive carbonyl species sequestering effect. Sci. Rep. 2016, 6, 27224. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, G.; Sayre, L.M. Carnosine Inhibits (E)-4-Hydroxy-2-nonenal-Induced Protein Cross-Linking: Structural Characterization of Carnosine- HNE Adducts1. Chem. Res. Toxicol. 2003, 16, 1589–1597. [Google Scholar] [CrossRef]
- Marchette, L.D.; Wang, H.; Li, F.; Babizhayev, M.A.; Kasus-Jacobi, A. Carcinine has 4-hydroxynonenal scavenging property and neuroprotective effect in mouse retina. Invest. Ophthalmol. Vis. Sci. 2012, 53, 3572–3583. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, S.; Lv, X.; Sun, H.; Weng, J.; Liang, Y.; Zhou, D. The mechanism of mesna in protection from cisplatin-induced ovarian damage in female rats. J. Gynecol. Oncol. 2013, 24, 177–185. [Google Scholar] [CrossRef]
- Kabasakal, L.; Şehirli, A.Ö.; Çetinel, Ş.; Cikler, E.; Gedik, N.; Şener, G. Mesna (2-mercaptoethane sulfonate) prevents ischemia/reperfusion induced renal oxidative damage in rats. Life Sci. 2004, 75, 2329–2340. [Google Scholar] [CrossRef]
- Yilmaz, E.R.; Kertmen, H.; Gürer, B.; Kanat, M.A.; Arikok, A.T.; Ergüder, B.I.; Hasturk, A.E.; Ergil, J.; Sekerci, Z. The protective effect of 2-mercaptoethane sulfonate (MESNA) against traumatic brain injury in rats. Acta Neurochir. 2013, 155, 141–149. [Google Scholar] [CrossRef]
- Aluise, C.D.; Miriyala, S.; Noel, T.; Sultana, R.; Jungsuwadee, P.; Taylor, T.J.; Cai, J.; Pierce, W.M.; Vore, M.; Moscow, J.A. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-α release: Implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic. Biol. Med. 2011, 50, 1630–1638. [Google Scholar] [CrossRef]
- Dolgun, H.; Sekerci, Z.; Turkoglu, E.; Kertmen, H.; Yilmaz, E.R.; Anlar, M.; Erguder, I.B.; Tuna, H. Neuroprotective effect of mesna (2-mercaptoethane sulfonate) against spinal cord ischemia/reperfusion injury in rabbits. J. Clin. Neurosci. 2010, 17, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, A.; Morse, M.A.; Chung, F.-L. Inhibitory effects of 2-mercaptoethane sulfonate and 6-phenylhexyl isothiocyanate on urinary bladder tumorigenesis in rats induced by N-butyl-N-(4-hydroxybutyl) nitrosamine. Cancer Lett. 2003, 193, 11–16. [Google Scholar] [CrossRef]
- Sener, G.; Sehirli, O.; Ercan, F.; Sirvancı, S.; Gedik, N.; Kacmaz, A. Protective effect of MESNA (2-mercaptoethane sulfonate) against hepatic ischemia/reperfusion injury in rats. Surg. Today 2005, 35, 575–580. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, Z.; Jiang, Y.; Chen, F.; Wang, M. Acrolein scavengers: Reactivity, mechanism and impact on health. Mol. Nutr. Food Res. 2011, 55, 1375–1390. [Google Scholar] [CrossRef]
- Bispo, V.S.; de Arruda Campos, I.P.; Di Mascio, P.; Medeiros, M.H. Structural elucidation of a carnosine-acrolein adduct and its quantification in human urine samples. Sci. Rep. 2016, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.-S. Molecular mechanisms behind free radical scavengers function against oxidative stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef]
- Gu, J.; Zhu, S.; Li, X.; Wu, H.; Li, Y.; Hua, F. Effect of amifostine in head and neck cancer patients treated with radiotherapy: A systematic review and meta-analysis based on randomized controlled trials. PLoS ONE 2014, 9, e95968. [Google Scholar] [CrossRef]
- Buentzel, J.; Micke, O.; Adamietz, I.A.; Monnier, A.; Glatzel, M.; de Vries, A. Intravenous amifostine during chemoradiotherapy for head-and-neck cancer: A randomized placebo-controlled phase III study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 684–691. [Google Scholar] [CrossRef]
- Jellema, A.P.; Slotman, B.J.; Muller, M.J.; Leemans, C.R.; Smeele, L.E.; Hoekman, K.; Aaronson, N.K.; Langendijk, J.A. Radiotherapy alone, versus radiotherapy with amifostine 3 times weekly, versus radiotherapy with amifostine 5 times weekly: A prospective randomized study in squamous cell head and neck cancer. Cancer 2006, 107, 544–553. [Google Scholar] [CrossRef]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent modifiers: A chemical perspective on the reactivity of α, β-unsaturated carbonyls with thiols via hetero-Michael addition reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- LoPachin, R.M.; Barber, D.S.; Gavin, T. Molecular mechanisms of the conjugated α, β-unsaturated carbonyl derivatives: Relevance to neurotoxicity and neurodegenerative diseases. Toxicol. Sci. 2008, 104, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.P.; Podgorski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The thiol-Michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Hipkiss, A.R. Depression, diabetes and dementia: Formaldehyde may be a common causal agent; could carnosine, a pluripotent peptide, be protective? Aging Dis. 2017, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R. Carnosine, diabetes and Alzheimer’s disease. Expert Rev. Neurother. 2009, 9, 583–585. [Google Scholar] [CrossRef]
- Ramis, R.; Ortega-Castro, J.; Caballero, C.; Casasnovas, R.; Cerrillo, A.; Vilanova, B.; Adrover, M.; Frau, J. How does pyridoxamine inhibit the formation of advanced glycation end products? The role of its primary antioxidant activity. Antioxidants 2019, 8, 344. [Google Scholar] [CrossRef]
- Voziyan, P.A.; Hudson, B.G. Pyridoxamine as a multifunctional pharmaceutical: Targeting pathogenic glycation and oxidative damage. Cell. Mol. Life Sci. CMLS 2005, 62, 1671–1681. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Rao, L.; Yang, C.; Yuan, H.; Gao, T.; Chen, X.; Sun, H.; Xian, M.; Liu, C. Ratiometric Fluorescent Probe for Monitoring Endogenous Methylglyoxal in Living Cells and Diabetic Blood Samples. Anal. Chem. 2019, 91, 5646–5653. [Google Scholar] [CrossRef]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B6 and its role in cell metabolism and physiology. Cells 2018, 7, 84. [Google Scholar] [CrossRef]
- Mooney, S.; Leuendorf, J.-E.; Hendrickson, C.; Hellmann, H. Vitamin B6: A long known compound of surprising complexity. Molecules 2009, 14, 329–351. [Google Scholar] [CrossRef]
- Mascolo, E.; Vernì, F. Vitamin B6 and Diabetes: Relationship and Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 3669. [Google Scholar] [CrossRef]
- Schenone, M.; Dančík, V.; Wagner, B.K.; Clemons, P.A. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Chem. Biol. 2013, 9, 232. [Google Scholar] [CrossRef]
- Sztuba-Solinska, J.; Chavez-Calvillo, G.; Cline, S.E. Unveiling the druggable RNA targets and small molecule therapeutics. Bioorg. Med. Chem. 2019, 27, 2149–2165. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Matsunaga, A.; Oe, T. Inhibition effect of pyridoxamine on lipid hydroperoxide-derived modifications to human serum albumin. PLoS ONE 2018, 13, e0196050. [Google Scholar] [CrossRef]
- Vallon, V.; Komers, R. Pathophysiology of the diabetic kidney. Compr. Physiol. 2011, 1, 1175–1232. [Google Scholar]
- Tanimoto, M.; Gohda, T.; Kaneko, S.; Hagiwara, S.; Murakoshi, M.; Aoki, T.; Yamada, K.; Ito, T.; Matsumoto, M.; Horikoshi, S. Effect of pyridoxamine (K-163), an inhibitor of advanced glycation end products, on type 2 diabetic nephropathy in KK-Ay/Ta mice. Metabolism 2007, 56, 160–167. [Google Scholar] [CrossRef]
- Zagol-Ikapitte, I.; Matafonova, E.; Amarnath, V.; Bodine, C.L.; Boutaud, O.; Tirona, R.G.; Oates, J.A.; Roberts II, L.J.; Davies, S.S. Determination of the pharmacokinetics and oral bioavailability of salicylamine, a potent γ-ketoaldehyde scavenger, by LC/MS/MS. Pharmaceutics 2010, 2, 18–29. [Google Scholar] [CrossRef]
- Sane, R.; Agarwal, S.; Elmquist, W.F. Brain distribution and bioavailability of elacridar after different routes of administration in the mouse. Drug Metab. Dispos. 2012, 40, 1612–1619. [Google Scholar] [CrossRef]
- Zhao, J.; Lu, Y.; Du, S.; Song, X.; Bai, J.; Wang, Y. Comparative pharmacokinetic studies of borneol in mouse plasma and brain by different administrations. J. Zhejiang Univ. Sci. B 2012, 13, 990–996. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moghadam-Kia, S.; Werth, V.P. Prevention and treatment of systemic glucocorticoid side effects. Int. J. Dermatol. 2010, 49, 239–248. [Google Scholar] [CrossRef]
- Fong, T.G.; Tulebaev, S.R.; Inouye, S.K. Delirium in elderly adults: Diagnosis, prevention and treatment. Nat. Rev. Neurol. 2009, 5, 210. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Wassertheil-Smoller, S.; Thomson, C.; Aragaki, A.; Anderson, G.L.; Manson, J.E.; Patterson, R.E.; Rohan, T.E.; Van Horn, L.; Shikany, J.M. Multivitamin use and risk of cancer and cardiovascular disease in the Women’s Health Initiative cohorts. Arch. Intern. Med. 2009, 169, 294–304. [Google Scholar] [CrossRef]
- Miller, E.R., III; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef]
- Cararo, J.H.; Streck, E.L.; Schuck, P.F.; da C Ferreira, G. Carnosine and related peptides: Therapeutic potential in age-related disorders. Aging Dis. 2015, 6, 369. [Google Scholar] [PubMed]
- Haus, J.M.; Thyfault, J.P. Therapeutic potential of carbonyl-scavenging carnosine derivative in metabolic disorders. J. Clin. Investig. 2018, 128, 5198–5200. [Google Scholar] [CrossRef] [PubMed]
- Schön, M.; Mousa, A.; Berk, M.; Chia, W.L.; Ukropec, J.; Majid, A.; Ukropcová, B.; De Courten, B. The potential of carnosine in brain-related disorders: A comprehensive review of current evidence. Nutrients 2019, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Brings, S.; Fleming, T.; Freichel, M.; Muckenthaler, M.U.; Herzig, S.; Nawroth, P.P. Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int. J. Mol. Sci. 2017, 18, 984. [Google Scholar] [CrossRef]
- Sharma, C.; Kaur, A.; Thind, S.S.; Singh, B.; Raina, S. Advanced glycation End-products (AGEs): An emerging concern for processed food industries. J. Food Sci. Technol. 2015, 52, 7561–7576. [Google Scholar] [CrossRef]
- Anderson, E.J.; Vistoli, G.; Katunga, L.A.; Funai, K.; Regazzoni, L.; Monroe, T.B.; Gilardoni, E.; Cannizzaro, L.; Colzani, M.; De Maddis, D. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J. Clin. Investig. 2018, 128, 5280–5293. [Google Scholar] [CrossRef]
- Schaur, R.J.; Siems, W.; Bresgen, N.; Eckl, P.M. 4-Hydroxy-nonenal—A bioactive lipid peroxidation product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef]
- Henning, C.; Liehr, K.; Girndt, M.; Ulrich, C.; Glomb, M.A. Extending the spectrum of α-dicarbonyl compounds in vivo. J. Biol. Chem. 2014, 289, 28676–28688. [Google Scholar] [CrossRef]
- Aldini, G.; Carini, M.; Beretta, G.; Bradamante, S.; Facino, R.M. Carnosine is a quencher of 4-hydroxy-nonenal: Through what mechanism of reaction? Biochem. Biophys. Res. Commun. 2002, 298, 699–706. [Google Scholar] [CrossRef]
- Aldini, G.; Orioli, M.; Rossoni, G.; Savi, F.; Braidotti, P.; Vistoli, G.; Yeum, K.-J.; Negrisoli, G.; Carini, M. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J. Cell. Mol. Med. 2011, 15, 1339–1354. [Google Scholar] [CrossRef]
- Gallant, S.; Semyonova, M.; Yuneva, M. Carnosine as a potential anti-senescence drug. Biochem. Mosc. 2000, 65, 866–868. [Google Scholar]
- Lee, Y.; Hsu, C.; Lin, M.; Liu, K.; Yin, M. Histidine and carnosine delay diabetic deterioration in mice and protect human low density lipoprotein against oxidation and glycation. Eur. J. Pharmacol. 2005, 513, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Kurata, H.; Fujii, T.; Tsutsui, H.; Katayama, T.; Ohkita, M.; Takaoka, M.; Tsuruoka, N.; Kiso, Y.; Ohno, Y.; Fujisawa, Y. Renoprotective effects of l-carnosine on ischemia/reperfusion-induced renal injury in rats. J. Pharmacol. Exp. Ther. 2006, 319, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L.E.; Fontaine-Bisson, B.; El-Sohemy, A. Functional genetic variants of glutathione S-transferase protect against serum ascorbic acid deficiency. Am. J. Clin. Nutr. 2009, 90, 1411–1417. [Google Scholar] [CrossRef]
- Milman, U.; Blum, S.; Shapira, C.; Aronson, D.; Miller-Lotan, R.; Anbinder, Y.; Alshiek, J.; Bennett, L.; Kostenko, M.; Landau, M. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: A prospective double-blinded clinical trial. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 341–347. [Google Scholar] [CrossRef]
- Janssen, B.; Hohenadel, D.; Brinkkoetter, P.; Peters, V.; Rind, N.; Fischer, C.; Rychlik, I.; Cerna, M.; Romzova, M.; de Heer, E. Carnosine as a protective factor in diabetic nephropathy: Association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 2005, 54, 2320–2327. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Han, S.-I.; Won, Y.-J.; Lee, E.; Park, E.; Hwang, S.-Y.; Yeum, K.-J. Black rice with giant embryo attenuates obesity-associated metabolic disorders in ob/ob mice. J. Agric. Food Chem. 2016, 64, 2492–2497. [Google Scholar] [CrossRef]
- Colzani, M.; Criscuolo, A.; De Maddis, D.; Garzon, D.; Yeum, K.-J.; Vistoli, G.; Carini, M.; Aldini, G. A novel high resolution MS approach for the screening of 4-hydroxy-trans-2-nonenal sequestering agents. J. Pharm. Biomed. Anal. 2014, 91, 108–118. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A. Opinion paper: Nanotechnology: A successful approach to improve oral bioavailability of phytochemicals. Recent Pat. Drug Deliv. Formul. 2016, 10, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed]
- De Pace, R.C.C.; Liu, X.; Sun, M.; Nie, S.; Zhang, J.; Cai, Q.; Gao, W.; Pan, X.; Fan, Z.; Wang, S. Anticancer activities of (-)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res. 2013, 23, 187–196. [Google Scholar] [CrossRef]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Bioactive peptides/chitosan nanoparticles enhance cellular antioxidant activity of (-)-epigallocatechin-3-gallate. J. Agric. Food Chem. 2013, 61, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.-J.; Liu, Y.; Chang, K.-L.; Lim, B.K.; Chiu, G.N. Perorally active nanomicellar formulation of quercetin in the treatment of lung cancer. Int. J. Nanomedicine 2012, 7, 651. [Google Scholar]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef]
- Li, W.; Yi, S.; Wang, Z.; Chen, S.; Xin, S.; Xie, J.; Zhao, C. Self-nanoemulsifying drug delivery system of persimmon leaf extract: Optimization and bioavailability studies. Int. J. Pharm. 2011, 420, 161–171. [Google Scholar] [CrossRef]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA nanoparticles improve the oral bioavailability of curcumin in rats: Characterizations and mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Bielawski, C.W. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene based electrochemical sensors and biosensors: A review. Electroanalalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Lu, C.-H.; Yang, H.-H.; Zhu, C.-L.; Chen, X.; Chen, G.-N. A graphene platform for sensing biomolecules. Angew. Chem. 2009, 121, 4879–4881. [Google Scholar] [CrossRef]
- Cho, Y.; Shi, R.; Borgens, R.B.; Ivanisevic, A. Functionalized mesoporous silica nanoparticle-based drug delivery system to rescue acrolein-mediated cell death. Nanomedicine 2008, 3, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shi, R.; Borgens, R.; Ivanisevic, A. Repairing the damaged spinal cord and brain with nanomedicine. Small 2008, 4, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shi, R.; Borgens, R.B. Chitosan nanoparticle-based neuronal membrane sealing and neuroprotection following acrolein-induced cell injury. J. Biol. Eng. 2010, 4, 2. [Google Scholar] [CrossRef]
- Wojsiat, J.; Zoltowska, K.M.; Laskowska-Kaszub, K.; Wojda, U. Oxidant/antioxidant imbalance in Alzheimer’s disease: Therapeutic and diagnostic prospects. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Aldini, G.; Domingues, M.R.; Spickett, C.M.; Domingues, P.; Altomare, A.; Sánchez-Gómez, F.J.; Oeste, C.L.; Pérez-Sala, D. Protein lipoxidation: Detection strategies and challenges. Redox Biol. 2015, 5, 253–266. [Google Scholar] [CrossRef]
- Cordis, G.A.; Das, D.K.; Riedel, W. High-performance liquid chromatographic peak identification of 2, 4-dinitrophenylhydrazine derivatives of lipid peroxidation aldehydes by photodiode array detection. J. Chromatogr. A 1998, 798, 117–123. [Google Scholar] [CrossRef]
- Mateos, R.; Lecumberri, E.; Ramos, S.; Goya, L.; Bravo, L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress: Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from fruits. J. Chromatogr. B 2005, 827, 76–82. [Google Scholar]
- Zhu, H.; Li, X.; Shoemaker, C.F.; Wang, S.C. Ultrahigh performance liquid chromatography analysis of volatile carbonyl compounds in virgin olive oils. J. Agric. Food Chem. 2013, 61, 12253–12259. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Meinema, A.C.; Permentier, H.; Hopfgartner, G.; Bischoff, R. Integrated quantification and identification of aldehydes and ketones in biological samples. Anal. Chem. 2014, 86, 5089–5100. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fang, H.; Han, X. Shotgun lipidomics analysis of 4-hydroxyalkenal species directly from lipid extracts after one-step in situ derivatization. Anal. Chem. 2012, 84, 4580–4586. [Google Scholar] [CrossRef]
- Milic, I.; Hoffmann, R.; Fedorova, M. Simultaneous detection of low and high molecular weight carbonylated compounds derived from lipid peroxidation by electrospray ionization-tandem mass spectrometry. Anal. Chem. 2013, 85, 156–162. [Google Scholar] [CrossRef]
- Ni, Z.; Milic, I.; Fedorova, M. Identification of carbonylated lipids from different phospholipid classes by shotgun and LC-MS lipidomics. Anal. Bioanal. Chem. 2015, 407, 5161–5173. [Google Scholar] [CrossRef]
- Tomono, S.; Miyoshi, N.; Ohshima, H. Comprehensive analysis of the lipophilic reactive carbonyls present in biological specimens by LC/ESI-MS/MS. J. Chromatogr. B 2015, 988, 149–156. [Google Scholar] [CrossRef]
- García-Gómez, D.; Martínez-Lozano Sinues, P.; Barrios-Collado, C.; Vidal-de-Miguel, G.; Gaugg, M.; Zenobi, R. Identification of 2-alkenals, 4-hydroxy-2-alkenals, and 4-hydroxy-2, 6-alkadienals in exhaled breath condensate by UHPLC-HRMS and in breath by real-time HRMS. Anal. Chem. 2015, 87, 3087–3093. [Google Scholar] [CrossRef]
- El-Maghrabey, M.H.; Kishikawa, N.; Ohyama, K.; Kuroda, N. Analytical method for lipoperoxidation relevant reactive aldehydes in human sera by high-performance liquid chromatography–fluorescence detection. Anal. Biochem. 2014, 464, 36–42. [Google Scholar] [CrossRef]
- Ali, M.F.B.; Kishikawa, N.; Ohyama, K.; Mohamed, H.A.-M.; Abdel-Wadood, H.M.; Mahmoud, A.M.; Imazato, T.; Ueki, Y.; Wada, M.; Kuroda, N. Chromatographic determination of low-molecular mass unsaturated aliphatic aldehydes with peroxyoxalate chemiluminescence detection after fluorescence labeling with 4-(N, N-dimethylaminosulfonyl)-7-hydrazino-2, 1, 3-benzoxadiazole. J. Chromatogr. B 2014, 953, 147–152. [Google Scholar] [CrossRef]
- Cui, T.; Schopfer, F.J.; Zhang, J.; Chen, K.; Ichikawa, T.; Baker, P.R.; Batthyany, C.; Chacko, B.K.; Feng, X.; Patel, R.P. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J. Biol. Chem. 2006, 281, 35686–35698. [Google Scholar] [CrossRef]
- Colzani, M.; Aldini, G.; Carini, M. Mass spectrometric approaches for the identification and quantification of reactive carbonyl species protein adducts. J. Proteom. 2013, 92, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, G.; Heinonen, M. LC–MS investigations on interactions between isolated β-lactoglobulin peptides and lipid oxidation product malondialdehyde. Food Chem. 2015, 175, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Steen, H.; Mann, M. The ABC’s (and XYZ’s) of peptide sequencing. Nat. Rev. Mol. Cell Biol. 2004, 5, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.M., Jr.; Rauniyar, N.; Prokai, L. Rapid characterization of covalent modifications to rat brain mitochondrial proteins after ex vivo exposure to 4-hydroxy-2-nonenal by liquid chromatography–tandem mass spectrometry using data-dependent and neutral loss-driven MS3 acquisition. J. Mass Spectrom. 2007, 42, 1599–1605. [Google Scholar] [CrossRef]
- Rauniyar, N.; Stevens, S.M., Jr.; Prokai-Tatrai, K.; Prokai, L. Characterization of 4-hydroxy-2-nonenal-modified peptides by liquid chromatography- tandem mass spectrometry using data-dependent acquisition: Neutral loss-driven MS3 versus neutral loss-driven electron capture dissociation. Anal. Chem. 2009, 81, 782–789. [Google Scholar] [CrossRef]
- Méndez, D.; Hernáez, M.L.; Kamali, A.N.; Diez, A.; Puyet, A.; Bautista, J.M. Differential carbonylation of cytoskeletal proteins in blood group O erythrocytes: Potential role in protection against severe malaria. Infect. Genet. Evol. 2012, 12, 1780–1787. [Google Scholar] [CrossRef]
- Vasil’ev, Y.V.; Tzeng, S.-C.; Huang, L.; Maier, C.S. Protein modifications by electrophilic lipoxidation products: Adduct formation, chemical strategies and tandem mass spectrometry for their detection and identification. Mass Spectrom. Rev. 2014, 33, 157–182. [Google Scholar] [CrossRef]
- Fritz, K.S.; Kellersberger, K.A.; Gomez, J.D.; Petersen, D.R. 4-HNE adduct stability characterized by collision-induced dissociation and electron transfer dissociation mass spectrometry. Chem. Res. Toxicol. 2012, 25, 965–970. [Google Scholar] [CrossRef]
- Carini, M.; Aldini, G.; Facino, R.M. Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrom. Rev. 2004, 23, 281–305. [Google Scholar] [CrossRef]
- Domingues, R.M.; Domingues, P.; Melo, T.; Pérez-Sala, D.; Reis, A.; Spickett, C.M. Lipoxidation adducts with peptides and proteins: Deleterious modifications or signaling mechanisms? J. Proteom. 2013, 92, 110–131. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Reed, T.; Sultana, R. Roles of 3-nitrotyrosine-and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer’s disease. Free Radic. Res. 2011, 45, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Orioli, M.; Carini, M. Protein modification by acrolein: Relevance to pathological conditions and inhibition by aldehyde sequestering agents. Mol. Nutr. Food Res. 2011, 55, 1301–1319. [Google Scholar] [CrossRef]
- Garzón, B.; Oeste, C.L.; Díez-Dacal, B.; Pérez-Sala, D. Proteomic studies on protein modification by cyclopentenone prostaglandins: Expanding our view on electrophile actions. J. Proteom. 2011, 74, 2243–2263. [Google Scholar] [CrossRef] [PubMed]
- Spickett, C.M.; Reis, A.; Pitt, A.R. Use of narrow mass-window, high-resolution extracted product ion chromatograms for the sensitive and selective identification of protein modifications. Anal. Chem. 2013, 85, 4621–4627. [Google Scholar] [CrossRef]
- Milic, I.; Melo, T.; Domingues, M.R.; Domingues, P.; Fedorova, M. Heterogeneity of peptide adducts with carbonylated lipid peroxidation products. J. Mass Spectrom. 2015, 50, 603–612. [Google Scholar] [CrossRef]
- Gao, D.; Willard, B.; Podrez, E.A. Analysis of covalent modifications of proteins by oxidized phospholipids using a novel method of peptide enrichment. Anal. Chem. 2014, 86, 1254–1262. [Google Scholar] [CrossRef]
- Uchida, K.; Stadtman, E. Covalent attachment of 4-hydroxynonenal to glyceraldehyde-3-phosphate dehydrogenase. A possible involvement of intra-and intermolecular cross-linking reaction. J. Biol. Chem. 1993, 268, 6388–6393. [Google Scholar]
- Golizeh, M.; Abusarah, J.; Benderdour, M.; Sleno, L. Covalent binding of 4-hydroxynonenal to matrix metalloproteinase 13 studied by liquid chromatography–mass spectrometry. Chem. Res. Toxicol. 2014, 27, 1556–1565. [Google Scholar] [CrossRef]
- Smathers, R.L.; Fritz, K.S.; Galligan, J.J.; Shearn, C.T.; Reigan, P.; Marks, M.J.; Petersen, D.R. Characterization of 4-HNE modified L-FABP reveals alterations in structural and functional dynamics. PLoS ONE 2012, 7, 38459. [Google Scholar] [CrossRef]
- Anavi, S.; Ni, Z.; Tirosh, O.; Fedorova, M. Steatosis-induced proteins adducts with lipid peroxidation products and nuclear electrophilic stress in hepatocytes. Redox Biol. 2015, 4, 158–168. [Google Scholar] [CrossRef]
- Rogowska-Wrzesinska, A.; Wojdyla, K.; Nedić, O.; Baron, C.P.; Griffiths, H.R. Analysis of protein carbonylation—Pitfalls and promise in commonly used methods. Free Radic. Res. 2014, 48, 1145–1162. [Google Scholar] [CrossRef]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Carini, M.; Orioli, M.; Vistoli, G.; Regazzoni, L.; Colombo, G.; Rossi, R.; Milzani, A.; Aldini, G. Protein carbonylation: 2, 4-dinitrophenylhydrazine reacts with both aldehydes/ketones and sulfenic acids. Free Radic. Biol. Med. 2009, 46, 1411–1419. [Google Scholar] [CrossRef]
- Bollineni, R.C.; Fedorova, M.; Hoffmann, R. Qualitative and quantitative evaluation of derivatization reagents for different types of protein-bound carbonyl groups. Analyst 2013, 138, 5081–5088. [Google Scholar] [CrossRef]
- Bollineni, R.C.; Hoffmann, R.; Fedorova, M. Proteome-wide profiling of carbonylated proteins and carbonylation sites in HeLa cells under mild oxidative stress conditions. Free Radic. Biol. Med. 2014, 68, 186–195. [Google Scholar] [CrossRef]
- Aluise, C.D.; Camarillo, J.M.; Shimozu, Y.; Galligan, J.J.; Rose, K.L.; Tallman, K.A.; Marnett, L.J. Site-specific, intramolecular cross-linking of Pin1 active site residues by the lipid electrophile 4-oxo-2-nonenal. Chem. Res. Toxicol. 2015, 28, 817–827. [Google Scholar] [CrossRef]
- Fritz, K.S.; Galligan, J.J.; Smathers, R.L.; Roede, J.R.; Shearn, C.T.; Reigan, P.; Petersen, D.R. 4-Hydroxynonenal inhibits SIRT3 via thiol-specific modification. Chem. Res. Toxicol. 2011, 24, 651–662. [Google Scholar] [CrossRef]
- Vunta, H.; Davis, F.; Palempalli, U.D.; Bhat, D.; Arner, R.J.; Thompson, J.T.; Peterson, D.G.; Reddy, C.C.; Prabhu, K.S. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Δ12, 14-prostaglandin J2 in macrophages. J. Biol. Chem. 2007, 282, 17964–17973. [Google Scholar] [CrossRef]
- Cernuda-Morollón, E.; Pineda-Molina, E.; Pérez-Sala, D. 15-Deoxy-Δ12, 14-prostaglandin J2Inhibition of NF-κB-DNA Binding through Covalent Modification of the p50 Subunit. J. Biol. Chem. 2001, 276, 35530–35536. [Google Scholar] [CrossRef]
- Takahashi, N.; Mizuno, Y.; Kozai, D.; Yamamoto, S.; Kiyonaka, S.; Shibata, T.; Uchida, K.; Mori, Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels 2008, 2, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Parker, J. Prostaglandin A2 protein interactions and inhibition of cellular proliferation. Prostaglandins 1995, 50, 359–375. [Google Scholar] [CrossRef]
- Díez-Dacal, B.; Pérez-Sala, D. Anti-inflammatory prostanoids: Focus on the interactions between electrophile signaling and resolution of inflammation. Sci. World J. 2010, 10, 655–675. [Google Scholar] [CrossRef]
- Higdon, A.N.; Dranka, B.P.; Hill, B.G.; Oh, J.-Y.; Johnson, M.S.; Landar, A.; Darley-Usmar, V.M. Methods for imaging and detecting modification of proteins by reactive lipid species. Free Radic. Biol. Med. 2009, 47, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Cummins, T.D.; Higdon, A.N.; Kramer, P.A.; Chacko, B.K.; Riggs, D.W.; Salabei, J.K.; Dell’Italia, L.J.; Zhang, J.; Darley-Usmar, V.M.; Hill, B.G. Utilization of fluorescent probes for the quantification and identification of subcellular proteomes and biological processes regulated by lipid peroxidation products. Free Radic. Biol. Med. 2013, 59, 56–68. [Google Scholar] [CrossRef]
- Vila, A.; Tallman, K.A.; Jacobs, A.T.; Liebler, D.C.; Porter, N.A.; Marnett, L.J. Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives. Chem. Res. Toxicol. 2008, 21, 432–444. [Google Scholar] [CrossRef]
- Pérez-Sala, D. Electrophilic eicosanoids: Signaling and targets. Chem. Biol. Interact. 2011, 192, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Gayarre, J.; Stamatakis, K.; Renedo, M.; Pérez-Sala, D. Differential selectivity of protein modification by the cyclopentenone prostaglandins PGA1 and 15-deoxy-Δ12, 14-PGJ2: Role of glutathione. FEBS Lett. 2005, 579, 5803–5808. [Google Scholar] [CrossRef]
- Oeste, C.L.; Pérez-Sala, D. Modification of cysteine residues by cyclopentenone prostaglandins: Interplay with redox regulation of protein function. Mass Spectrom. Rev. 2014, 33, 110–125. [Google Scholar] [CrossRef]
- Diers, A.R.; Higdon, A.N.; Ricart, K.C.; Johnson, M.S.; Agarwal, A.; Kalyanaraman, B.; Landar, A.; Darley-Usmar, V.M. Mitochondrial targeting of the electrophilic lipid 15-deoxy-Δ12, 14prostaglandin J2 increases apoptotic efficacy via redox cell signalling mechanisms. Biochem. J. 2010, 426, 31–41. [Google Scholar] [CrossRef]
- Sánchez-Gómez, F.J.; Gayarre, J.; Avellano, M.I.; Pérez-Sala, D. Direct evidence for the covalent modification of glutathione-S-transferase P1-1 by electrophilic prostaglandins: Implications for enzyme inactivation and cell survival. Arch. Biochem. Biophys. 2007, 457, 150–159. [Google Scholar] [CrossRef]
- Oeste, C.L.; Díez-Dacal, B.; Bray, F.; De Lacoba, M.G.; Beatriz, G.; Andreu, D.; Ruiz-Sánchez, A.J.; Pérez-Inestrosa, E.; García-Domínguez, C.A.; Rojas, J.M. The C-terminus of H-Ras as a target for the covalent binding of reactive compounds modulating Ras-dependent pathways. PLoS ONE 2011, 6, 15866. [Google Scholar] [CrossRef]
- Sánchez-Gómez, F.J.; Dorado, C.G.; Ayuso, P.; Agúndez, J.A.; Pajares, M.A.; Pérez-Sala, D. Modulation of GSTP1-1 oligomerization by electrophilic inflammatory mediators and reactive drugs. Inflamm. Allergy Drug Targets 2013, 12, 162–171. [Google Scholar] [CrossRef]
- Uchida, K.; Szweda, L.I.; Chae, H.-Z.; Stadtman, E.R. Immunochemical detection of 4-hydroxynonenal protein adducts in oxidized hepatocytes. Proc. Natl. Acad. Sci. USA 1993, 90, 8742–8746. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Kondo, M.; Osawa, T.; Shibata, N.; Kobayashi, M.; Uchida, K. 15-deoxy-Δ12, 14-prostaglandin J2 a prostaglandin D2 metabolite generated during inflammatory processes. J. Biol. Chem. 2002, 277, 10459–10466. [Google Scholar] [CrossRef] [PubMed]
- Charles, R.L.; Burgoyne, J.R.; Mayr, M.; Weldon, S.M.; Hubner, N.; Dong, H.; Morisseau, C.; Hammock, B.D.; Landar, A.; Eaton, P. Redox regulation of soluble epoxide hydrolase by 15-deoxy-δ-prostaglandin J2 controls coronary hypoxic vasodilation. Circ. Res. 2011, 108, 324–334. [Google Scholar] [CrossRef]
- Shiraki, T.; Kamiya, N.; Shiki, S.; Kodama, T.S.; Kakizuka, A.; Jingami, H. α, β-unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2005, 280, 14145–14153. [Google Scholar] [CrossRef] [PubMed]
- Díez-Dacal, B.; Gayarre, J.; Gharbi, S.; Timms, J.F.; Coderch, C.; Gago, F.; Pérez-Sala, D. Identification of aldo-keto reductase AKR1B10 as a selective target for modification and inhibition by prostaglandin A1: Implications for antitumoral activity. Cancer Res. 2011, 71, 4161–4171. [Google Scholar] [CrossRef]
- Garzón, B.; Gayarre, J.; Gharbi, S.; Díez-Dacal, B.; Sánchez-Gómez, F.J.; Timms, J.F.; Pérez-Sala, D. A biotinylated analog of the anti-proliferative prostaglandin A1 allows assessment of PPAR-independent effects and identification of novel cellular targets for covalent modification. Chem. Biol. Interact. 2010, 183, 212–221. [Google Scholar] [CrossRef]
- Wall, S.B.; Smith, M.R.; Ricart, K.; Zhou, F.; Vayalil, P.K.; Oh, J.-Y.; Landar, A. Detection of electrophile-sensitive proteins. Biochim. Biophys. Acta 2014, 1840, 913–922. [Google Scholar] [CrossRef]
- Gharbi, S.; Garzón, B.; Gayarre, J.; Timms, J.; Pérez-Sala, D. Study of protein targets for covalent modification by the antitumoral and anti-inflammatory prostaglandin PGA1: Focus on vimentin. J. Mass Spectrom. 2007, 42, 1474–1484. [Google Scholar] [CrossRef]
- Chavez, J.; Chung, W.-G.; Miranda, C.L.; Singhal, M.; Stevens, J.F.; Maier, C.S. Site-specific protein adducts of 4-hydroxy-2 (E)-nonenal in human THP-1 monocytic cells: Protein carbonylation is diminished by ascorbic acid. Chem. Res. Toxicol. 2010, 23, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, K.; Sánchez-Gómez, F.J.; Pérez-Sala, D. Identification of novel protein targets for modification by 15-deoxy-Δ12, 14-prostaglandin J2 in mesangial cells reveals multiple interactions with the cytoskeleton. J. Am. Soc. Nephrol. 2006, 17, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Ha, S.; Lee, H.Y.; Lee, K.-J. ROSics: Chemistry and proteomics of cysteine modifications in redox biology. Mass Spectrom. Rev. 2015, 34, 184–208. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sala, D.; Oeste, C.L.; Martínez, A.E.; Carrasco, M.J.; Garzón, B.; Canada, F.J. Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat. Commun. 2015, 6, 1–17. [Google Scholar] [CrossRef]
- Tsapara, A.; Kardassis, D.; Moustakas, A.; Gravanis, A.; Stournaras, C. Expression and characterization of Cys374 mutated human β-actin in two different mammalian cell lines: Impaired microfilament organization and stability. FEBS Lett. 1999, 455, 117–122. [Google Scholar] [CrossRef]






| Activity | Approach | Reference |
|---|---|---|
| Identification and Characterization of Vimentin | ||
| Identification as putative target for lipoxidation | 15d-PGJ2B→Electrophoresis → WB → Detection of biotin → MALDI-TOF-MS | Stamatakis et al. [284] |
| HNE → Electrophoresis → Anti-HNE-WB → Nano-LC-MALDI-MS/MS | Chavez et al. [283] | |
| Cell Senescence→Electrophoresis → Anti-HNE-WB → MALDI-TOF-MS | Aldini et al. [220] | |
| Confirmation of lipoxidation site in cell | cyPG(biotinylated) → Immunoprecipitation → Detection Biotin | Aldini et al. [220] |
| cyPG(biotinylation) → Immunoprecipitation → SDSPAGE → LCMS/MS | Gharbi S et al. [282] | |
| Carbonyl derivatization → Avidin enrichment → MS/MS | Chavez et al. [283] | |
| Validation of lipoxidation site (Cys328) | Transfection → cyPG(biotinylation) → Avidin enrichment → SDSPAGE → WB | Gharbi et al. [275] |
| Carbonyl derivatization → Avidin enrichment → MS/MS | Chavez et al. [283] | |
| Analysis of lipoxidation in vitro | cyPG(biotinylation) → SDSPAGE → Biotin Detection | Gharbi et al. [282] |
| cyPG(biotinylation)/HNE → SDSPAGE → Biotin Detection/Anti-HNEWB | Aldini et al. [220] | |
| Confirmation of lipoxidation site in vitro (Cys328) | cyPG(biotinylation) → Digestion and Avidin enrichment → MALDI-TOF-TOF MS/MS | Gharbi et al. [282] |
| In vitro competition assays | Aldini et al. [220] | |
| Functional Assessment of Vimentin Lipoxidation | ||
| In vitro assessment of lipoxidation: Filaments derangement | HNE → Polymerization (NaCl Induced) → Filaments electron microscopy | Aldini et al. [220] |
| In cell assessment of lipoxidation (Condensation of network by HNE treatment) | Transfection of cells (expressing vimentin) with GFP → HNE treatment → Confocal microscopy | Aldini et al. [220] |
| Cys328 in function consequences of vimentin lipoxidation: network condensation and filaments preservation in vimentin Cys328ser | Transfection of vimentin or Cys328ser in cell (vimentin deficient) → HNE treatment → Immuno-fluorescence → Confocal microscopy | Aldini et al. [220] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuloria, S.; Subramaniyan, V.; Karupiah, S.; Kumari, U.; Sathasivam, K.; Meenakshi, D.U.; Wu, Y.S.; Guad, R.M.; Udupa, K.; Fuloria, N.K. A Comprehensive Review on Source, Types, Effects, Nanotechnology, Detection, and Therapeutic Management of Reactive Carbonyl Species Associated with Various Chronic Diseases. Antioxidants 2020, 9, 1075. https://doi.org/10.3390/antiox9111075
Fuloria S, Subramaniyan V, Karupiah S, Kumari U, Sathasivam K, Meenakshi DU, Wu YS, Guad RM, Udupa K, Fuloria NK. A Comprehensive Review on Source, Types, Effects, Nanotechnology, Detection, and Therapeutic Management of Reactive Carbonyl Species Associated with Various Chronic Diseases. Antioxidants. 2020; 9(11):1075. https://doi.org/10.3390/antiox9111075
Chicago/Turabian StyleFuloria, Shivkanya, Vetriselvan Subramaniyan, Sundram Karupiah, Usha Kumari, Kathiresan Sathasivam, Dhanalekshmi Unnikrishnan Meenakshi, Yuan Seng Wu, Rhanye Mac Guad, Kaviraja Udupa, and Neeraj Kumar Fuloria. 2020. "A Comprehensive Review on Source, Types, Effects, Nanotechnology, Detection, and Therapeutic Management of Reactive Carbonyl Species Associated with Various Chronic Diseases" Antioxidants 9, no. 11: 1075. https://doi.org/10.3390/antiox9111075
APA StyleFuloria, S., Subramaniyan, V., Karupiah, S., Kumari, U., Sathasivam, K., Meenakshi, D. U., Wu, Y. S., Guad, R. M., Udupa, K., & Fuloria, N. K. (2020). A Comprehensive Review on Source, Types, Effects, Nanotechnology, Detection, and Therapeutic Management of Reactive Carbonyl Species Associated with Various Chronic Diseases. Antioxidants, 9(11), 1075. https://doi.org/10.3390/antiox9111075