Inhibition of Osteoclast Differentiation by Carotenoid Derivatives through Inhibition of the NF-?B Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ethanolic Extract of Lycopene
2.3. Synthetic Carotenoid Derivatives
2.4. Energy Calculations
2.5. Solubilization of the Test Compounds
2.6. Cell Culture
2.7. Differentiation Assays
2.8. Cell Fractionation
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
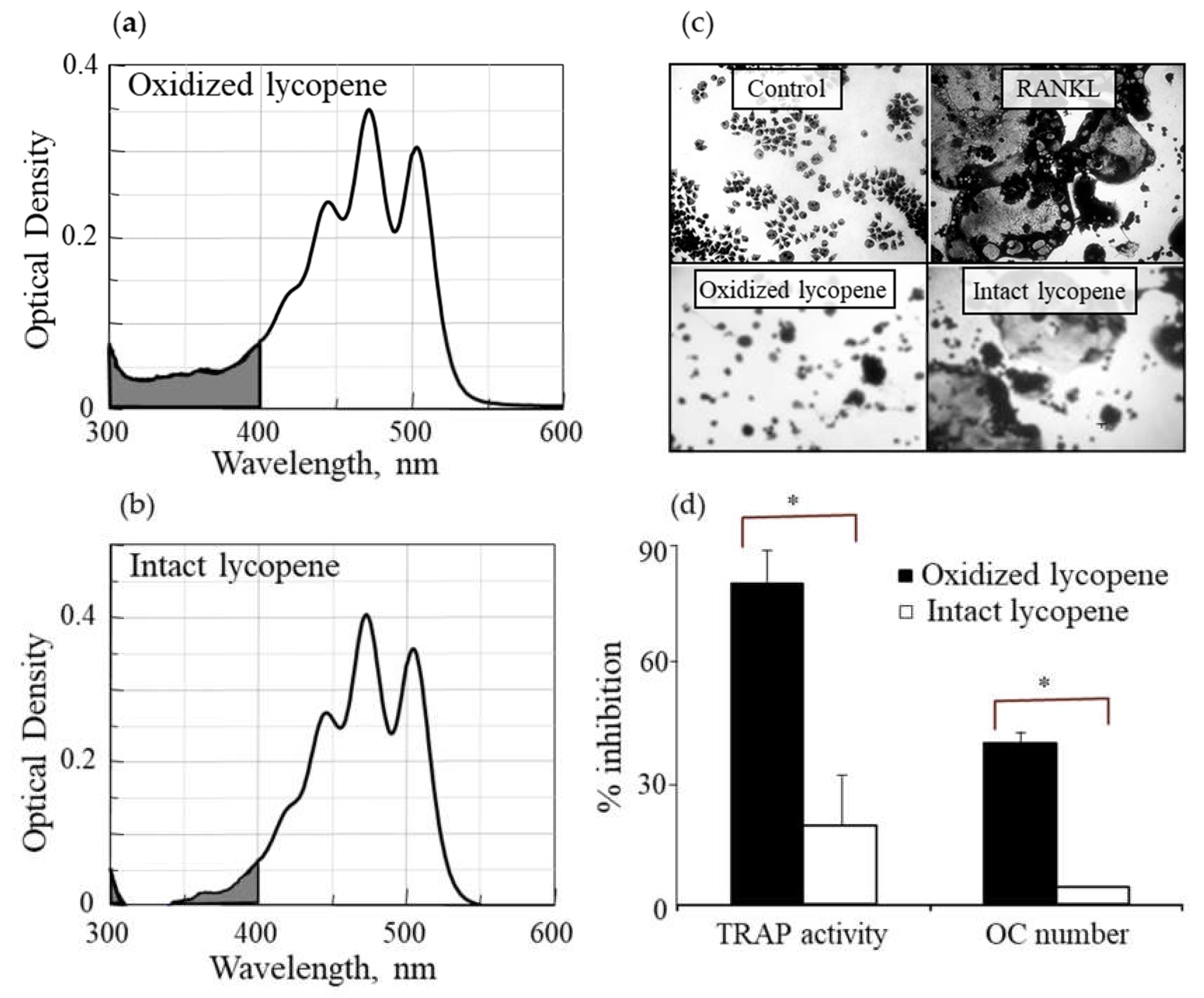
3.1. Oxidized Lycopene Is More Potent than Intact Lycopene in Inhibiting RANKL-Induced Osteoclast Differentiation
3.2. Diapocarotene-Dials Inhibition of RANKL-Induced Osteoclast Differentiation Depends on the Electron Density around the Reactive Carbon Atoms of the Molecules
3.3. Diapocarotene-Dials Inhibit RANKL-Induced NF-κB Activation in Osteoclast Precursors
3.4. Active Diapocarotene-Dials Inhibit RANKL-Induced TRAP Activity Synergistically with Curcumin and with Carnosic Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tucker, K.L.; Hannan, M.T.; Chen, H.; Cupples, L.A.; Wilson, P.W.; Kiel, D.P. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am. J. Clin. Nutr. 1999, 69, 727–736. [Google Scholar] [CrossRef]
- Muhlbauer, R.C.; Li, F. Effect of vegetables on bone metabolism. Nature 1999, 401, 343–344. [Google Scholar] [CrossRef] [PubMed]
- New, S.A.; Robins, S.P.; Campbell, M.K.; Martin, J.C.; Garton, M.J.; Bolton-Smith, C.; Grubb, D.A.; Lee, S.J.; Reid, D.M. Dietary influences on bone mass and bone metabolism: Further evidence of a positive link between fruit and vegetable consumption and bone health? Am. J. Clin. Nutr. 2000, 71, 142–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arscott, S.A. Food sources of carotenoids. In Carotenoids and Human Health; Tanumihardjo, S.A., Ed.; Springer: New York, NY, USA, 2013; pp. 1–331. [Google Scholar] [CrossRef] [Green Version]
- Khachik, F. Distribution and metabolism of dietary carotenoids in humans as a criterion for development of nutritional supplements. Pure Appl. Chem. 2006, 78, 1551–1557. [Google Scholar] [CrossRef]
- Parker, R.S. Carotenoids in human blood and tissues. J. Nutr. 1989, 119, 101–104. [Google Scholar] [CrossRef] [Green Version]
- El-Sohemy, A.; Baylin, A.; Kabagambe, E.; Ascherio, A.; Spiegelman, D.; Campos, H. Individual carotenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. Am. J. Clin. Nutr. 2002, 76, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Le Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Biotechnol. 2000, 20, 293–334. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Shen, C.L.; Von Bergen, V.; Chyu, M.C.; Jenkins, M.R.; Mo, H.; Chen, C.H.; Kwun, I.S. Fruits and dietary phytochemicals in bone protection. Nutr. Res. 2012, 32, 897–910. [Google Scholar] [CrossRef]
- Cheong, S.H.; Chang, K.J. The preventive effect of fermented milk supplement containing tomato (Lycopersion esculentum) and taurine on bone loss in ovariectomized rats. Adv. Exp. Med. Biol. 2009, 643, 333–340. [Google Scholar] [CrossRef]
- Rao, L.G.; Krishnadev, N.; Banasikowska, K.; Rao, A.V. Lycopene I—Effect on osteoclasts: Lycopene inhibits basal and parathyroid hormone-stimulated osteoclast formation and mineral resorption mediated by reactive oxygen species in rat bone marrow cultures. J. Med. Food 2003, 6, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Rao, A.V.; Rao, L.G. Lycopene II—Effect on osteoblasts: The carotenoid lycopene stimulates cell proliferation and alkaline phosphatase activity of SaOS-2 cells. J. Med. Food 2003, 6, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, J.; Fernandes, M.H.; Pinho, O.; Monteiro, P.R.R. Modulation of human osteoclastogenesis and osteoblastogenesis by lycopene. J. Nutr. Biochem. 2018, 57, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Yavropoulou, M.P.; Yovos, J.G. Osteoclastogenesis—Current knowledge and future perspectives. J. Musculoskelet. Neuronal Interact. 2008, 8, 204–216. [Google Scholar]
- Boyce, B.F.; Xiu, Y.; Li, J.; Xing, L.; Yao, Z. NF-κB-mediated regulation of osteoclastogenesis. Endocrinol. Metab. 2015, 30, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef]
- Franzoso, G.; Carlson, L.; Xing, L.; Poljak, L.; Shores, E.W.; Brown, K.D.; Leonardi, A.; Tran, T.; Boyce, B.F.; Siebenlist, U. Requirement for NF-κB in osteoclast and B-cell development. Genes Dev. 1997, 11, 3482–3496. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, T.; Fukushima, H.; Nakao, K.; Shin, M.; Yasuda, H.; Weih, F.; Doi, T.; Aoki, K.; Alles, N.; Ohya, K.; et al. Processing of the NF-κB2 precursor p100 to p52 is critical for RANKL-induced osteoclast differentiation. J. Bone Miner. Res. 2010, 25, 1058–1067. [Google Scholar]
- Joo, Y.E.; Karrasch, T.; Muhlbauer, M.; Allard, B.; Narula, A.; Herfarth, H.H.; Jobin, C. Tomato lycopene extract prevents lipopolysaccharide-induced NF-κB signaling but worsens dextran sulfate sodium-induced colitis in NF-κBEGFP mice. PLoS ONE 2009, 4, e4562. [Google Scholar] [CrossRef] [Green Version]
- Hadad, N.; Levy, R. The synergistic anti-inflammatory effects of lycopene, lutein, beta-carotene, and carnosic acid combinations via redox-based inhibition of NF-κB signaling. Free Radic. Biol. Med. 2012, 53, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Simone, R.E.; Russo, M.; Catalano, A.; Monego, G.; Froehlich, K.; Boehm, V.; Palozza, P. Lycopene inhibits NF-kB-mediated IL-8 expression and changes redox and PPARgamma signalling in cigarette smoke-stimulated macrophages. PLoS ONE 2011, 6, e19652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnewiel, K.; Ernst, H.; Caris-Veyrat, C.; Ben-Dor, A.; Kampf, A.; Salman, H.; Danilenko, M.; Levy, J.; Sharoni, Y. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic. Biol. Med. 2009, 47, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Linnewiel-Hermoni, K.; Motro, Y.; Miller, Y.; Levy, J.; Sharoni, Y. Carotenoid derivatives inhibit nuclear factor κB activity in bone and cancer cells by targeting key thiol groups. Free Radic. Biol. Med. 2014, 75, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Nara, E.; Hayashi, H.; Kotake, M.; Miyashita, K.; Nagao, A. Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leukemia cells. Nutr. Cancer 2001, 39, 273–283. [Google Scholar] [CrossRef]
- Caris-Veyrat, C.; Schmid, A.; Carail, M.; Bohm, V. Cleavage products of lycopene produced by in vitro oxidations: Characterization and mechanisms of formation. J. Agric. Food Chem. 2003, 51, 7318–7325. [Google Scholar] [CrossRef]
- Giuliano, G.; Al-Babili, S.; Von Lintig, J. Carotenoid oxygenases: Cleave it or leave it. Trends Plant. Sci. 2003, 8, 145–149. [Google Scholar] [CrossRef]
- Ben-Aziz, A.; Britton, G.; Goodwin, T.W. Carotene epoxides of Lycopersicon esculentum. Phytochemistry 1973, 12, 2759–2764. [Google Scholar] [CrossRef]
- Veprik, A.; Khanin, M.; Linnewiel-Hermoni, K.; Danilenko, M.; Levy, J.; Sharoni, Y. Polyphenols, isothiocyanates, and carotenoid derivatives enhance estrogenic activity in bone cells but inhibit it in breast cancer cells. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E815–E824. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the Phenol-Explorer database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Cao, F.; Liu, T.; Xu, Y.; Xu, D.; Feng, S. Curcumin inhibits cell proliferation and promotes apoptosis in human osteoclastoma cell through MMP-9, NF-κB and JNK signaling pathways. Int. J. Clin. Exp. Pathol. 2015, 8, 6037–6045. [Google Scholar] [PubMed]
- Cheng, T.; Zhao, Y.; Li, B.; Cheng, M.; Wang, J.; Zhang, X. Curcumin attenuation of wear particle-induced osteolysis via RANKL signaling pathway suppression in mouse calvarial model. Mediat. Inflamm. 2017, 2017, 5784374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zhou, X.; Zhou, L.; Liu, Z.; Yuan, J.; Cheng, J.; Zhao, J.; Wu, L.; Li, H.; Qiu, H.; et al. Carnosic acid inhibits inflammation response and joint destruction on osteoclasts, fibroblast-like synoviocytes, and collagen-induced arthritis rats. J. Cell. Physiol. 2018, 233, 6291–6303. [Google Scholar] [CrossRef] [PubMed]
- Thummuri, D.; Naidu, V.G.M.; Chaudhari, P. Carnosic acid attenuates RANKL-induced oxidative stress and osteoclastogenesis via induction of Nrf2 and suppression of NF-κB and MAPK signalling. J. Mol. Med. 2017, 95, 1065–1076. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Mark Gordon’s Quantum Theory Group. Available online: http://www.msg.ameslab.gov/GAMESS/GAMESS.html (accessed on 2 November 2020).
- Mulliken, R.S. Electronic population analysis on LCAO–MO molecular wave functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef] [Green Version]
- Na, H.K.; Surh, Y.J. Transcriptional regulation via cysteine thiol modification: A novel molecular strategy for chemoprevention and cytoprotection. Mol. Carcinog. 2006, 45, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Pande, V.; Sousa, S.F.; Ramos, M.J. Direct covalent modification as a strategy to inhibit nuclear factor-κB. Curr. Med. Chem. 2009, 16, 4261–4273. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satoh, T.; Kosaka, K.; Itoh, K.; Kobayashi, A.; Yamamoto, M.; Shimojo, Y.; Kitajima, C.; Cui, J.; Kamins, J.; Okamoto, S.; et al. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1. J. Neurochem. 2008, 104, 1116–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felemban, A.; Braguy, J.; Zurbriggen, M.D.; Al-Babili, S. Apocarotenoids involved in plant development and stress response. Front. Plant Sci. 2019, 10, 1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, K.-P.; Dickinson, A.J.; Mi, J.; Cui, G.; Kharbatia, N.M.; Guo, X.; Sugiono, E.; Aranda, M.; Rueping, M.; Benfey, P.N.; et al. Anchorene is an endogenous diapocarotenoid required for anchor root formation in Arabidopsis. bioRxiv 2018. [Google Scholar] [CrossRef]
- Harrison, E.H.; dela Sena, C.; Eroglu, A.; Fleshman, M.K. The formation, occurrence, and function of beta-apocarotenoids: Beta-carotene metabolites that may modulate nuclear receptor signaling. Am. J. Clin. Nutr. 2012, 96, 1189S–1192S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopec, R.E.; Riedl, K.M.; Harrison, E.H.; Curley, R.W., Jr.; Hruszkewycz, D.P.; Clinton, S.K.; Schwartz, S.J. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J. Agric. Food Chem. 2010, 58, 3290–3296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajic, M.; Zaripheh, S.; Sun, F.; Erdman, J.W., Jr. Apo-8’-lycopenal and apo-12′-lycopenal are metabolic products of lycopene in rat liver. J. Nutr. 2006, 136, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, M.; Giuffrida, D.; Salafia, F.; Giofrè, S.V.; Mondello, L. Carotenoids and apocarotenoids determination in intact human blood samples by online supercritical fluid extraction-supercritical fluid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2018, 1032, 40–47. [Google Scholar] [CrossRef]
- Eroglu, A.; Harrison, E.H. Carotenoid metabolism in mammals, including man: Formation, occurrence, and function of apocarotenoids. J. Lipid Res. 2013, 54, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Cooperstone, J.L.; Novotny, J.A.; Riedl, K.M.; Cichon, M.J.; Francis, D.M.; Curley, R.W., Jr.; Schwartz, S.J.; Harrison, E.H. Limited appearance of apocarotenoids is observed in plasma after consumption of tomato juices: A randomized human clinical trial. Am. J. Clin. Nutr. 2018, 108, 784–792. [Google Scholar] [CrossRef]
- Lobo, G.P.; Amengual, J.; Palczewski, G.; Babino, D.; von Lintig, J. Mammalian carotenoid-oxygenases: Key players for carotenoid function and homeostasis. Biochim. Biophys. Acta 2012, 1821, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, C.; Hessel, S.; Lampert, J.M.; Vogt, K.; Lederer, M.O.; Breithaupt, D.E.; von Lintig, J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 2001, 276, 14110–14116. [Google Scholar] [CrossRef] [Green Version]
- Amengual, J.; Lobo, G.P.; Golczak, M.; Li, H.N.; Klimova, T.; Hoppel, C.L.; Wyss, A.; Palczewski, K.; von Lintig, J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 2011, 25, 948–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, J.L.; Ricupero, D.A.; Huang, S.; Fatma, N.; Singh, D.P.; Romero, J.R.; Chattopadhyay, N. Differential activity of kaempferol and quercetin in attenuating tumor necrosis factor receptor family signaling in bone cells. Biochem. Pharmacol. 2006, 71, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.C.; Takada, Y.; Aggarwal, B.B. Curcumin (diferuloylmethane) inhibits receptor activator of NF-κB ligand-induced NF-κB activation in osteoclast precursors and suppresses osteoclastogenesis. J. Immunol. 2004, 172, 5940–5947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Andersson, G.; Lindgren, U.; Li, Y. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem. Biophys. Res. Commun. 2010, 401, 356–362. [Google Scholar] [CrossRef]
- Kim, S.J.; Kang, S.Y.; Shin, H.H.; Choi, H.S. Sulforaphane inhibits osteoclastogenesis by inhibiting nuclear factor-κB. Mol. Cells 2005, 20, 364–370. [Google Scholar]
- Murakami, A.; Song, M.; Ohigashi, H. Phenethyl isothiocyanate suppresses receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis by blocking activation of ERK1/2 and p38 MAPK in RAW264.7 macrophages. BioFactors 2007, 30, 1–11. [Google Scholar] [CrossRef]
- Shang, W.; Zhao, L.J.; Dong, X.L.; Zhao, Z.M.; Li, J.; Zhang, B.B.; Cai, H. Curcumin inhibits osteoclastogenic potential in PBMCs from rheumatoid arthritis patients via the suppression of MAPK/RANK/c-Fos/NFATc1 signaling pathways. Mol. Med. Rep. 2016, 14, 3620–3626. [Google Scholar] [CrossRef] [Green Version]
- Calniquer, G.; Khanin, M.; Ovadia, H.; Linnewiel-Hermoni, K.; Stepensky, D.; Trachtenberg, A.; Levy, J.; Sharoni, Y. Combined effects of carotenoids and polyphenols in balancing the response of skin cells to UV irradiation. Eur. J. Nutr. 2020. submitted. [Google Scholar]



| Derivative 1 | Structure | Mulliken Population Values (Electron Density) | HOMO-LUMO 2 Energy Gap (kcal/mol) | |
|---|---|---|---|---|
| Left | Right | |||
| 6,14′ |  | 6.16 | 6.10 | 189.51 |
| 10,10′ |  | 6.17 | 6.17 | 191.39 |
| 8,8′ |  | 6.21 | 6.21 | 178.21 |
| 8,12′ |  | 6.23 | 6.22 | 210.84 |
| 12,12′ |  | 6.24 | 6.24 | 214.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odes-Barth, S.; Khanin, M.; Linnewiel-Hermoni, K.; Miller, Y.; Abramov, K.; Levy, J.; Sharoni, Y. Inhibition of Osteoclast Differentiation by Carotenoid Derivatives through Inhibition of the NF-?B Pathway. Antioxidants 2020, 9, 1167. https://doi.org/10.3390/antiox9111167
Odes-Barth S, Khanin M, Linnewiel-Hermoni K, Miller Y, Abramov K, Levy J, Sharoni Y. Inhibition of Osteoclast Differentiation by Carotenoid Derivatives through Inhibition of the NF-?B Pathway. Antioxidants. 2020; 9(11):1167. https://doi.org/10.3390/antiox9111167
Chicago/Turabian StyleOdes-Barth, Shlomit, Marina Khanin, Karin Linnewiel-Hermoni, Yifat Miller, Karina Abramov, Joseph Levy, and Yoav Sharoni. 2020. "Inhibition of Osteoclast Differentiation by Carotenoid Derivatives through Inhibition of the NF-?B Pathway" Antioxidants 9, no. 11: 1167. https://doi.org/10.3390/antiox9111167
APA StyleOdes-Barth, S., Khanin, M., Linnewiel-Hermoni, K., Miller, Y., Abramov, K., Levy, J., & Sharoni, Y. (2020). Inhibition of Osteoclast Differentiation by Carotenoid Derivatives through Inhibition of the NF-?B Pathway. Antioxidants, 9(11), 1167. https://doi.org/10.3390/antiox9111167









