The Sulfilimine Analogue of Allicin, S-Allyl-S-(S-allyl)-N-Cyanosulfilimine, Is Antimicrobial and Reacts with Glutathione
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and LC-MS of S-allyl-S-(S-allyl)-N-cyanosulfilimine
2.2. Stability of CSA Compared to Allicin
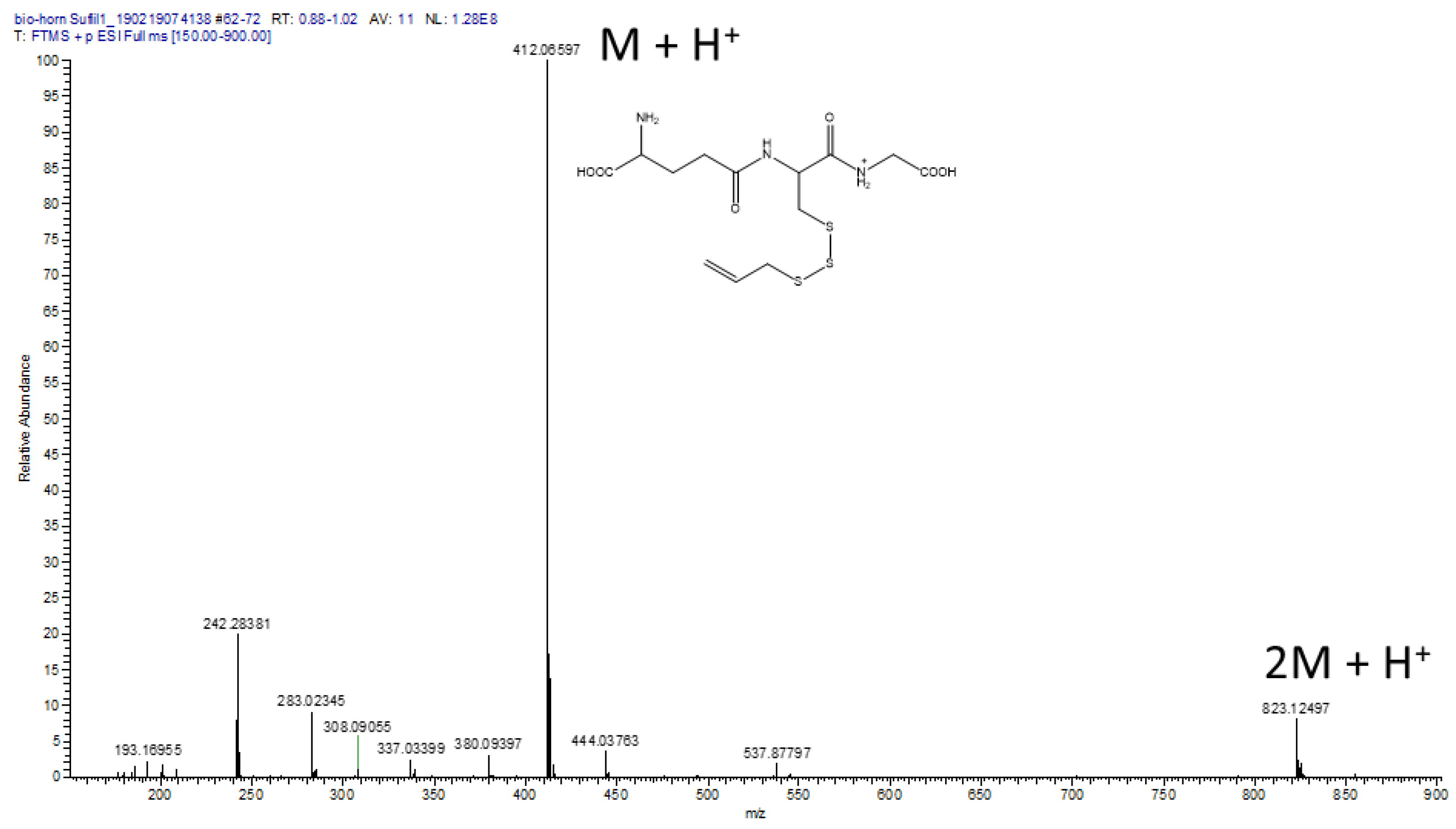
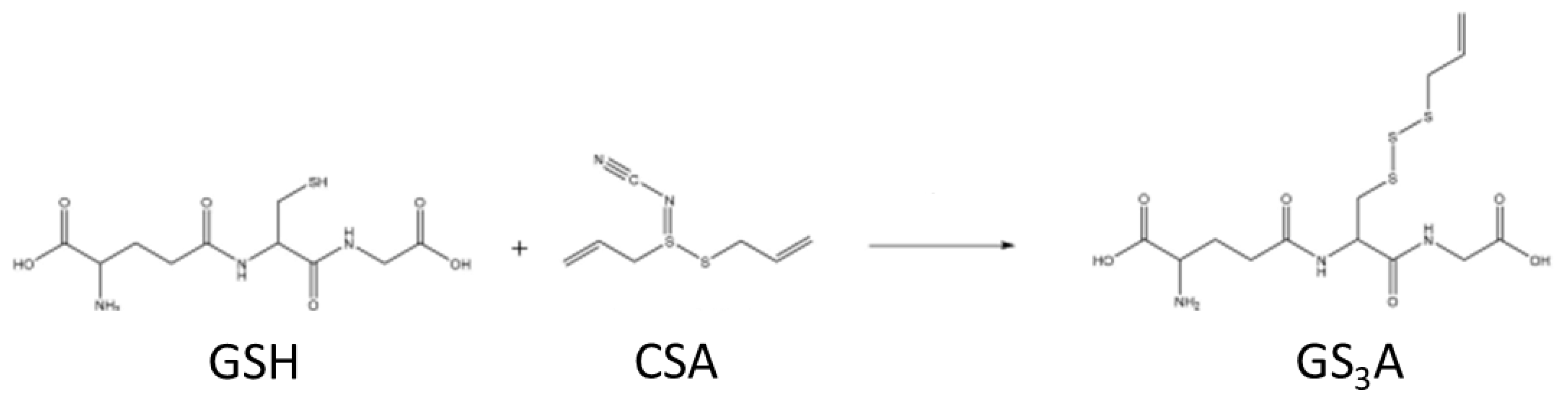
2.3. Reaction of GSH with CSA and LC/MS of the GS3A Product
2.4. Experiments with Microorganisms
2.5. Experiments with Arabidopsis Roots
3. Results and Discussion
3.1. Stability of CSA Compared to Allicin
3.2. CSA Inhibits the Growth of Bacteria and Yeast in Agar-Diffusion Tests
3.3. Chemogenetic Screen of CSA Compared to Allicin in the Yeast Mutants Δglr1, Δtrx2, Δyap1, and Δzwf1
3.4. Effect of CSA on Arabidopsis Root Growth
3.5. Reaction of CSA with GSH and Characterization of GS3A as the Reaction Product
3.6. A Δgsh1 Mutant can Grow on Medium without GSH when Supplemented with GS3A
4. Conclusions/Highlights
- CSA is antimicrobial in a dose-dependent manner.
- CSA probably has a similar mechanism of action to allicin.
- CSA reacts with GSH to make GS3A.
- GS3A can complement a Δgsh1 yeast mutant and, therefore, may be a substrate for Glr.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kohrt, E.; Hartke, K. Neuartige sulfimide. Arch. Pharm. 1993, 326, 267–270. [Google Scholar] [CrossRef]
- Gilchrist, T.L.; Moody, C.J. The chemistry of sulfilimines. Chem. Rev. 1977, 77, 409–435. [Google Scholar] [CrossRef]
- Block, E. Sulfilimines and Related Derivatives. In ACS Monograph Series; Oae, S., Furukawa, N., Eds.; ACS: Washington, DC, USA, 1983; Volume 179, p. 340. [Google Scholar]
- Garciá Mancheño, O.; Bolm, C. Synthesis of N-(1H)-tetrazole sulfoximines. Org. Lett. 2007, 9, 2951–2954. [Google Scholar] [CrossRef] [PubMed]
- Garciá Mancheño, O.; Bistri, O.; Bolm, C. Iodinane- and metal-free synthesis of N-cyano sulfilimines: Novel and easy access of NH-sulfoximines. Org. Lett. 2007, 9, 3809–3811. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Bolm, C. Metal-free synthesis of N-cyano-substituted sulfilimines and sulfoximines. Synthesis 2010, 2922–2925. [Google Scholar] [CrossRef]
- Steinkamp, A.-D.; Seling, N.; Lee, S.; Boedtkjer, E.; Bolm, C. Synthesis of N-Cyano-substituted sulfilimine and sulfoximine derivatives of S0859 and their biological evaluation as sodium bicarbonate Co-transport Inhibitors. Med. Chem. Commun. 2015, 6, 2163–2169. [Google Scholar] [CrossRef]
- Karpel-Massler, G.; Kast, R.E.; Siegelin, M.D.; Dwucet, A.; Schneider, E.; Westhoff, M.-A.; Wirtz, C.R.; Chen, X.Y.; Halatsch, M.-E.; Bolm, C. Anti-glioma activity of dapsone and its enhancement by synthetic chemical modification. Neurochem. Res. 2017, 42, 3382–3389. [Google Scholar] [CrossRef] [PubMed]
- Cavallito, C.J.; Bailey, J.H. Allicin, the antibacterial principle of Allium sativum I.; isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Cavallito, C.J.; Buck, J.S.; Suter, C.M. Allicin, the antibacterial principle of Allium sativum. II. Determination of the chemical structure. J. Am. Chem. Soc. 1944, 66, 1952–1954. [Google Scholar] [CrossRef]
- Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M.; Glozman, S.; Yavin, E.; Weiner, L. S-allylmercaptoglutathione: The reaction product of allicin with glutathione possesses SH-modifying and antioxidant properties. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2000, 1499, 144–153. [Google Scholar] [CrossRef]
- Horn, T.; Bettray, W.; Slusarenko, A.J.; Gruhlke, M.C.H. S-allylmercaptoglutathione Is a substrate for glutathione reductase (EC. 1.8.1.7) from Yeast (Saccharomyces cerevisiae). Antioxidants 2018, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.K.; Huyen, N.T.T.; Van Loi, V.; Gruhlke, M.C.H.; Mäder, U.; Maaß, S.; Becher, D.; Bernhardt, J.; Arbach, M.; Hamilton, C.J. The disulfide stress response and protein S-thioallylation caused by allicin and diallyl polysulfanes in B. subtilis as revealed by transcriptomics and proteomics. Antioxidants 2019, 8, 605. [Google Scholar] [CrossRef]
- Lawson, L.D.; Wood, S.G.; Hughes, B.G. HPLC analysis of allicin and other thiosulfinates in garlic clove homogenates. Planta Med. 1991, 57, 263–270. [Google Scholar] [CrossRef]
- Block, E. Garlic and Other Alliums—The Lore and the Science; RSC Publishing: Cambridge, UK, 2010. [Google Scholar]
- Reiter, J.; Levina, N.; Van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a volatile Antimicrobial from GARLIC (Allium sativum), Kills human lung pathogenic Bacteria, Including MDR strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef]
- Reiter, J.; Hübbers, A.M.; Albrecht, F.; Leichert, L.I.O.; Slusarenko, A.J. Allicin, a natural antimicrobial defence substance from garlic, inhibits DNA gyrase activity in bacteria. Int. J. Med. Microbiol. 2020, 310, 151359. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Borlinghaus, J.; Dörner, P.; Schröder, W.; Gruhlke, M.C.H.; Klaas, M.; Slusarenko, A.J. Investigation of the deposition behaviour and antibacterial effectivity of allicin aerosols and vapour using a lung model. Exp. Ther. Med. 2020, 19, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The mode of action of allicin: Its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1463, 20–30. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Portz, D.; Stitz, M.; Anwar, A.; Schneider, T.; Jacob, C.; Schlaich, N.L.; Slusarenko, A.J. Allicin disrupts the cell’s electrochemical potential and induces apoptosis in yeast. Free Radic. Biol. Med. 2012, 49, 1916–1924. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Slusarenko, A.J. The biology of reactive sulfur species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef]
- Müller, A.; Eller, J.; Albrecht, F.; Prochnow, P.; Kuhlmann, K.; Bandow, J.E.; Slusarenko, A.J.; Leichert, L.I. Allicin induces thiol Stress in bacteria through S-allylmercapto modification of protein cysteines. J. Biol. Chem. 2016, 291, 11477–11490. [Google Scholar] [CrossRef]
- Leontiev, R.; Slusarenko, A.J. Finding the starting point for mode-of-action studies of novel selenium compounds: Yeast as a genetic toolkit. Curr. Org. Synth. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Gruhlke, M.C.H.; Schlembach, I.; Leontiev, R.; Uebachs, A.; Gollwitzer, P.; Weiss, A.; Delaunay, A.; Toledano, M.B.; Slusarenko, A.J. Yap1p, the central regulator of the S. cerevisiae oxidative stress response, is activated by allicin, a natural oxidant and defence substance of garlic. Free Radic. Biol. Med. 2017, 108, 793–802. [Google Scholar] [CrossRef]
- Leontiev, R.; Hohaus, N.; Jacob, C.; Gruhlke, M.C.H.; Slusarenko, A.J. A comparison of the antibacterial and antifungal activities of thiosulfinate analogues of allicin. Sci. Rep. 2018, 8, 6763. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Bolger, A.; Schier, C.; Vogel, A.; Usadel, B.; Gruhlke, M.C.H.; Slusarenko, A.J. Genetic and molecular characterization of multi-component resistance of Pseudomonas against allicin. Life Sci. Alliance 2020, 3, e202000670. [Google Scholar] [CrossRef]
- Kuge, S.; Jones, N. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 1994, 13, 655–664. [Google Scholar] [CrossRef]
- Jamieson, D.J. Oxidative stress responses of the yeast Saccharomyces cerevisiae. Yeast 1998, 14, 1511–1527. [Google Scholar] [CrossRef]
- Toledano, M.B.; Delaunay, A.; Biteau, B.; Spector, D.; Azevedo, D. Oxidative stress responses in yeast. In Yeast Stress Responses; Hohmann, S., Mager, W.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 241–303. [Google Scholar]
- Morano, K.A.; Grant, C.M.; Moye-Rowley, W.S. The response to heat Shock and oxidative stress in saccharomyces cerevisiae. Genetics 2011, 190, 1157–1195. [Google Scholar] [CrossRef]
- Brachmann, C.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.; Hieter, P.; Boeke, J.D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998, 14, 115–132. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Sarfraz, M.; Nasim, M.J.; Jacob, C.; Gruhlke, M.C. Yeast chemogenetic screening as a tool to unravel the antifungal mode of action of Two selected selenocyanates. Appl. Sci. 2019, 9, 3728. [Google Scholar] [CrossRef]
- Parisy, V.; Poinssot, B.; Owsianowski, L.; Buchala, A.; Glazebrook, J.; Mauch, F. Identification of PAD2 as a gamma-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis. Plant J. 2007, 49, 159–172. [Google Scholar] [CrossRef]
- Mhamdi, A.; Hager, J.; Chaouch, S.; Queval, G.; Han, Y.; Taconnat, L.; Saindrenan, P.; Gouia, H.; Issakidis-Bourguet, E.; Renou, J.-P. Arabidopsis glutathione reductase1 plays a crucial role in leaf responses to intracellular Hydrogen Peroxide and in ensuring appropriate gene expression through both salicylic acid and jasmonic acid signaling pathways. Plant Physiol. 2010, 153, 1144–1160. [Google Scholar] [CrossRef]
- Moutiez, M.; Aumercier, M.; Parmentier, B.; Tartar, A.; Sergheraert, C. Compared recognition of di- and trisulfides substrates by glutathione and trypanothione reductases. Biochim. Biophys. Acta 1995, 1245, 161–166. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horn, T.; Bettray, W.; Noll, U.; Krauskopf, F.; Huang, M.-R.; Bolm, C.; Slusarenko, A.J.; Gruhlke, M.C.H. The Sulfilimine Analogue of Allicin, S-Allyl-S-(S-allyl)-N-Cyanosulfilimine, Is Antimicrobial and Reacts with Glutathione. Antioxidants 2020, 9, 1086. https://doi.org/10.3390/antiox9111086
Horn T, Bettray W, Noll U, Krauskopf F, Huang M-R, Bolm C, Slusarenko AJ, Gruhlke MCH. The Sulfilimine Analogue of Allicin, S-Allyl-S-(S-allyl)-N-Cyanosulfilimine, Is Antimicrobial and Reacts with Glutathione. Antioxidants. 2020; 9(11):1086. https://doi.org/10.3390/antiox9111086
Chicago/Turabian StyleHorn, Tobias, Wolfgang Bettray, Ulrike Noll, Felix Krauskopf, Meng-Ruo Huang, Carsten Bolm, Alan J. Slusarenko, and Martin C. H. Gruhlke. 2020. "The Sulfilimine Analogue of Allicin, S-Allyl-S-(S-allyl)-N-Cyanosulfilimine, Is Antimicrobial and Reacts with Glutathione" Antioxidants 9, no. 11: 1086. https://doi.org/10.3390/antiox9111086
APA StyleHorn, T., Bettray, W., Noll, U., Krauskopf, F., Huang, M.-R., Bolm, C., Slusarenko, A. J., & Gruhlke, M. C. H. (2020). The Sulfilimine Analogue of Allicin, S-Allyl-S-(S-allyl)-N-Cyanosulfilimine, Is Antimicrobial and Reacts with Glutathione. Antioxidants, 9(11), 1086. https://doi.org/10.3390/antiox9111086






