Hydrogen Sulfide Relaxes Human Uterine Artery via Activating Smooth Muscle BKCa Channels
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Human Uterine Artery Collection
2.2. Antibodies and Chemicals
2.3. Isolation and Culture of Primary UA Smooth Muscle Cells (hUASMC)
2.4. Immunofluorescence Microscopy
2.5. RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.6. Western Blot
2.7. Electrophysiology
2.8. Organ Bath Studies
2.9. Statistics
3. Results
3.1. Expression of BKCa Channels in UA In Vitro and Primary UASMC In Vitro
3.2. Functional BKCa Channels in Primary hUASMC In Vitro
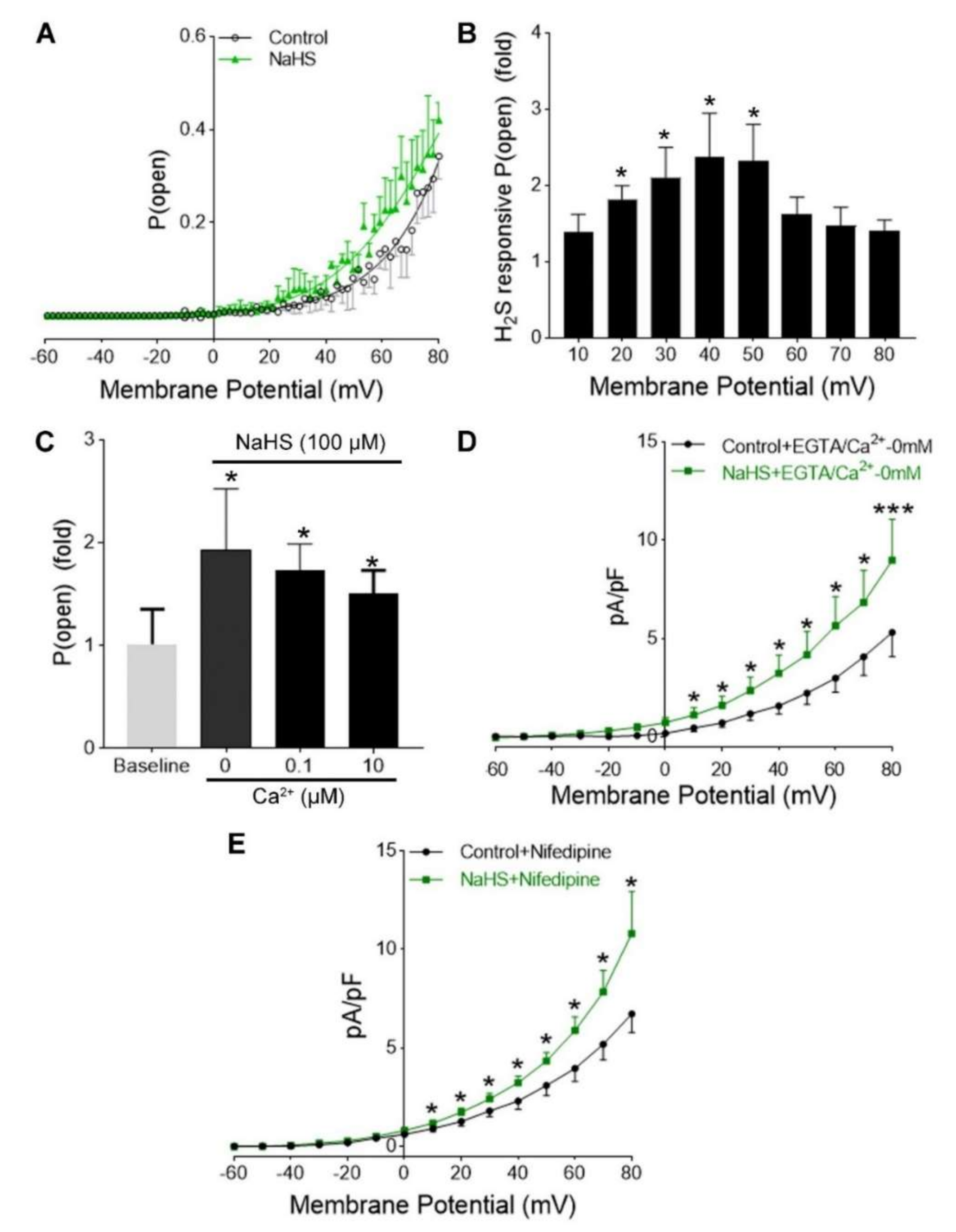
3.3. H2S Increased Ca2+-Activated and Voltage-Dependent K+ Currents in hUASMC
3.4. H2S Activation of hUASMC BKCa Was Independent of Extracellular Ca2+
3.5. H2S-Induced BKCa Activation Is Redox-Sensitive
3.6. H2S Relaxed Human UA via BKCa Channel
3.7. H2S Did Not Activate KATP Channels in hUASMC
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Greiss, F.C. Uterine blood flow during pregnnacy. MCV Q. 1972, 8, 52–60. [Google Scholar]
- Thornburg, K.L.; Jacobson, S.L.; Giraud, G.D.; Morton, M.J. Hemodynamic changes in pregnancy. Semin. Perinatol. 2000, 24, 11–14. [Google Scholar] [CrossRef]
- Osol, G.; Mandala, M. Maternal uterine vascular remodeling during pregnancy. Physiology 2009, 24, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. Intrauterine programming of adult disease. Mol. Med. Today 1995, 1, 418–423. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Kimura, T.; Yoshida, Y.; Toda, N. Mechanisms of relaxation induced by prostaglandins in isolated canine uterine arteries. Am. J. Obstet. Gynecol. 1992, 167, 1409–1416. [Google Scholar] [CrossRef]
- Magness, R.R.; Shaw, C.E.; Phernetton, T.M.; Zheng, J.; Bird, I.M. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am. J. Physiol. 1997, 272, H1730–H17340. [Google Scholar] [CrossRef]
- Gokina, N.I.; Kuzina, O.Y.; Vance, A.M. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1642–H1652. [Google Scholar] [CrossRef]
- Naden, R.P.; Iliya, C.A.; Arant, B.S., Jr.; Gant, N.F., Jr.; Rosenfeld, C.R. Hemodynamic effects of indomethacin in chronically instrumented pregnant sheep. Am. J. Obstet. Gynecol. 1985, 151, 484–494. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Cox, B.E.; Roy, T.; Magness, R.R. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J. Clin. Investig. 1996, 98, 2158–2166. [Google Scholar] [CrossRef]
- Lechuga, T.J.; Qi, Q.R.; Magness, R.R.; Chen, D.B. Ovine uterine artery hydrogen sulfide biosynthesis in vivo: Effects of ovarian cycle and pregnancy. Biol. Reprod. 2019, 100, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Sheibani, L.; Lechuga, T.J.; Zhang, H.; Hameed, A.; Wing, D.A.; Kumar, S.; Rosenfeld, C.R.; Chen, D.B. Augmented H2S production via cystathionine-beta-synthase upregulation plays a role in pregnancy-associated uterine vasodilation. Biol. Reprod. 2017, 96, 664–672. [Google Scholar] [CrossRef]
- Bai, J.; Qi, Q.R.; Li, Y.; Day, R.; Makhoul, J.; Magness, R.R.; Chen, D.B. Estrogen receptors and estrogen-induced uterine vasodilation in pregnancy. Int. J. Mol. Sci. 2020, 21, 4349. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide and polysulfides as biological mediators. Molecules 2014, 19, 16146–16157. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R.; et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Chen, J.C.; Sheibani, L.; Lechuga, T.J.; Chen, N.-B. Pregnancy Augments VEGF-Stimulated In Vitro Angiogenesis and Vasodilator (NO and H2S) Production in Human Uterine Artery Endothelial Cells. J. Clin. Endocrinol. Metab. 2017, 102, 2382–2393. [Google Scholar] [CrossRef]
- Tang, G.; Wu, L.; Liang, W.; Wang, R. Direct Stimulation of KATP Channels by Exogenous and Endogenous Hydrogen Sulfide in Vascular Smooth Muscle Cells. Mol. Pharmacol. 2005, 68, 1757–1764. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Xiao, D.; Longo, L.D.; Zhang, L. Role of KATP and L-type Ca2+ channel activities in regulation of ovine uterine vascular contractility: Effect of pregnancy and chronic hypoxia. Am. J. Obstet. Gynecol. 2010, 203, 596.e6–596.e12. [Google Scholar] [CrossRef]
- Jackson-Weaver, O.; Osmond, J.M.; Riddle, M.A.; Naik, J.S.; Bosc, L.V.G.; Walker, B.R.; Kanagy, N.L. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca2+-activated K+ channels and smooth muscle Ca2+ sparks. Am. J. Physiol. Circ. Physiol. 2013, 304, H1446–H1454. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, V.; Lingle, C.J. Regulation of BK Channels by Beta and Gamma Subunits. Annu. Rev. Physiol. 2019, 81, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy-Natarajan, G.; Koide, M. BK Channels in the Vascular System. Int. Rev. Neurobiol. 2016, 128, 401–438. [Google Scholar] [CrossRef]
- Brayden, J.E.; Nelson, M.T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 1992, 256, 532–535. [Google Scholar] [CrossRef]
- Yan, J.; Aldrich, R.W. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 7917–7922. [Google Scholar] [CrossRef]
- Yan, J.; Aldrich, R.W. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 2010, 466, 513–516. [Google Scholar] [CrossRef]
- Hu, X.Q.; Dasgupta, C.; Chen, M.; Xiao, D.; Huang, X.; Han, L.; Yang, S.; Xu, Z.; Zhang, L. Pregnancy reprograms large-conductance Ca2+-activated K+ channel in uterine arteries: Roles of ten-eleven translocation methylcytosine dioxygenase 1-mediated active demethylation. Hypertension 2017, 69, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.R.; Cornfield, D.N.; Roy, T. Ca2+-activated K+ channels modulate basal and E2beta-induced rises in uterine blood flow in ovine pregnancy. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H422–H431. [Google Scholar] [CrossRef]
- Rosenfeld, C.R.; Liu, X.T.; DeSpain, K. Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1878–H1887. [Google Scholar] [CrossRef][Green Version]
- Rosenfeld, C.R.; Roy, T.; DeSpain, K.; Cox, B.E. Large-conductance Ca2+-dependent K+ channels regulate basal uteroplacental blood flow in ovine pregnancy. J. Soc. Gynecol. Investig. 2005, 12, 402–408. [Google Scholar] [CrossRef]
- Lorca, R.A.; Wakle-Prabagaran, M.; Freeman, W.E.; Pillai, M.K.; England, S.K. The large-conductance voltage- and Ca2+-activated K+ channel and its gamma1-subunit modulate mouse uterine artery function during pregnancy. J. Physiol. 2018, 596, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, T.J.; Zhang, H.H.; Sheibani, L.; Karim, M.; Jia, J.; Magness, R.R.; Rosenfeld, C.R.; Chen, D.B. Estrogen replacement therapy in ovariectomized nonpregnant ewes stimulates uterine artery hydrogen sulfide biosynthesis by selectively up-regulating cystathionine beta-synthase expression. Endocrinology 2015, 156, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.L.; Kang, S.; Hoshi, N. XE991 and linopirdine are state-dependent inhibitors for Kv7/KCNQ channels that favor activated single subunits. J. Pharmacol. Exp. Ther. 2017, 362, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kay, H.Y.; Greene, D.L.; Kang, S.; Kosenko, A.; Hoshi, N. M-current preservation contributes to anticonvulsant effects of valproic acid. J. Clin. Investig. 2015, 125, 3904–3914. [Google Scholar] [CrossRef] [PubMed]
- Pallotta, B.S.; Magleby, K.L.; Barrett, J.N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature 1981, 293, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, R. H(2)S-induced vasorelaxation and underlying cellular and molecular mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H474–H480. [Google Scholar] [CrossRef]
- Hu, X.Q.; Dasgupta, C.; Xiao, D.; Huang, X.; Yang, S.; Zhang, L. MicroRNA-210 targets ten-eleven translocation methylcytosine dioxygenase 1 and suppresses pregnancy-mediated adaptation of large conductance Ca2+-activated K+ channel expression and function in ovine uterine arteries. Hypertension 2017, 70, 601–612. [Google Scholar] [CrossRef]
- Nelson, M.T.; Quayle, J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995, 268, C799–C822. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Hu, X.Q.; Zhang, L. Function and regulation of large conductance Ca2+-activated K+ channel in vascular smooth muscle cells. Drug Discov. Today 2012, 17, 974–987. [Google Scholar] [CrossRef]
- Salkoff, L.; Butler, A.; Ferreira, G.; Santi, C.; Wei, A. High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. 2006, 7, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wu, L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J. Biol. Chem. 1997, 272, 8222–8226. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.D.; Daggett, H.; Hanner, M.; Garcia, M.L.; McManus, O.B.; Brot, N.; Weissbach, H.; Heinemann, S.H.; Hoshi, T. Oxidative regulation of large conductance calcium-activated potassium channels. J. Gen. Physiol. 2001, 117, 253–274. [Google Scholar] [CrossRef] [PubMed]
- DiChiara, T.J.; Reinhart, P.H. Redox modulation of hslo Ca2+-activated K+ channels. J. Neurosci. 1997, 17, 4942–4955. [Google Scholar] [CrossRef] [PubMed]
- Erxleben, C.; Everhart, A.L.; Romeo, C.; Florance, H.; Bauer, M.B.; Alcorta, D.A.; Rossie, S.; Shipston, M.J.; Armstrong, D.L. Interacting effects of N-terminal variation and strex exon splicing on slo potassium channel regulation by calcium, phosphorylation, and oxidation. J. Biol. Chem. 2002, 277, 27045–27052. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Gao, T.M.; Huang, H.; Tong, Z. Redox modulation of large conductance calcium-activated potassium channels in CA1 pyramidal neurons from adult rat hippocampus. Neurosci. Lett. 2000, 286, 191–194. [Google Scholar] [CrossRef]
- Jiang, B.; Tang, G.; Cao, K.; Wu, L.; Wang, R. Molecular mechanism for H2S-induced activation of K(ATP) channels. Antioxid Redox Signal. 2010, 12, 1167–1178. [Google Scholar] [CrossRef]
- Liang, G.H.; Adebiyi, A.; Leo, M.D.; McNally, E.M.; Leffler, C.W.; Jaggar, J.H. Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2088–H2095. [Google Scholar] [CrossRef]
- Materazzi, S.; Zagli, G.; Nassini, R.; Bartolini, I.; Romagnoli, S.; Chelazzi, C.; Benemei, S.; Coratti, A.; De Gaudio, A.R.; Patacchini, R. Vasodilator activity of hydrogen sulfide (H2S) in human mesenteric arteries. Microvasc Res. 2017, 109, 38–44. [Google Scholar] [CrossRef]
- Ariyaratnam, P.; Loubani, M.; Morice, A.H. Hydrogen sulphide vasodilates human pulmonary arteries: A possible role in pulmonary hypertension? Microvasc Res. 2013, 90, 135–137. [Google Scholar] [CrossRef]
- Olson, K.R. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim. Biophys. Acta 2009, 1787, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, R.O., Jr.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef] [PubMed]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of hydrogen sulfide. Annu. Rev. Pharmacol. Toxicol. 1992, 32, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. The therapeutic potential of hydrogen sulfide: Separating hype from hope. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R297–R312. [Google Scholar] [CrossRef]
- Hu, X.Q.; Xiao, D.; Zhu, R.; Huang, X.; Yang, S.; Wilson, S.; Zhang, L. Pregnancy upregulates large-conductance Ca2+-activated K+ channel activity and attenuates myogenic tone in uterine arteries. Hypertension 2011, 58, 1132–1139. [Google Scholar] [CrossRef]
- Toro, L.; Li, M.; Zhang, Z.; Singh, H.; Wu, Y.; Stefani, E. MaxiK channel and cell signalling. Pflugers Arch. 2014, 466, 875–886. [Google Scholar] [CrossRef]
- Meera, P.; Wallner, M.; Song, M.; Toro, L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0-S6), an extracellular N terminus, and an intracellular (S9-S10) C terminus. Proc. Natl. Acad. Sci. USA 1997, 94, 14066–14071. [Google Scholar] [CrossRef]
- Meera, P.; Wallner, M.; Jiang, Z.; Toro, L. A calcium switch for the functional coupling between alpha (hslo) and beta subunits (Kv,cabeta) of maxi K channels. FEBS Lett. 1996, 385, 127–128. [Google Scholar] [CrossRef]
- Marty, A.; Tan, Y.P.; Trautmann, A. Three types of calcium-dependent channel in rat lacrimal glands. J. Physiol. 1984, 357, 293–325. [Google Scholar] [CrossRef]
- Ransom, C.B.; Liu, X.; Sontheimer, H. BK channels in human glioma cells have enhanced calcium sensitivity. Glia 2002, 38, 281–291. [Google Scholar] [CrossRef]
- Gessner, G.; Schonherr, K.; Soom, M.; Hansel, A.; Asim, M.; Baniahmad, A.; Derst, C.; Hoshi, T.; Heinemann, S.H. BKCa channels activating at resting potential without calcium in LNCaP prostate cancer cells. J. Membr. Biol. 2005, 208, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Dopico, A.M.; Bukiya, A.N.; Jaggar, J.H. Calcium- and voltage-gated BK channels in vascular smooth muscle. Pflugers Arch. 2018, 470, 1271–1289. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.R.; Word, R.A.; DeSpain, K.; Liu, X.T. Large conductance Ca2+-activated K+ channel channels contribute to vascular function in nonpregnant human uterine arteries. Reprod. Sci. 2008, 15, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.D.; Reeve, H.L.; Huang, J.M.; Tolarova, S.; Nelson, D.P.; Weir, E.K.; Archer, S.L. Potassium channel diversity in vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 1997, 75, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Nagar, D.; Liu, X.T.; Rosenfeld, C.R. Estrogen regulates {beta}1-subunit expression in Ca2+-activated K+ channel in arteries from reproductive tissues. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1417–H1427. [Google Scholar] [CrossRef] [PubMed]
- Schubert, R.; Nelson, M.T. Protein kinases: Tuners of the BKCa channel in smooth muscle. Trends Pharmacol. Sci. 2001, 22, 505–512. [Google Scholar] [CrossRef]
- Bagur, R.; Hajnoczky, G. Intracellular Ca2+ sensing: Its role in calcium homeostasis and signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef]
- Perez, G.J.; Bonev, A.D.; Nelson, M.T. Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am. J. Physiol. Cell Physiol. 2001, 281, C1769–C1775. [Google Scholar] [CrossRef]
- Nelson, S.H.; Steinsland, O.S.; Johnson, R.L.; Suresh, M.S.; Gifford, A.; Ehardt, J.S. Pregnancy-induced alterations of neurogenic constriction and dilation of human uterine artery. Am. J. Physiol. 1995, 268, H1694–H1701. [Google Scholar] [CrossRef]
- Asano, M.; Masuzawa-Ito, K.; Matsuda, T.; Suzuki, Y.; Oyama, H.; Shibuya, M.; Sugita, K. Functional role of charybdotoxin-sensitive K+ channels in the resting state of cerebral, coronary and mesenteric arteries of the dog. J. Pharmacol. Exp. Ther. 1993, 267, 1277–1285. [Google Scholar]
- Sitdikova, G.F.; Weiger, T.M.; Hermann, A. Hydrogen sulfide increases calcium-activated potassium (BK) channel activity of rat pituitary tumor cells. Pflugers Arch. 2010, 459, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Q.; Song, R.; Romero, M.; Dasgupta, C.; Huang, X.; Holguin, M.A.; Williams, V.; Xiao, D.; Wilson, S.M.; Zhang, L. Pregnancy increases Ca2+ sparks/spontaneous transient outward currents and reduces uterine arterial myogenic tone. Hypertension 2019, 73, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.K.; Sikka, G.; Gazi, S.K.; Steppan, J.; Jung, S.M.; Bhunia, A.K.; Barodka, V.M.; Gazi, F.K.; Barrow, R.K.; Wang, R.; et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011, 109, 1259–1268. [Google Scholar] [CrossRef]






| Subunits | Forward (5′-3′) | Reverse (5′-3′) | Amplicon (bp) |
|---|---|---|---|
| α | CTTCGTGGGTCTGTCCTTCC | TCTCTCGGTTGGCAGACTTG | 98 |
| β1 | AAGTGCCACCTGATTGAGACC | CACAGGCATGGGTACTGGG | 80 |
| β2 | GCACCGGATCGCTGTCATTA | TGGCAAAAAGACCTCCGGTA | 76 |
| β3 | GAGAGGACCGAGCCGTGATG | CACCACCTAGCAGAGTCAGTGAAG | 513 |
| β4 | GCGTTCTCATTGTGGTCC | TTCCAGTTGTGCCTGTTTC | 243 |
| γ1 | CGCGTCAGAGGCCGAG | TGGCTAAAGGCGGCGTC | 90 |
| γ2 | TCCTGGACTTCGCCATCTTC | TCAGCTCTGTGGGCTCCAC | 81 |
| γ3 | TTGGGGCTCAACCCTAACAC | GAATTCCAGGGCCCCACTAC | 98 |
| γ4 | TGGATCCAGGAGAACGCATC | TATCCTCCTGCTCTCCATGGG | 87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Bai, J.; Yang, Y.-h.; Hoshi, N.; Chen, D.-b. Hydrogen Sulfide Relaxes Human Uterine Artery via Activating Smooth Muscle BKCa Channels. Antioxidants 2020, 9, 1127. https://doi.org/10.3390/antiox9111127
Li Y, Bai J, Yang Y-h, Hoshi N, Chen D-b. Hydrogen Sulfide Relaxes Human Uterine Artery via Activating Smooth Muscle BKCa Channels. Antioxidants. 2020; 9(11):1127. https://doi.org/10.3390/antiox9111127
Chicago/Turabian StyleLi, Yan, Jin Bai, Yi-hua Yang, Naoto Hoshi, and Dong-bao Chen. 2020. "Hydrogen Sulfide Relaxes Human Uterine Artery via Activating Smooth Muscle BKCa Channels" Antioxidants 9, no. 11: 1127. https://doi.org/10.3390/antiox9111127
APA StyleLi, Y., Bai, J., Yang, Y.-h., Hoshi, N., & Chen, D.-b. (2020). Hydrogen Sulfide Relaxes Human Uterine Artery via Activating Smooth Muscle BKCa Channels. Antioxidants, 9(11), 1127. https://doi.org/10.3390/antiox9111127






