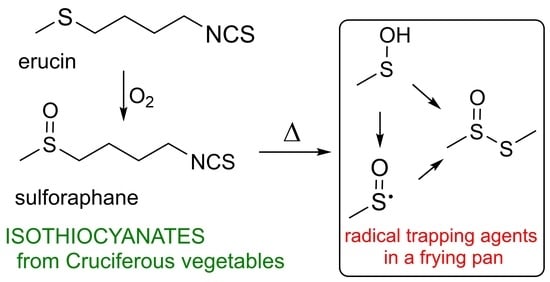
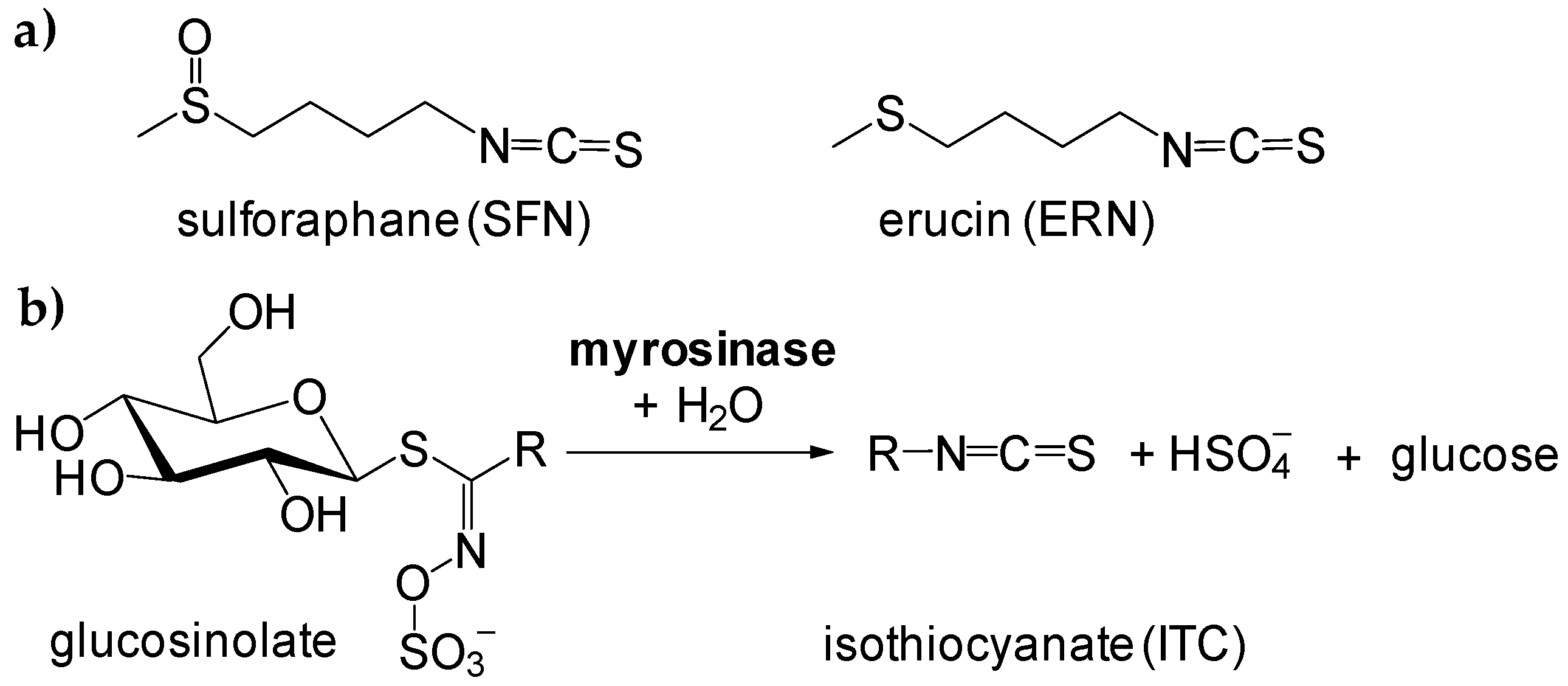
A Lesson Learnt from Food Chemistry—Elevated Temperature Triggers the Antioxidant Action of Two Edible Isothiocyanates: Erucin and Sulforaphane
Abstract
:

Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [Green Version]
- Posner, G.H.; Cho, C.G.; Green, J.V.; Zhang, Y.; Talalay, P. Design and synthesis of bifunctional isothiocyanate analogs of sulforaphane: Correlation between structure and potency as inducers of anticarcinogenic detoxication enzymes. J. Med. Chem. 1994, 37, 170–176. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Palliyaguru, D.L.; Yuan, J.M.; Kensler, T.W.; Fahey, J.W. Isothiocyanates: Translating the Power of Plants to People. Mol. Nutr. Food Res. 2018, 62, e1700965. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Heaney, R.K.; Mullin, W.J.; VanEtten, C.H. Glucosinolates and their breakdown products in food and food plants. CRC Crit. Rev. Food Sci. Nutr. 1983, 18, 123–201. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef] [Green Version]
- Azarenko, O.; Jordan, M.A.; Wilson, L. Erucin, the Major Isothiocyanate in Arugula (Eruca sativa), Inhibits Proliferation of MCF7 Tumor Cells by Suppressing Microtubule Dynamics. PLoS ONE 2014, 9, e100599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or sulforaphane: Is it the source or dose that matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur. J. Nutr. 2008, 47, 73–88. [Google Scholar] [CrossRef]
- Houghton, C.A. Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxidative Med. Cell. Longev. 2019, 2019, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Melchini, A.; Traka, M.H. Biological Profile of Erucin: A New Promising Anticancer Agent from Cruciferous Vegetables. Toxins 2010, 2, 593–612. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Lee, K.W.; Park, J.H.Y. Erucin Exerts Anti-Inflammatory Properties in Murine Macrophages and Mouse Skin: Possible Mediation through the Inhibition of NFκB Signaling. Int. J. Mol. Sci. 2013, 14, 20564–20577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citi, V.; Piragine, E.; Pagnotta, E.; Ugolini, L.; Mannelli, L.D.C.; Testai, L.; Ghelardini, C.; Lazzeri, L.; Calderone, V.; Martelli, A. Anticancer properties of erucin, an H2S-releasing isothiocyanate, on human pancreatic adenocarcinoma cells (AsPC-1). Phytother. Res. 2019, 33, 845–855. [Google Scholar] [CrossRef] [Green Version]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef]
- Valgimigli, L.; Iori, R. Antioxidant and pro-oxidant capacities of ITCs. Environ. Mol. Mutagen. 2009, 50, 222–237. [Google Scholar] [CrossRef]
- Barillari, J.; Canistro, D.; Paolini, M.; Ferroni, F.; Pedulli, G.F.; Iori, R.; Valgimigli, L. Direct Antioxidant Activity of Purified Glucoerucin, the Dietary Secondary Metabolite Contained in Rocket (Eruca sativa Mill.) Seeds and Sprouts. J. Agric. Food Chem. 2005, 53, 2475–2482. [Google Scholar] [CrossRef]
- Papi, A.; Orlandi, M.; Bartolini, G.; Barillari, J.; Iori, R.; Paolini, M.; Ferroni, F.; Fumo, M.G.; Pedulli, G.F.; Valgimigli, L. Cytotoxic and Antioxidant Activity of 4-Methylthio-3-butenyl Isothiocyanate from Raphanus sativus L. (Kaiware Daikon) Sprouts. J. Agric. Food Chem. 2008, 56, 875–883. [Google Scholar] [CrossRef]
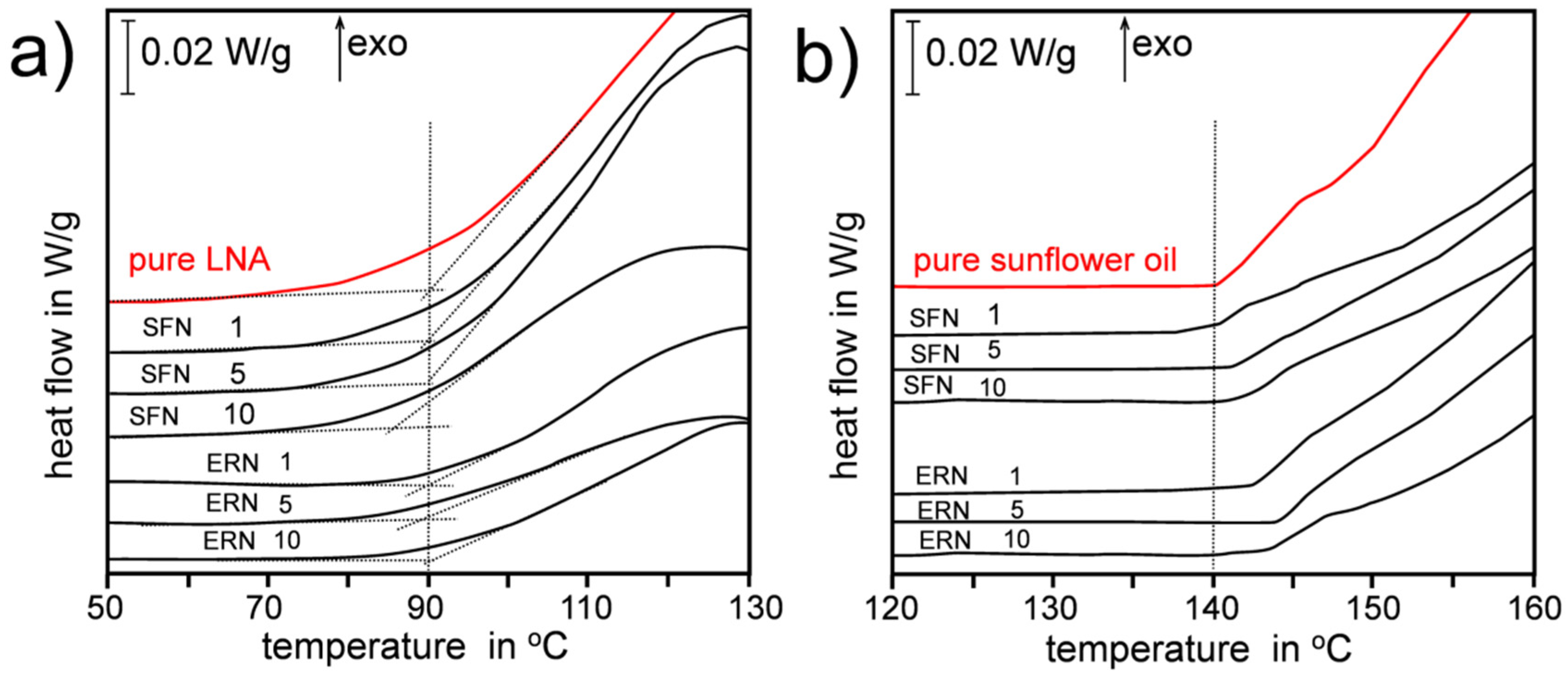
- Litwinienko, G.; Kasprzycka-Guttman, T. A DSC study on thermoxidation kinetics of mustard oil. Thermochim. Acta 1998, 319, 185–191. [Google Scholar] [CrossRef]
- Litwinienko, G.; Kasprzycka-Guttman, T.; Studzinski, M. Effects of selected phenol derivatives on the autoxidation of linolenic acid investigated by DSC non-isothermal methods. Thermochim. Acta 1997, 307, 97–106. [Google Scholar] [CrossRef]
- Litwinienko, G.; Kasprzycka-Guttman, T. The Influence of some Chain-Breaking Antioxidants on Thermal-Oxidative Decomposition of Linolenic Acid. J. Therm. Anal. Calorim. 1998, 54, 203–210. [Google Scholar] [CrossRef]
- Litwinienko, G.; Kasprzycka-Guttman, T.; Jamanek, D. DSC study of antioxidant properties of dihydroxyphenols. Thermochim. Acta 1999, 331, 79–86. [Google Scholar] [CrossRef]
- Musialik, M.; Litwinienko, G. DSC study of linolenic acid autoxidation inhibited by BHT, dehydrozingerone and olivetol. J. Therm. Anal. Calorim. 2007, 88, 781–785. [Google Scholar] [CrossRef]
- Jin, Y.; Ou, J.; Rosen, R.T.; Ho, C.T. Thermal degradation of sulforaphane in aqueous solution. J. Agric. Food Chem. 1999, 47, 3121–3123. [Google Scholar] [CrossRef]
- Vaidya, V.; Ingold, K.U.; Pratt, D.A. Garlic: Source of the Ultimate Antioxidants-Sulfenic Acids. Angew. Chem. Int. Ed. 2009, 48, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Lynett, P.T.; Butts, K.; Vaidya, V.; Garrett, G.E.; Pratt, D.A. The mechanism of radical-trapping antioxidant activity of plant-derived thiosulfinates. Org. Biomol. Chem. 2011, 9, 3320–3330. [Google Scholar] [CrossRef]
- Block, E. The Organosulfur Chemistry of the Genus Allium—Implications for the Organic Chemistry of Sulfur. Angew. Chem. Int. Ed. Engl. 1992, 31, 1135–1178. [Google Scholar] [CrossRef]
- Koelewijn, P.; Berger, H. Mechanism of the antioxidant action of dialkyl sulfoxides. Recl. Trav. Chim. Pays-Bas 1972, 91, 1275–1286. [Google Scholar] [CrossRef]
- Bateman, L.; Cain, M.; Colclough, T.; Cunneen, J.I. Oxidation of organic sulphides. Part XIII. The antioxidant action of sulphoxides and thiolsulphinates in autoxidizing squalene. J. Chem. Soc. 1962, 3570–3578. [Google Scholar] [CrossRef]
- Block, M.R. Chemistry of alkyl thiosulfinate esters. II. Sulfenic acids from dialkyl thiolsulfinate esters. J. Am. Chem. Soc. 1972, 94, 642–644. [Google Scholar] [CrossRef]
- Block, M.R. Chemistry of alkyl thiolsulfinate esters. III. tert-Butanethiosulfoxylic acid. J. Am. Chem. Soc. 1972, 94, 644–645. [Google Scholar] [CrossRef]
- Gupta, V.; Carroll, K.S. Sulfenic acid chemistry, detection and cellular lifetime. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 847–875. [Google Scholar] [CrossRef] [Green Version]
- Kubec, R.; Drhová, V.; Velíšek, J. Thermal Degradation of S-Methylcysteine and its Sulfoxide Important Flavor Precursors of Brassica and Allium Vegetables. J. Agric. Food Chem. 1998, 46, 4334–4340. [Google Scholar] [CrossRef]

| Compound, Symbol, RT 1 | Experiment 5 | MS Spectral Data m/z (Relative Intensity) |
|---|---|---|
| 4-methylthio-3-butenyl isothiocyanate 2 3A, RT = 34.2 min | SFN(100 °C, 160 °C), ERN(160 °C) | 161 [(M+2)+, 9], 87 (22), 85 (24), 72 (42), 61 (100), 45 (34) |
| but-3-enyl isothiocyanate 3 3B, RT = 11.6 min | SFN(100 °C, 160 °C), ERN(160 °C) | 113 (M+, 50), 85 (10), 72 (100), 55 (30), 39 (71) |
| S-methyl methylthiosulfonate 4 3C, RT = 10.5 min | SFN(160 °C), ERN(160 °C) | 128 [(M+2)+, 8], 126 (M+, 73), 111 (11), 81 (5), 79 (50), 64 (40), 47 (10), 45 (20) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cedrowski, J.; Dąbrowa, K.; Krogul-Sobczak, A.; Litwinienko, G. A Lesson Learnt from Food Chemistry—Elevated Temperature Triggers the Antioxidant Action of Two Edible Isothiocyanates: Erucin and Sulforaphane. Antioxidants 2020, 9, 1090. https://doi.org/10.3390/antiox9111090
Cedrowski J, Dąbrowa K, Krogul-Sobczak A, Litwinienko G. A Lesson Learnt from Food Chemistry—Elevated Temperature Triggers the Antioxidant Action of Two Edible Isothiocyanates: Erucin and Sulforaphane. Antioxidants. 2020; 9(11):1090. https://doi.org/10.3390/antiox9111090
Chicago/Turabian StyleCedrowski, Jakub, Kajetan Dąbrowa, Agnieszka Krogul-Sobczak, and Grzegorz Litwinienko. 2020. "A Lesson Learnt from Food Chemistry—Elevated Temperature Triggers the Antioxidant Action of Two Edible Isothiocyanates: Erucin and Sulforaphane" Antioxidants 9, no. 11: 1090. https://doi.org/10.3390/antiox9111090
APA StyleCedrowski, J., Dąbrowa, K., Krogul-Sobczak, A., & Litwinienko, G. (2020). A Lesson Learnt from Food Chemistry—Elevated Temperature Triggers the Antioxidant Action of Two Edible Isothiocyanates: Erucin and Sulforaphane. Antioxidants, 9(11), 1090. https://doi.org/10.3390/antiox9111090