Catechins within the Biopolymer Matrix—Design Concepts and Bioactivity Prospects
Abstract
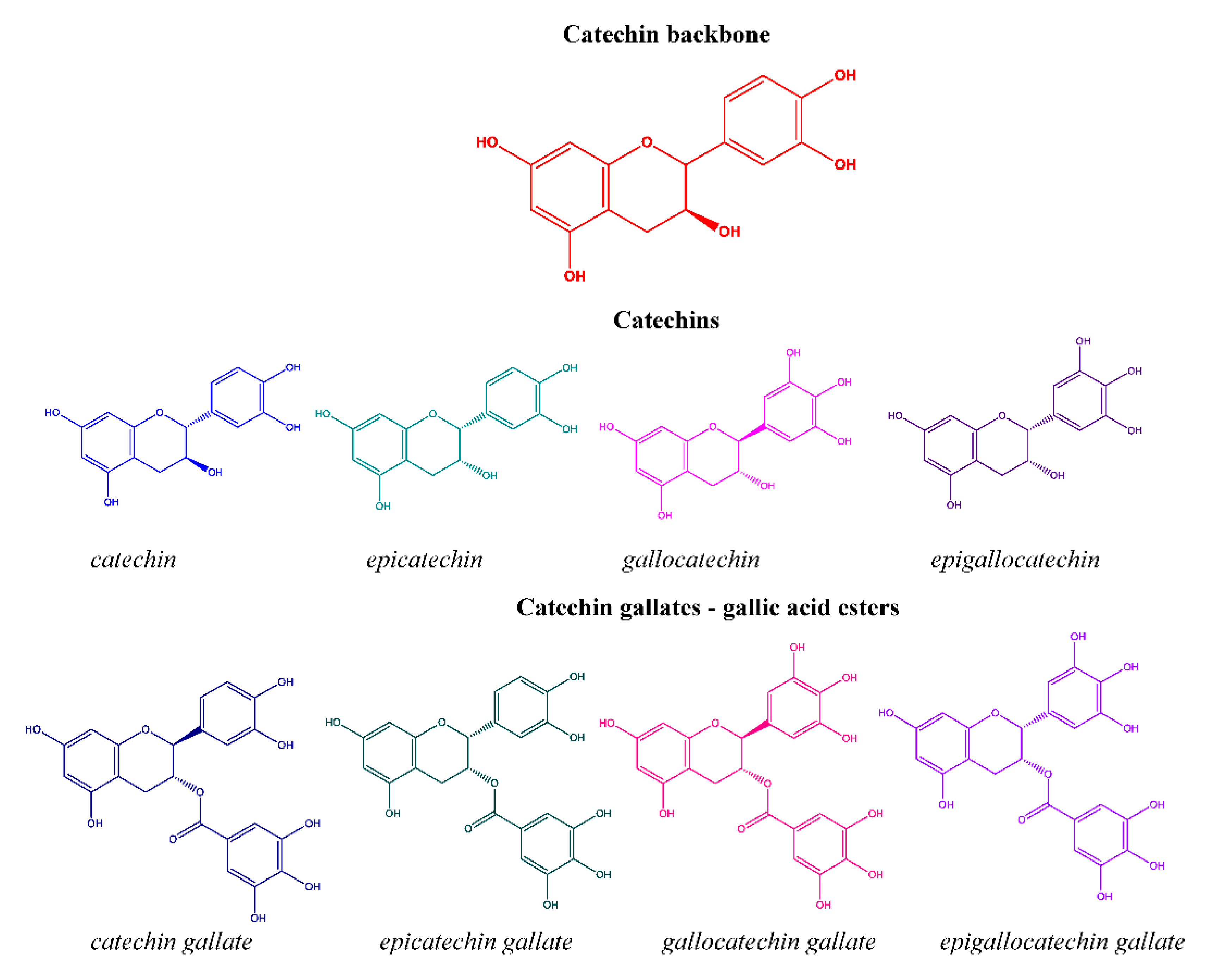
:1. Introduction
2. Catechins Encapsulation Methodologies
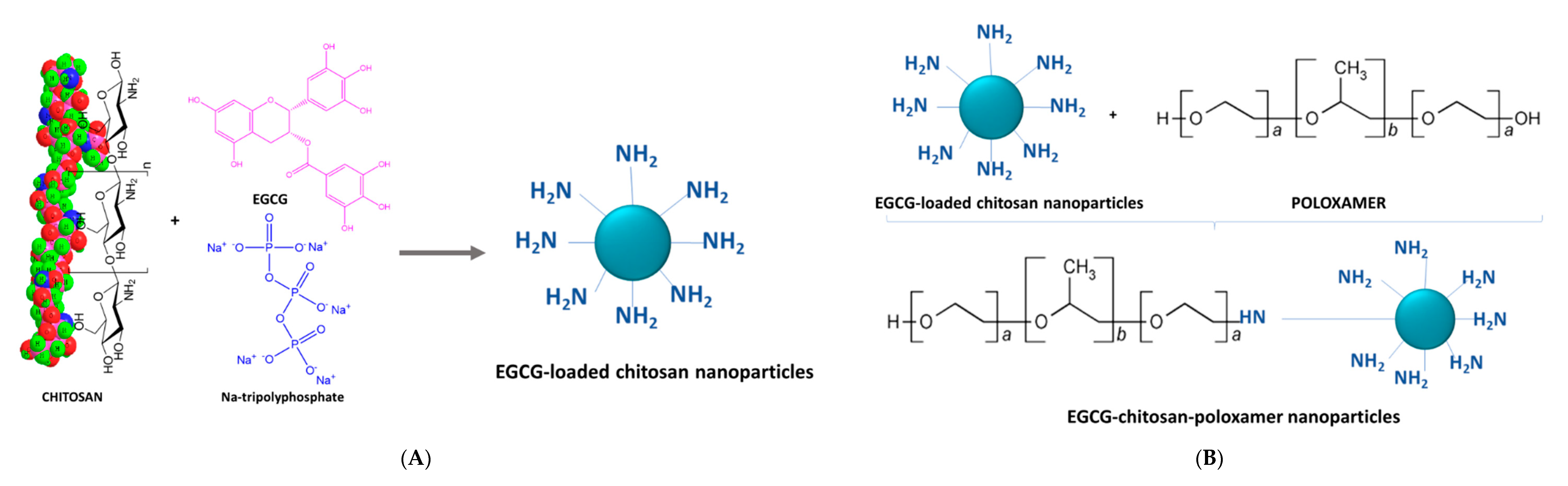
2.1. Ionic Gelation
2.2. Electrospinning
2.3. Solvent Displacement Method
2.4. Spray-Drying and Homogenisation
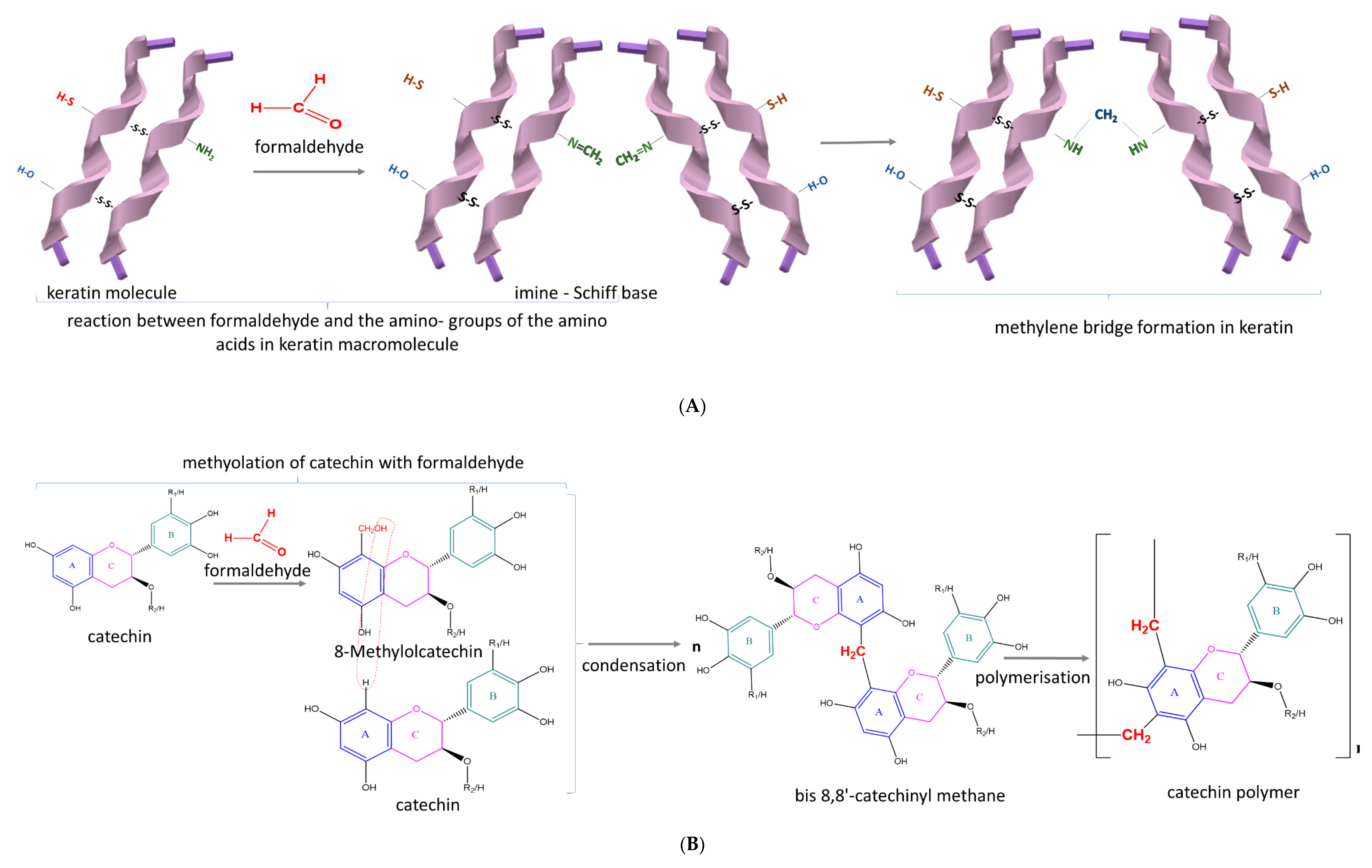
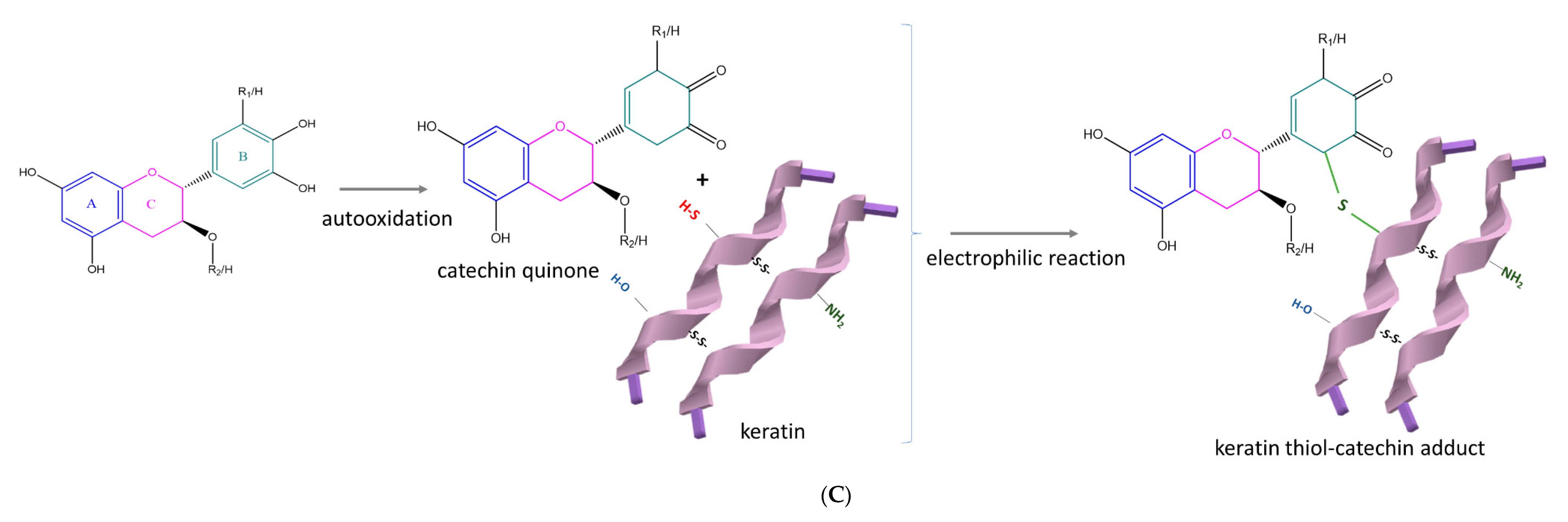
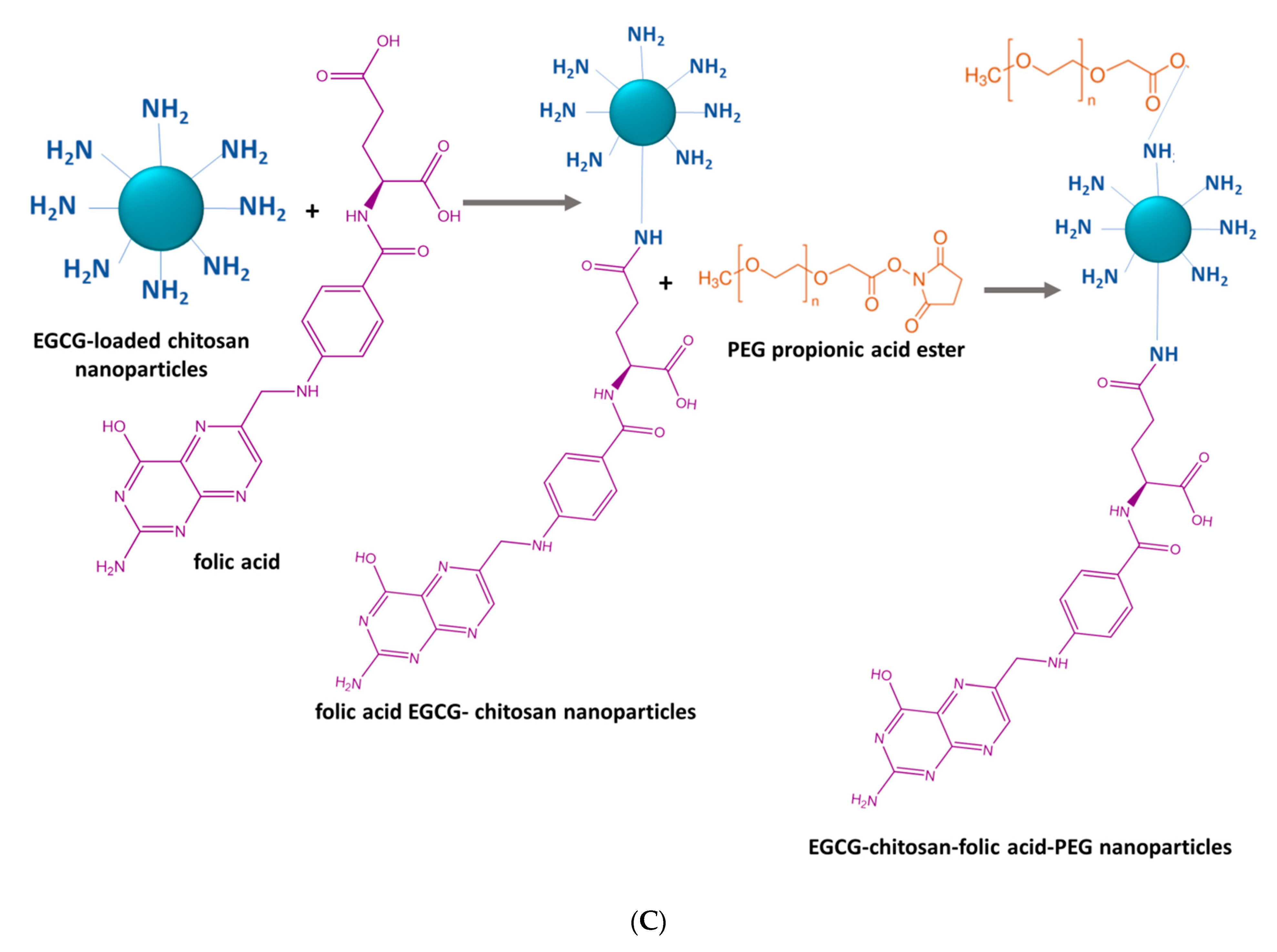
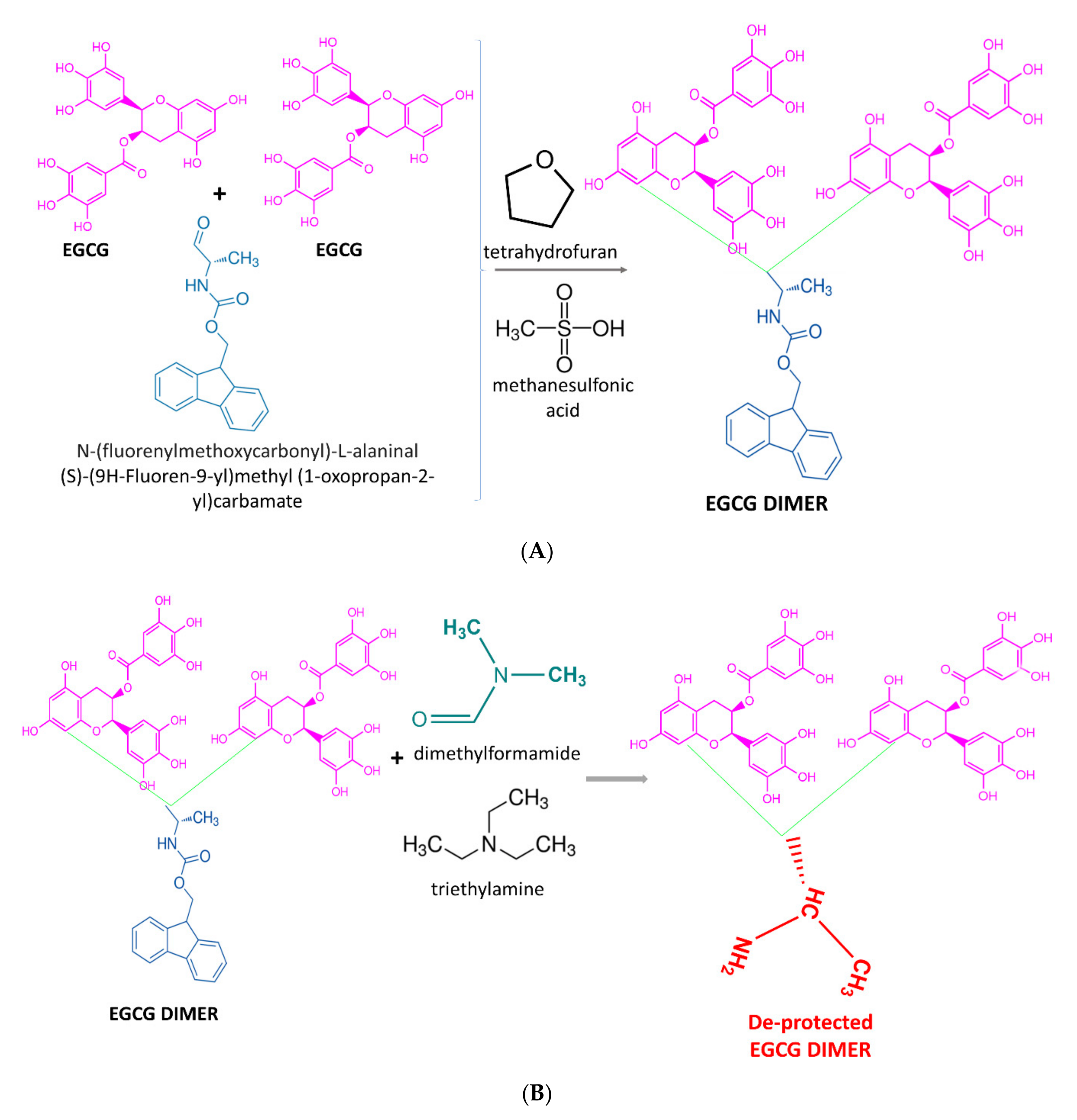
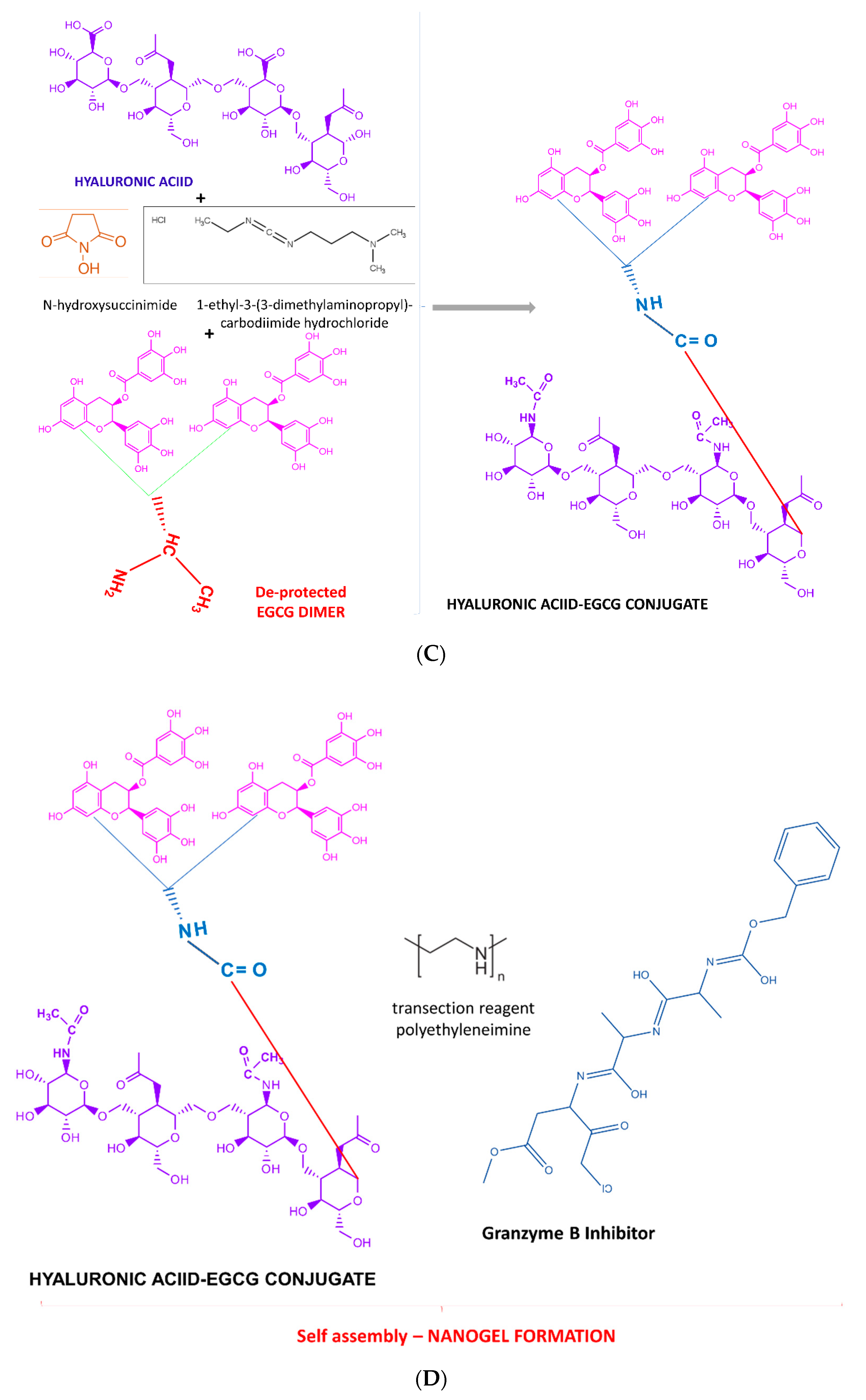
2.5. Chemical Synthesis Methods
2.6. Direct Addition, Layer-by-Layer Assembly and Soaking Methods
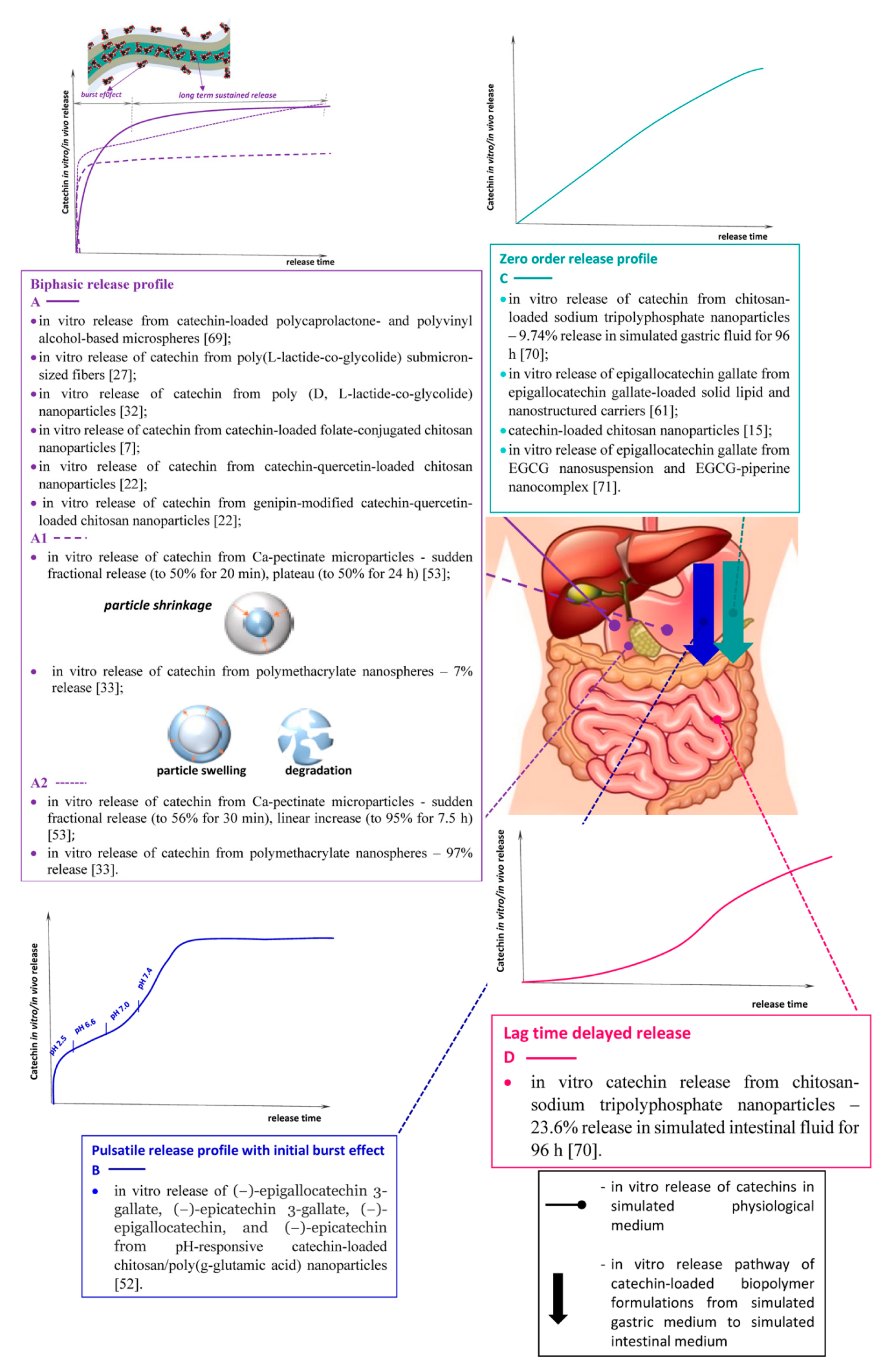
3. Modes of In Vitro Catechins Release
4. Bioactivity Enhancements of Catechins-Loaded Biopolymer Formulations
4.1. Antioxidant Activity
4.2. Antidiabetic Activity
4.3. Antiproliferative Activity
4.4. Antibacterial Activity
4.5. Neuroprotective Activity
5. Synergism, Mode of Delivery and Safety Aspects
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Sawicka, B.; Ziarati, P.; Krochmal-Marczak, B.; Skiba, D. Nutraceuticals in food and pharmacy. Rev. Agron. Sci. 2019, 74, 7–31. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of tea catechins and its improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.H.; Augustin, M.A. Nano- and micro-particles for delivery of catechins: Physical and biological performance. Crit. Rev. Food Sci. Nutr. 2019, 59, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.J.B.; Tayo, L.L. Synthesis of poly (lactic-co-glycolic acid) microspheres loaded with (−)-epigallocatechin-3-gallate-β-cyclodextrin inclusion complex using double solvent emulsification. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2018; Volume 2045, p. 020056. [Google Scholar]
- Helal, N.A.; Eassa, H.A.; Amer, A.M.; Eltokhy, M.A.; Edafiogho, I.; Nounou, M.I. Nutraceuticals’ Novel Formulations: The Good, the Bad, the Unknown and Patents Involved Involved. Recent Pat. Drug Deliv. Formul. 2019, 13, 105–156. [Google Scholar] [CrossRef] [PubMed]
- Yaneva, Z.; Ivanova, D.; Nikolova, N.; Tzanova, M. The 21st century revival of chitosan in service to bio-organic chemistry. Biotechnol. Biotechnol. Equip. 2020, 34, 221–237. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Yu, Q.; Li, D.; Li, F. Synthesis, characterization of catechin-loaded folate-conjugated chitosan nanoparticles and their anti-proliferative effect. CyTA J. Food 2018, 16, 868–876. [Google Scholar] [CrossRef]
- Chung, J.E.; Tan, S.; Gao, S.J.; Yongvongsoontorn, N.; Kim, S.H.; Lee, J.H.; Choi, S.H.; Yano, H.; Zhuo, L.; Kurisawa, M.; et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat. Nanotechnol. 2014, 9, 907–912. [Google Scholar] [CrossRef]
- Haratifar, S.; Meckling, K.A.; Corredig, M. Antiproliferative activity of tea catechins associated with casein micelles, using HT29 colon cancer cells. J. Dairy Sci. 2014, 97, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Scholl, C.; Lepper, A.; Lehr, T.; Hanke, N.; Schneider, K.L.; Brockmoeller, J.; Seufferlein, T.; Stinglet, J.K. Population nutrikinetics of green tea extract. PLoS ONE 2018, 13, e0193074.56. [Google Scholar] [CrossRef]
- Yi, Z.; Sun, Z.; Chen, G.; Zhang, H.; Ma, H.; Su, W.; Cui, X.; Li, X. Size-controlled, colloidally stable and functional nanoparticles based on the molecular assembly of green tea polyphenols and keratins for cancer therapy. J. Mater. Chem. B 2018, 6, 1373–1386. [Google Scholar] [CrossRef]
- Yaneva, Z.; Georgieva, N.; Staleva, M. Development of d,l-α-tocopherol acetate/zeolite carrier system: Equilibrium study. Mon. Chem.-Chem. Mon. 2016, 147, 1167–1175. [Google Scholar] [CrossRef]
- Lyubenova Yaneva, Z. Nonsteroidal anti-inflammatory drug solid-state microencapsulation on green activated carbon—Mass transfer and host-guest interactions. Chem. Biochem. Eng. Q. 2019, 33, 249–269. [Google Scholar] [CrossRef]
- Haratifar, S.; Meckling, K.A.; Corredig, M. Bioefficacy of tea catechins encapsulated in casein micelles tested on a normal mouse cell line (4D/WT) and its cancerous counterpart (D/v-src) before and after in vitro digestion. Food Funct. 2014, 5, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Rajput, R.; Nag, P.; Kumar, S.; Rachana Singh, M. Synthesis, characterization and evaluation of antioxidant properties of catechin hydrate nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 39, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Szekalska, M.; Sosnowska, K.; Czajkowska-Ko’snik, A.; Winnicka, K. Calcium chloride modified alginate microparticles formulated by the spray drying process: A strategy to prolong the release of freely soluble drugs. Materials 2018, 11, 1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.B.; Patel, J.K.; Chakraborty, S. Review of patents and application of spray drying in pharmaceutical, food and flavor industry. Recent Pat. Drug Deliv. Formul. 2014, 8, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, L.; Madadlou, A.; Yarmand, M.; Mousavi, M.E. Spray-dried alginate microparticles carrying caffeine-loaded and potentially bioactive nanoparticles. Food Res. Int. 2014, 62, 1113–1119. [Google Scholar] [CrossRef]
- Santa-Maria, M.; Scher, H.; Jeoh, T. Microencapsulation of bioactives in cross-linked alginate matrices by spray drying. J. Microencapsul. 2012, 29, 286–295. [Google Scholar] [CrossRef]
- Zhang, H.; Jung, J.; Zhao, Y. Preparation, characterization and evaluation of antibacterial activity of catechins and catechins–Zn complex loaded β-chitosan nanoparticles of different particle sizes. Carbohydr. Polym. 2016, 137, 82–91. [Google Scholar] [CrossRef]
- Li, F.; Jina, H.; Xiao, J.; Yin, X.; Liu, X.; Lia, D.; Huang, Q. The simultaneous loading of catechin and quercetin on chitosan-based nanoparticles as effective antioxidant and antibacterial agent. Food Res. Int. 2018, 111, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, E.; Chung, D.; Lee, H.G. Characteristics and antioxidant activity of catechin-loaded calcium pectinate gel beads prepared by internal gelation. Colloids Surf. B Biointerfaces 2009, 74, 17–22. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, J.S.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar]
- Ghitescu, P.; Popa, A.; Schipanski, A.; Hirsch, C.; Yazgana, G.; Popa, V.I.; Rossi, R.M.; Maniura-Weber, K. Catechin loaded PLGA submicron-sized fibers reduce levels of reactive oxygen species induced by MWCNT in vitro. Eur. J. Pharm. Biopharm. 2018, 122, 78–86. [Google Scholar] [CrossRef]
- Hoseyni, S.Z.; Jafari, S.M.; Tabarestani, H.S.; Ghorbani, M.; Assadpour, E.; Sabaghi, M. Production and characterization of catechin-loaded electrospun nanofibers from Azivash gum-polyvinyl alcohol. Carbohydr. Polym. 2020, 235, 115979. [Google Scholar] [CrossRef]
- Hoseyni, S.Z.; Jafari, S.M.; Tabarestani, H.S.; Ghorbani, M.; Assadpour, E.; Sabaghi, M. Release of catechin from Azivash gum-polyvinyl alcohol electrospun nanofibers in simulated food and digestion media. Food Hydrocoll. 2021, 112, 106366. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Maver, U.; Glaser, T.K.; Bren, U.; Hrnčič, M.K.; Petek, G.; Peršin, Z. Electrospun Composite nanofibrous materials based on (poly)-phenol-polysaccharide formulations for potential wound treatment. Materials 2020, 13, 2631. [Google Scholar] [CrossRef]
- Barreras-Urbina, C.G.; Ramírez-Wong, B.; López-Ahumada, G.A.; Burruel-Ibarra, S.E.; Martínez-Cruz, O.; Tapia-Hernández, J.A.; Félix, F.R. Nano- and micro-particles by nanoprecipitation: Possible application in the food and agricultural industries. Int. J. Food Prop. 2016, 19, 1912–1923. [Google Scholar] [CrossRef]
- Pool, H.; Quintanar, D.; Figueroa, J.D.; Marinho Mano, C.; Bechara, J.E.; Godínez, L.A.; Mendoza, S. Antioxidant effects of quercetin and catechin encapsulated into PLGA nanoparticles. J. Nanomater. 2012, 2012, 145380. [Google Scholar] [CrossRef]
- Pool, H.; Luna-Barcenas, G.; McClements, D.J.; Mendoza, S. Development of polymethacrylate nanospheres as targeted delivery systems for catechin within the gastrointestinal tract. J. Nanopart. Res. 2017, 19, 324–339. [Google Scholar] [CrossRef]
- Rodrigues, S.; Rosa da Costa, A.M.; Flórez-Fernández, N.; Torres, M.D.; Faleiro, M.L.; Buttini, F.; Grenha, A. Inhalable spray-dried chondroitin sulphate microparticles: Effect of different solvents on particle properties and drug activity. Polymers 2020, 12, 425. [Google Scholar] [CrossRef] [Green Version]
- Peres, I.; Rocha, S.; Gomesa, J.; Morais, S.; Pereira, M.C.; Coelho, M. Preservation of catechin antioxidant properties loaded in carbohydrate nanoparticles. Carbohydr. Polym. 2011, 86, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Paximada, P.; Echegoyen, Y.; Koutinas, A.A.; Mandal, I.G.; Lagaron, J.M. Encapsulation of hydrophilic and lipophilized catechin into nanoparticles through emulsion electrospraying. Food Hydrocoll. 2017, 64, 123–132. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balaraman, M. Extraction of catechins from decaffeinated green tea for development of nanoemulsion using palm oil and sunflower oil-based lipid carrier systems. J. Food Eng. 2015, 147, 14–23. [Google Scholar] [CrossRef]
- Huang, Y.B.; Tsai, M.J.; Wu, P.C.; Tsai, Y.H.; Wu, Y.H.; Fang, J.Y. Elastic liposomes as carriers for oral delivery and the brain distribution of (+)-catechin. J. Drug Target. 2011, 19, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Liu, J.; Ren, J.; Huang, F.; Ou, H.; Ding, Y.; Zhang, Y.; Ma, R.; An, Y.; Liu, J.; et al. Green tea catechin-based complex micelles combined with doxorubicin to overcome cardiotoxicity and multidrug resistance. Theranostics 2016, 6, 1277–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraguchi, I.; Ooya, T. Catechin-albumin conjugates: Potentials as an antioxidant-functionalized drug carrier. In Proceedings of the 10th World Biomaterials Congress 2016, Montréal, QC, Canada, 17–22 May 2016. [Google Scholar] [CrossRef]
- Sistanipour, E.; Meshkinia, A.; Oveisi, H. Catechin-conjugated mesoporous hydroxyapatite nanoparticle: A novel nano-antioxidant with enhanced osteogenic property. Colloids Surf. B Biointerfaces 2018, 169, 329–339. [Google Scholar] [CrossRef]
- Takano, T.; Murakami, T.; Kamitakahara, H.; Nakatsubo, F. Mechanism of formaldehyde adsorption of (+)-catechin. J. Wood Sci. 2008, 54, 329–331. [Google Scholar] [CrossRef]
- Mori, T.; Ishii, T.; Akagawa, M.; Nakamura, Y.; Nakayama, T. Covalent binding of tea catechins to protein thiols: The relationship between stability and electrophilic reactivity. Biosci. Biotechnol. Biochem. 2010, 74, 2451–2456. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yong, H.; Yao, X.; Hu, H.; Yun, D.; Xiao, L. Recent advances in phenolic–protein conjugates: Synthesis, characterization, biological activities and potential applications. RSC Adv. 2019, 9, 35825–35840. [Google Scholar] [CrossRef] [Green Version]
- Safer, A.M.; Leporatti, S.; Jose, J.; Soliman, M.S. Conjugation of EGCG and Chitosan NPs as a novel nano-drug delivery system. Int. J. Nanomed. 2019, 14, 8033–8046. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Yan, J.; Luo, L.; Ma, M.; Zhu, H. Preparation and characterization of (−)-Epigallocatechin-3-gallate (EGCG)-loaded nanoparticles and their inhibitory effects on human breast cancer MCF-7 cells. Sci. Rep. 2017, 7, 45521. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, J.H.; Wang, H.; Wang, C.X.; Zhang, J.Y.; Tao, Y.W.; Zheng, G.D.; Xie, H.Y. Synthesis and characterization of folate-poly (ethylene glycol) chitosan graft-polyethylenimine as a non-viral carrier for tumor-targeted gene delivery. Afr. J. Biotechnol. 2011, 10, 6120–6129. [Google Scholar]
- Liang, K.; Ng, S.; Lee, F.; Lim, J.; Chung, J.E.; Lee, S.S.; Kurisawa, M. Targeted intracellular protein delivery based on hyaluronic acid–green tea catechin nanogels. Acta Biomater. 2016, 33, 142–152. [Google Scholar] [CrossRef]
- Delcea, M.; Möhwald, H.; Skirtach, A.G. Stimuli-responsive LbL capsules and nanoshells for drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 730–747. [Google Scholar] [CrossRef]
- Shutava, T.G.; Balkundia, S.S.; Lvov, Y.M. (−)-Epigallocatechin gallate/gelatin layer-by-layer assembled films and microcapsules. J. Colloid Interface Sci. 2009, 330, 276–283. [Google Scholar] [CrossRef]
- Mandal, R.S.K.; Bhatt, S.; Jithin, M.V.; Lekshman, A.; Raguvaran, R.; Mondal, D.B. Evaluation of encapsulated catechin in chitosan-sodium tripolyphosphate nanoparticle. J. Pharmacogn. Phytochem. 2019, 8, 4153–4158. [Google Scholar]
- Tang, D.W.; Yu, S.H.; Ho, Y.C.; Huang, B.Q.; Tsai, G.J.; Hsieh, H.Y.; Sung, H.W.; Mi, F.L. Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocoll. 2013, 30, 33–41. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.W.; Chung, D.; Lee, H.G. Catechin-loaded calcium pectinate microparticles reinforced with liposome and hydroxypropylmethylcellulose: Optimization and in vivo antioxidant activity. Food Hydrocoll. 2009, 23, 2226–2233. [Google Scholar] [CrossRef]
- Meena, K.P.; Vijayakumar, M.R.; Dwibedy, P.S. Catechin-loaded Eudragit microparticles for the management of diabetes: Formulation, characterization and in vivo evaluation of antidiabetic efficacy. J. Microencapsul. 2017, 34, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, J. Enhanced oral bioavailability of EGCG using pH-sensitive polymeric nanoparticles: Characterization and in vivo investigation on nephrotic syndrome rats. Drug Des. Dev. Ther. 2018, 12, 2509–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, V.S.S.; Poejo, J.; Matias, A.A.; Rodríguez-Rojo, S.; Coceroc, M.J.; Duarte, C.M.M. Using different natural origin carriers for development of epigallocatechin gallate (EGCG) solid formulations with improved antioxidant activity by PGSS-drying. RSC Adv. 2016, 6, 67599–67609. [Google Scholar] [CrossRef]
- Ahmad, M.; Mudgila, P.; Ganib, A.; Hamed, F.; Masoodi, F.A.; Maqsood, S. Nano-encapsulation of catechin in starch nanoparticles: Characterization, release behavior and bioactivity retention during simulated in-vitro digestion. Food Chem. 2019, 270, 95–104. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yu, S.H.; Tsai, G.J.; Tang, D.W.; Mi, F.L.; Peng, Y.P. Novel technology for the preparation of self-assembled catechin/gelatin nanoparticles and their characterization. J. Agric. Food Chem. 2010, 58, 6728–6734. [Google Scholar] [CrossRef]
- Perez-Ruiz, A.G.; Ganem, A.; Olivares-Corichi, I.M.; García-Sánchez, J.R. Lecithin–chitosan–TPGS nanoparticles as nanocarriers of (−)-epicatechin enhanced its anticancer activity in breast cancer cells. RSC Adv. 2018, 8, 34773–34782. [Google Scholar] [CrossRef] [Green Version]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef]
- Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Design, development, and characterization of lipid nanocarriers-based epigallocatechin gallate delivery system for preventive and therapeutic supplementation. Drug Des. Dev. Ther. 2016, 31, 3519–3528. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, N.; Mandal, A.K.A. Pharmacokinetic, toxicokinetic, and bioavailability studies of epigallocatechin-3-gallate loaded solid lipid nanoparticle in rat model. Drug Dev. Ind. Pharm. 2019, 45, 1506–1514. [Google Scholar] [CrossRef]
- Mandal, S.; Debnath, K.; Jana, N.R.; Jana, N.R.R. Trehalose conjugated, catechin loaded polylactide nanoparticle for improved neuroprotection against intracellular polyglutamine aggregate. Biomacromolecules 2020, 21, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Yaneva, Z.L. Drug mass transfer mechanism, thermodynamics, and in vitro release kinetics of antioxidant-encapsulated zeolite microparticles as a drug carrier system. Chem. Biochem. Eng. Q. 2018, 32, 281–298. [Google Scholar] [CrossRef]
- Yaneva, Z.; Georgieva, N. Physicochemical and morphological characterization of pharmaceutical nanocarriers and mathematical modeling of drug encapsulation/release mass transfer processes. In Nanoscale Fabrication, Optimization, Scale-up and Biological Aspects of Pharmaceutical Nanotechnology, 1st ed.; Grumezescu, A., Ed.; William Andrew An Imprint of Elsevier: Norwich, NY, USA, 2018; Chapter 5; ISBN 9780128136294. [Google Scholar]
- Yaneva, Z.; Staleva, M.; Georgieva, N. Study on the host-guest interactions during caffeine encapsulation into zeolite. Eur. J. Chem. 2015, 6, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Caro, R.; Veiga-Ochoa, M.D. Characterization and dissolution study of chitosan freeze-dried systems for drug-controlled release. Molecules 2009, 14, 4370–4386. [Google Scholar] [CrossRef] [PubMed]
- Indurkhya, A.; Patel, M.; Sharma, P.; Abed, S.N.; Shnoudeh, A.; Maheshwari, R.; Deb, P.K.; Tekade, R.K. Influence of Drug Properties and Routes of Drug Administration on the Design of Controlled Release System. In Dosage Form Design Considerations, Advances in Pharmaceutical Product Development and Research; Rakesh, K.T., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume I, Chapter 6; ISBN 978-0-12-814423-7. [Google Scholar]
- Sivabalan, M.; Gayathri, V.; Kiruthika, C.; Madhan, B. Formulation and evaluation of biodegradable polyphenolic microspheres for cancer. Int. J. Pharm. Technol. 2012, 4, 4493–4505. [Google Scholar]
- Samanta, A.; Bandyopadhyay, B.; Das, N. Formulation of catechin hydrate nanocapsule and study of its bioavailability. Med. Chem. 2016, 6, 399–404. [Google Scholar] [CrossRef]
- Dahiya, S.; Rani, R.; Dhingra, D.; Kumar, S.; Dilbaghi, N. Conjugation of epigallocatechin gallate and piperine into a zein nanocarrier: Implication on antioxidant and anticancer potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Jain, D.; Raturi, R.; Jain, V.; Bansal, P.; Singh, R. Recent technologies in pulsatile drug delivery systems. Biomatter 2011, 1, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beugeling, M.; Grasmeijer, N.; Born, P.A.; van der Meulen, M.; van der Kooij, R.S.; Schwengle, K.; Baert, L.; Amssoms, K.; Frijlink, H.W.; Hinrichs, W.L.J. The mechanism behind the biphasic pulsatile drug release from physically mixed poly(dl-lactic(-co-glycolic) acid)-based compacts. Int. J. Pharm. 2018, 551, 195–202. [Google Scholar] [CrossRef]
- Gandhi, S.; Nuxoll, E. Non-delaminating pulsatile release composites. Chem. Eng. Sci. 2016, 141, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Alfano, J.; Race, D.; Davé, R.N. Zero-order release of poorly water-soluble drug from polymeric films made via aqueous slurry casting. Eur. J. Pharm. Sci. 2018, 117, 245–254. [Google Scholar] [CrossRef]
- Montero Mirabet, M.; Skalsky, B. Advanced Approaches for Delayed-Release Formulations. ONdrugDelivery Mag. 2017, 4–9. [Google Scholar]
- Yaneva, Z.L.; Simeonov, E.B.; Georgieva, D.I. In vitro Ultraviolet-B radiation mediated antioxidant response of Bulgarian Goldenrod (Solidago virgaurea L.) extract. Bulg. Chem. Commun. 2020, 52, 33–40. [Google Scholar]
- Rani, V.; Gupta, K. ROS in carcinogenesis and anticancerous drug-induced toxicity. In Free Radicals in Human Health and Disease; Rani, V., Yadav, U., Eds.; Springer: New Delhi, India, 2015; pp. 209–225. [Google Scholar]
- Kailakua, S.I.; Mulyawantia, I.; Alamsyah, A.N. Formulation of nanoencapsulated catechin with chitosan as encapsulation material. Procedia Chem. 2014, 9, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Dang, S.; Gupta, S.; Bansal, R.; Ali, J.; Gabrani, R. Nano-encapsulation of a natural polyphenol, green tea catechins: Way to preserve its antioxidative potential. In Free Radicals in Human Health and Disease; Rani, V., Yadav, U., Eds.; Springer: New Delhi, India, 2015; pp. 397–415. [Google Scholar]
- Samanta, A.; Chanda, S.; Bandyopadhyay, B.; Das, N. Establishment of drug delivery system nanocapsulated with an antioxidant (+)-catechin hydrate and sodium meta borate chelator against sodium fluoride induced oxidative stress in rats. J. Trace Elem. Med. Biol. 2016, 33, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Hashemipour, M.A.; Lotfi, S.; Torabi, M.; Sharifi, F.; Ansari, M.; Ghassemi, A.; Sheikhshoaie, S. Evaluation of the effects of three plant species (Myrtus communis L., Camellia sinensis L., Zataria multiflora Boiss.) on the healing process of intraoral ulcers in rats. J. Dent. 2017, 18, 127–135. [Google Scholar]
- Pastoriza, S.; Mesías, M.; Cabrera, C.; Rufián-Henares, J.A. Healthy properties of green and white teas: An update. Food Funct. 2017, 8, 2650–2662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addepalli, V.; Suryavanshi, S.V. Catechin attenuates diabetic autonomic neuropathy in streptozotocin induced diabetic rats. Biomed. Pharmacother. 2018, 108, 1517–1523. [Google Scholar] [CrossRef]
- Grzesik, M.; Naparlo, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef]
- Kempegowda, P.K.; Zameer, F.; Murar, S.K. Delineating antidiabetic proficiency of catechin from Withania somnifera and its Inhibitory action on dipeptidyl peptidase-4 (DPP-4). Biomed. Res. 2018, 29, 3192–3200. [Google Scholar] [CrossRef] [Green Version]
- Cosarca, S.; Tanase, C.; Muntean, D.L. Therapeutic aspects of catechin and its derivatives—An update. Acta Biol. Marisiensis 2019, 2, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Lankatillake, C.; Huynh, T.; Dias, D.A. Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods 2019, 15, 105. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Zhangba, Z. Preparation and characterization of catechin-grafted chitosan with antioxidant and antidiabetic potential. Int. J. Biol. Macromol. 2014, 70, 150–155. [Google Scholar] [CrossRef]
- Ivanova, D.; Zhelev Zh Aoki, I.; Bakalova, R.; Higashi, T. Overproduction of reactive oxygen species—Obligatory or not for induction of apoptosis by anticancer drugs. Chin. J. Cancer Res. 2016, 28, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [Green Version]
- Thrachootam, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.V.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [Green Version]
- Coleman, W.B.; Tsongalis, G.T. Molecular mechanisms of human carcinogenesis. EXS 2006, 96, 321–349. [Google Scholar]
- Sanna, V.; Singh, C.K.; Jashari, R.; Adhami, V.M.; Chamcheu, J.C.; Rady, I.; Sechi, M.; Mukhtar, H.; Siddiqui, I.A. Targeted nanoparticles encapsulating (−)-epigallocatechin-3-gallate for prostate cancer prevention and therapy. Sci. Rep. 2017, 7, 41573. [Google Scholar] [CrossRef] [Green Version]
- Bae, K.H.; Tan, S.; Yamashita, A.; Ang, W.X.; Gao, S.J.; Wang, S.; Chung, J.E.; Kurisawa, M. Hyaluronic acid-green tea catechin micellar nanocomplexes: Fail-safe cisplatin nanomedicine for the treatment of ovarian cancer without off-target toxicity. Biomaterials 2017, 148, 41–53. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, Z.R.; Lai, C.H.; Hsieh, C.H.; Feng, C.L. Active targeted nanoparticles for oral administration of gastric cancer therapy. Biomacromolecules 2015, 16, 3021–3032. [Google Scholar] [CrossRef]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Bharali, D.J.; Nihal, M.; Adhami, V.M.; Khan, N.; Chamcheu, J.C.; Khan, M.I.; Shabana, S.; Mousa, S.A.; Mukhtar, H. Excellent antiproliferative and pro-apoptotic effects of -epigallocatechin-3gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1619–1626. [Google Scholar] [CrossRef]
- Singh, M.; Bhatnagar, P.; Mishra, S.; Kumar, P.; Shukla, Y.; Gupta, K.C. PLGA-encapsulated tea polyphenols enhance the chemotherapeutic efficacy of cisplatin against human cancer cells and mice bearing Ehrlich ascites carcinoma. Int. J. Nanomed. 2015, 10, 6789–6809. [Google Scholar] [CrossRef] [Green Version]
- Ernest, U.; Chen, H.Y.; Xu, M.J.; Taghipour, Y.D.; Asad, M.H.H.B.; Rahimi, R.; Murtaza, G. Anti-cancerous potential of polyphenol-loaded polymeric nanotherapeutics. Molecules 2018, 23, 2787. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.H.; Feng, C.L.; Lai, C.H.; Lin, J.H.; Chen, H.Y. Preparation of epigallocatechin gallate loaded nanoparticles and characterization of their inhibitory effects on Helicobacter pylori growth in vitro and in vivo. Sci. Technol. Adv. Mater. 2014, 15, 045006. [Google Scholar] [CrossRef] [Green Version]
- Atinderpal, K.; Kapoor, N.; Gupta, S.; Tyag, A.; Sharma, R.K.; Ali, J.; Gabrani, R.; Dang, S. Development and characterization of green tea catechins and ciprofloxacin-loaded nanoemulsion for intravaginal delivery to treat urinary tract infection. Indian J. Pharm. Sci. 2018, 80, 442–452. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2016, 15, 60. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Hembrom, S. Neuroprotective Effect of Flavonoids: A Systematic Review. Int. J. Aging Res. 2019, 2, 26. [Google Scholar]
- Ovais, M.; Zia, N.; Ahmad, I.; Khalil, A.T.; Raza, A.; Ayaz, M.; Sadiq, A.; Ullah, F.; Shinwari, Z.K. Phyto-therapeutic and nanomedicinal approaches to cure Alzheimer’s Disease: Present status and future opportunities. Front. Aging Neurosci. 2018, 10, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halevas, E.; Nday, C.M.; Salifoglou, A. Hybrid catechin silica nanoparticle influence on Cu(II) toxicity and morphological lesions in primary neuronal cells. J. Inorg. Biochem. 2016, 163, 240–249. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Prajapati, A.K. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers—A review. RSC Adv. 2015, 5, 97547–97562. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of natural antioxidants: An anti-aging perspective. Front. Bioeng. Biotechnol. 2020, 7, 447. [Google Scholar] [CrossRef] [Green Version]
- Bernatoniene, J.; Kopustinskiene, D.M. The role of catechins in cellular responses to oxidative stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La, X.; Zhang, L.; Li, Z.; Li, H.; Yang, Y. (−)-Epigallocatechin gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-κB/miR-155-5p/MDR1 pathway. J. Agric. Food Chem. 2019, 67, 2510–2518. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Q.; Shen, Y.; Gao, X.; Li, L.; Yan, Y.; Wang, H.; Cheng, Y. Green tea catechin dramatically promotes RNAi mediated by low-molecular-weight polymers. ACS Cent. Sci. 2018, 4, 1326–1333. [Google Scholar] [CrossRef]
- Liu, R.; Yan, X.; Liu, Z.; McClements, D.J.; Liu, F.; Liu, X. Fabrication and characterization of functional protein–polysaccharide–polyphenol complexes assembled from lactoferrin, hyaluronic acid and (−)-epigallocatechin gallate. Food Funct. 2019, 10, 1098–1108. [Google Scholar] [CrossRef]
- Apon, A.; Kamble, P. Systematic vs local drug delivery systems in the treatment of periodontal diseases—A review. World J. Adv. Sci. Res. 2018, 1, 1–15. [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific Opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar]
- Sergi, C.M. Epigallocatechin-3-Gallate toxicity in children: A potential and current toxicological event in the differential diagnosis with virus-triggered fulminant hepatic failure. Front. Pharmacol. 2020, 10, 1563. [Google Scholar] [CrossRef]
- Galati, G.; Lin, A.; Sultan, A.M.; O’Brien, P.J. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic. Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef]
- Jimenez-Saenz, M.; Martinez-Sanchez Mdel, C. Acute hepatitis associated with the use of green tea infusions. J. Hepatol. 2006, 44, 616–617. [Google Scholar] [CrossRef] [PubMed]
- Mazzanti, G.; Di Sotto, A.; Vitalone, A. Hepatotoxicity of green tea: An update. Arch. Toxicol. 2015, 89, 1175–1191. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Wan, X.; Yang, C.S.; Zhang, J. Green tea polyphenol (−)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol. Appl. Pharmacol. 2015, 283, 65–74. [Google Scholar] [CrossRef] [PubMed]

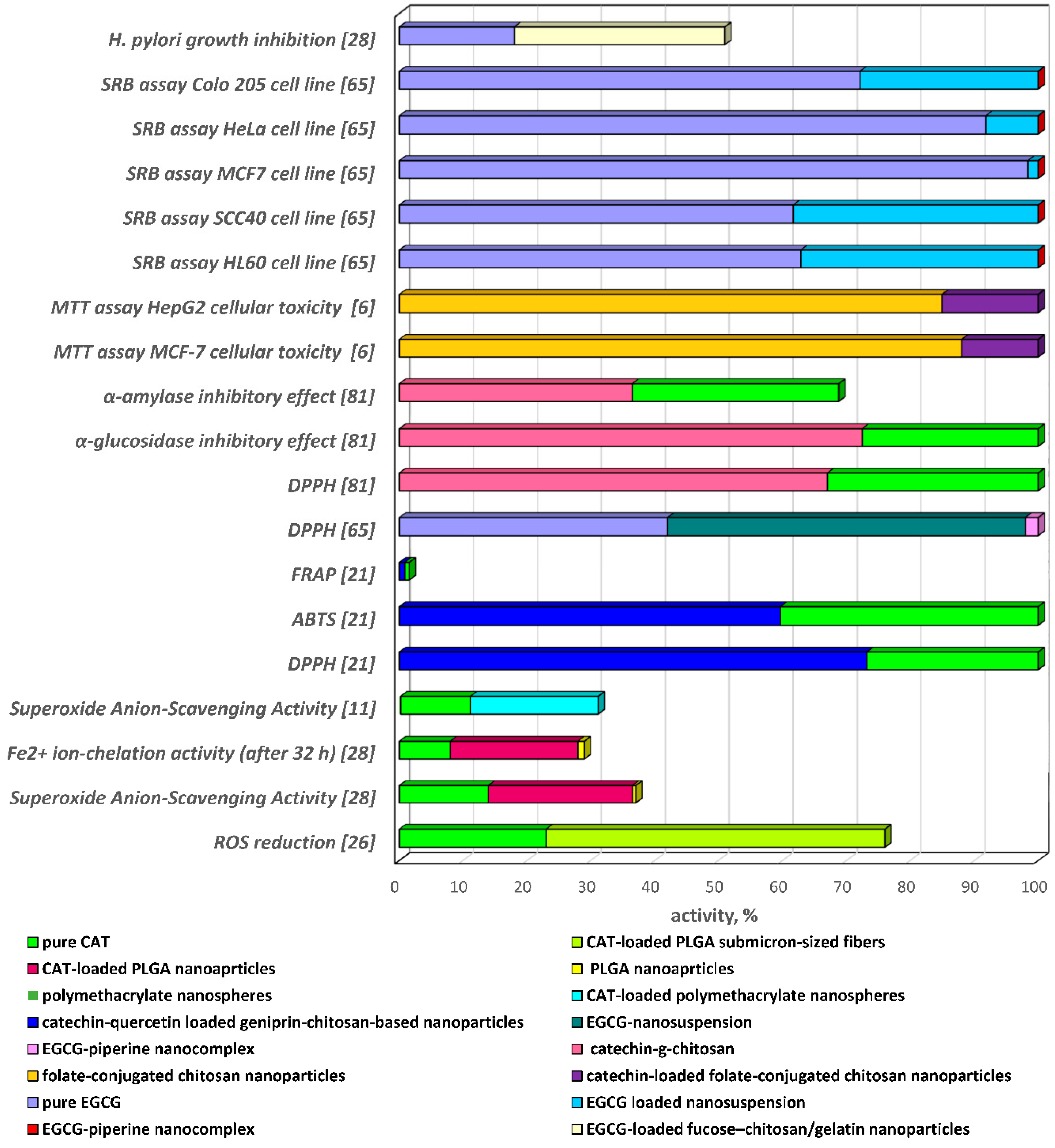
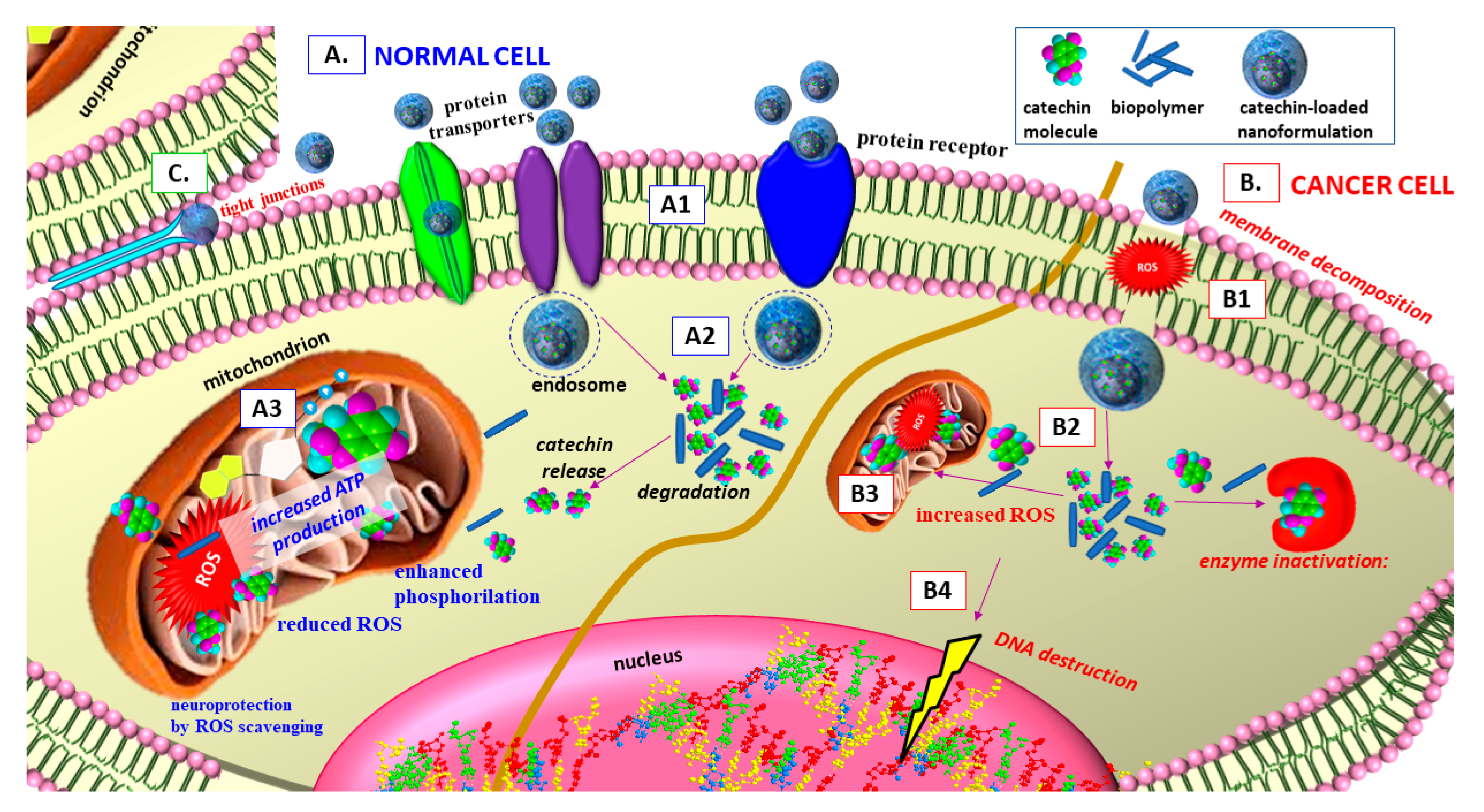
| Formulation Type | Preparation Method, Characteristics | EE, LE, EC * | In Vitro Release | Biological Activity | Reference |
|---|---|---|---|---|---|
| calcium-alginate microparticles | swelling absorption uniform morphological properties of particles | EE = 10.6–51.6% | sustained release in simulated gastric and intestinal fluids | - | [51] |
| chitosan-poly(γ-glutamic acid) (γ-PGA) self-assembled nanoparticles | polyelectrolyte self-assembly method particle size 134.3–147.8 nm | LE = 13.8–23.5% | 40% released in simulated gastric fluid | pH-responsive Sustained free radical antioxidant activity | [52] |
| Ca-pectinate microparticles reinforced with liposome and hydroxypropylmethylcellulose (HPMC) | CaCl2 conc. 5.20%, HPMC conc. 0.08%, hardening time: 12.63 min, mean particle size: 88 µm | EE = 9.5–30.19% | in simulated gastric fluid: 50% constant release from 20 min to 24 h incubation period, in simulated intestinal fluid: 56% for the initial 30 min, up to about 95% for the next 7.5 h, complete release after 8 h incubation. | increased antioxidant activity in rat plasma | [53] |
| Eudragit RS 100 microparticles | - | EE = 92.3% Production yield = 63.46 | - | in vivo antidiabetic efficacy | [54] |
| pH-sensitive polymeric nanoparticles | emulsion evaporation method, Poly(lactic-co-glycolic acid), lactic acid:glycolic acid, poly(vinyl alcohol, particle size = 91.3 ± 0.8 nm | EE = 80.8% | total released amount ~68% for 24 h | increased bioavailability of EGCG, lower proteinuria excretion of nephrotic syndrome in rats, lower kidney pathology scores | [55] |
| octenyl succinic anhydride (OSA)-starch, soybean lecithin and β-glucan solid microformulations | Gas-saturated solution drying | EE ≈ 80.5% | improved cellular antioxidant activity, improved storage stability, preservation of catechin bioactive properties | [56] | |
| starch-based nanoparticles | ultrasonic homogenization, horse chestnut: particle size 322.7 nm water chestnut: particle size 559.2 nm lotus stem: particle size: 615.6 nm zeta potential: (−21.5)–(−18.05) mV | EE: 59.09% EE: 48.30% EE: 55.00% encapsulation offers thermal protection of catechin | release after 4 h: 4.8 mg/100 mg 3.39 mg/100 mg 5.35 mg/100 mg | retained bioactive properties during in vitro digestion | [57] |
| self-assembled tea catechin/gelatin nanoparticles | direct mixing, mean particle size 200 nm, zeta potential < 0 | antioxidant activity, protection against enzymatic degradation | [58] | ||
| Ca-pectinate gel beads entrapping catechin-loaded liposomes | with/without hydroxypropylmethyl cellulose | EE decreased by 40–50% with 2–6%. CaCl2 concentration increase in gelling media | sustained oral delivery of catechins | [23] | |
| (−)-epicatechin-loaded lecithin–chitosan–D-alpha-tocopheryl polyethylene glycol succinate (TPGS) nanoparticles | molecular self-assembly | LE 3.42 ± 0.85% EE 56.1 ± 3.9% | inhibitory effect on human breast cancer cell line | [59] | |
| chitosan nanoparticles | mean particle size < 500 nm | EC = 4 µg/mg | increased bioavalability significantly enhanced in vitro intestinal absorption, stabilisation of catechins in the donor chamber | [60] | |
| solid lipid nanocarrier (SLN) nanostructured lipid carrier (NLC) | high-shear homogenisation and ultrasonication technique, unloaded particles size: SLN—206 nm, NLC—216 nm epigallocatechin gallate loaded particles size: SLN 364 nm, NLC 300 nm | EE (SLN) = 83% EE (NLC) = 90% | in simulated gastric medium: burst release, at intestinal conditions: slower, sustained release | low toxicity, biocompatibility, potential for oral preventive and therapeutic supplementation | [61] |
| solid lipid nanoparticles | spherical nanoparticles hydrodynamic diameter: 300.2 nm | EE = 81% | slow, sustained release, Fickian diffusion | improved bioavailability, protection from degradation, no acute or sub-chronic toxicity, suitable for oral administration | [62] |
| polylactide-based biodegradable nanoparticle | Polylactide is terminated with anti-amyloidogenic trehalose molecule or neurotransmitter dopamine and resultant nanoparticle is loaded with antiamyloidogenic catechin molecule nanoparticles, homogeneous morphology, average size ~180–220 nm | LE = 50% LC = 6% | In vitro release 80% for 35 h | biocompatibility, biodegradability, enhanced cellular/extracellular availability multivalent interaction | [63] |
| catechin-quercetin-loaded chitosan nanoparticles genipin-modified catechin-quercetin-loaded chitosan nanoparticles | mean diameter: 180.4 ± 3.1 nm 190.7 ± 2.8 nm ellipsoidal shape decreased zeta potential no particle aggregation | EE = 76.35% ± 1.37% | Release 63% in PBS for 80 h Release 76% in PBS for 80 h | antioxidant and antibacterial activity | [22] |
| catechin-loaded chitosan nanoparticles | average particle size 68.76 nm resistant to aggregation | EE = 60.42% | release 82.56% | enhanced antioxidant activity, no significant toxic effect | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaneva, Z.; Ivanova, D. Catechins within the Biopolymer Matrix—Design Concepts and Bioactivity Prospects. Antioxidants 2020, 9, 1180. https://doi.org/10.3390/antiox9121180
Yaneva Z, Ivanova D. Catechins within the Biopolymer Matrix—Design Concepts and Bioactivity Prospects. Antioxidants. 2020; 9(12):1180. https://doi.org/10.3390/antiox9121180
Chicago/Turabian StyleYaneva, Zvezdelina, and Donika Ivanova. 2020. "Catechins within the Biopolymer Matrix—Design Concepts and Bioactivity Prospects" Antioxidants 9, no. 12: 1180. https://doi.org/10.3390/antiox9121180




