Abstract
Extensive burns result in a local wound response and distant-organ injury (DOI) caused by oxidative-stress and inflammation. Melatonin (MT) shows promise in alleviating oxidative-stress and inflammation, but its role in thermal injury is largely unexplored. The present systematic review and meta-analysis were designed to assess the effects of MT on oxidative-stress and inflammatory markers against severe burn-induced DOI. Mean difference (MD)/standard mean difference (SMD) with 95% confidence interval (CI) were estimated using fixed-effect/random-effects models. Eighteen experimental studies met the inclusion criteria. Compared with the control group, MT significantly decreased the levels of malondialdehyde (SMD, −1.03; 95% CI, −1.30, −0.76, p < 0.00001) and 4-hydroxynonenal (MD, −1.06; 95% CI, −1.57, −0.56, p < 0.0001). Additionally, MT increased the levels of glutathione (SMD, 1.94; 95% CI, 1.27, 2.61, p < 0.00001) and superoxide-dismutase (SMD, 0.76; 95% CI, 0.08, 1.45, p = 0.03). Finally, MT significantly decreased the levels of tumor necrosis factor-α (SMD, −1.34; 95% CI, −1.92 to −0.77; p < 0.00001) and C-reactive protein (MD, −12.67; 95% CI, −16.72 to −8.62; p < 0.00001). Meta-analysis indicates that severe burn followed by immediate MT (10 mg/kg) intervention shows significant beneficial effects after 24-h against DOI by regulating oxidative-stress and the inflammatory response.
Keywords:
burns; wound healing; melatonin; oxidative stress; inflammation; systematic review; meta-analysis 1. Introduction
Burns represent complex traumatic skin injuries caused mainly by heat, radiation, electricity, abrasion, or exposure to chemicals [1]. In 2004, approximately 11 million people worldwide suffered burns [2], and the estimated average healthcare cost in developed countries was $88,218/patient [3]. According to the American Burn Association, about 72% of burns are thermal (41% from flames and 31% from scalds) [1], and the fire-related mortality rate is higher in developing countries than in developed nations [4,5]. Although recent advances in the management of severe burn care have improved significantly, multiple organ failure (MOF) is still considered a leading cause of mortality and morbidity following burn injuries, in particular when at least 30% of total body surface area (TBSA) burned [6].
Burn injuries is a critical care crisis, which not only damages the skin locally but also exerts detrimental effects on distant organs and can lead to MOF [7,8,9,10]. The development of complications and poor outcomes after burn injuries is often related to inflammation, blood coagulation disorders, hemorrhagic changes, and oxidative stress [8,9,10,11,12,13,14,15,16]. Additionally, the burn-induced inflammatory response creates a high demand for fluid resuscitation owing to excessive protein and fluid leakage into the interstitial spaces [8]. Accumulating evidence demonstrates that the inflammatory response and oxidative stress contribute to the progression of distant organ injury (DOI) following severe burns [8,11,12,13]. However, the pathophysiological mechanism underlying burn-induced DOI remains elusive. Oxygen radicals induce a local wound response and promote organ impairment, including of the liver [10,17], gastric mucosa [18], intestine [19,20], heart [21,22], and lung [23,24]. Burn injuries promote lipid peroxidation, an autocatalytic mechanism leading to oxidative damage to cellular membranes, the release of toxic, reactive metabolites, and cell death [25,26]. Likewise, Youn et al. [27] reported that burn injuries increase lipid peroxidation in plasma, liver, and lung. Because the oxidizing agent may originate from neutrophils sequestered in systemic organs as a systemic inflammatory reaction to a local burn insult [28], agents that suppress neutrophil activation and adherence might protect against thermal injury [29]. Moreover, antioxidant molecules reduce the excessive demand for fluid resuscitation [30]. Antioxidants are administered during the post-burn period to restore the oxidant-antioxidant balance and attenuate the inflammatory response, blood coagulation, and tissue injury [15,31,32]. Despite this knowledge, it is surprising that still, the clinical application of anti-oxidant therapy as an adjunct to burn care is limited [33]. In 2016, a systematic review by Adjepong et al. [34] reported that so far, only eleven studies devoted to the efficacy of micronutrients anti-oxidant therapy in burn patients had been published. Although this study reported the beneficial effects of antioxidant supplements in burn patients, the dose, timing, and the duration of the antioxidant use remain unclear. In addition, to date, only a limited number of antioxidants have been identified for the management of burn care [34,35]. The area of anti-oxidant therapy to prevent consequences of severe burn-induced DOI represents a virtually unexplored area that holds promise for effective adjunctive therapy. Hence, evidence synthesis is urgently required to study the role of anti-oxidants in burn injury, including potent antioxidants with anti-inflammatory, and immunomodulatory biomolecule like melatonin [36,37,38].
Melatonin (MT), a neurohormone produced mainly in the pineal gland, is a potent free radical scavenger and antioxidant. It scavenges reactive oxygen species (ROS) and reactive nitrogen species (RNS), stimulates the activity of antioxidant enzymes, inhibits proinflammatory cytokines, and activates adhesion molecules [39,40,41]. MT can preserve the level of glutathione (GSH) within cells and in mitochondria, suppressing oxidative damage [42,43]. MT not only attenuates oxidative damage in experimental burns [44,45,46,47,48] but also in burn patients [49]. While previous research indicates that MT could be beneficial in instances of DOI after severe skin burn, a comprehensive review on this topic has yet to be performed. Besides, MT shows promise in alleviating oxidative stress and inflammation following thermal injury in animals, the clinical use of MT is mostly limited to sleep-related outcomes, obesity [50,51], and dental diseases [52,53]. Therefore, we conducted a systematic review and meta-analysis to investigate the experimental data that support the clinical applicability of MT in the treatment of burn-induced DOI, with particular emphasis on oxidant-antioxidant balance and reduction of inflammation.
2. Materials and Methods
2.1. Search Strategy
To identify potentially relevant studies, a two-step systematic literature search was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [54]. Firstly, for comprehensive literature searching, the PubMed, Embase, and CINAHL electronic databases were searched for studies that assessed the effects of exogenous MT on oxidative stress and inflammatory markers in burn wound animal models up to August 2020. To identify additional relevant articles, the reference lists of identified studies were manually scanned. We used filters to retrieve only animal studies from PubMed and Embase. No limits (e.g., on language or publication date) were used.
The full search strategies for the Pubmed was: (“wound healing”[MeSH Terms] OR “wound healing”[Text Word] OR “wound healings”[Text Word] OR “wound repair”[Text Word] OR “wound*”[Text Word] OR “scar”[Text Word] OR “scar*”[Text Word] OR “thermal burn”[Text Word] OR “thermal injury”[Text Word] OR “burns”[MeSH Terms] OR “burn*”[Text Word] OR “cicatrix”[MeSH Terms] OR “cicatri*”[Text Word] OR “granulation tissue”[MeSH Terms] OR “granulation tissue”[Text Word] OR “reepithelialization”[Text Word]) AND (“melatonin”[MeSH Terms] OR “receptors, melatonin”[MeSH Terms] OR “tasimelteon”[Supplementary Concept] OR “ramelteon”[Supplementary Concept] OR “S-20098”[Supplementary Concept] OR “melatonin*”[Text Word] OR “tasimelteon”[Text Word] OR “ramelteon”[Text Word] OR “S-20098”[Text Word] OR “S20098”[Text Word] OR “circadin”[Text Word] OR “rozerem”[Text Word] OR “hetlioz”[Text Word] OR “valdoxan”[Text Word] OR “thymanax”[Text Word] OR “Melovine”[Text Word] OR “5-methoxy-n-acetyltryptamine”[Text Word] OR “n-acetyl-5-methoxytryptamine”[Text Word] OR “MT”[Title/Abstract] OR “MLT”[Title/Abstract]).
In Embase, the full search string was used: (‘wound healing’/exp OR ‘wound healing’:ab,ti,kw OR (granulation:ab,ti,kw AND wound:ab,ti,kw) OR (healing:ab,ti,kw AND wound:ab,ti,kw) OR (repair:ab,ti,kw AND wound:ab,ti,kw) OR (wound:ab,ti,kw AND regeneration:ab,ti,kw) OR (wound:ab,ti,kw AND repair:ab,ti,kw) OR (lenticular:ab,ti,kw AND wound:ab,ti,kw) OR ‘vulnus’/exp OR (wound:ab,ti,kw AND age:ab,ti,kw) OR ‘wound’/exp OR wound:ab,ti,kw OR ‘wounding’/exp OR ‘scar’/exp OR scar:ab,ti,kw OR ‘cicatrix’/exp OR cicatrix:ab,ti,kw OR (radiation:ab,ti,kw AND scar:ab,ti,kw) OR ‘burn’/exp OR burn:ab,ti,kw OR (burn:ab,ti,kw AND complication:ab,ti,kw) OR (burn:ab,ti,kw AND injury:ab,ti,kw) OR (burn:ab,ti,kw AND trauma:ab,ti,kw) OR (burn:ab,ti,kw AND wound:ab,ti,kw) OR ‘burning’/exp OR ‘burns’/exp OR (deep:ab,ti,kw AND burn:ab,ti,kw) OR (skin:ab,ti,kw AND burn:ab,ti,kw) OR (thermal:ab,ti,kw AND burn:ab,ti,kw) OR ‘granulation tissue’/exp OR (tissue:ab,ti,kw AND granulation:ab,ti,kw) OR ‘reepithelialization’/exp OR reepithelialization:ab,ti,kw) AND (‘melatonin’/exp OR ‘melatonin’:ab,ti,kw OR ‘melatonin receptor’:ab,ti,kw OR ‘tasimelteon’:ab,ti,kw OR ‘ramelteon’:ab,ti,kw OR (s:ab,ti,kw AND 20098:ab,ti,kw) OR melatonin*:ab,ti,kw OR ‘s 20098′:ab,ti,kw OR s20098:ab,ti,kw OR ‘tik 301′:ab,ti,kw OR tik301:ab,ti,kw OR circadin:ab,ti,kw OR ‘ly 15635′:ab,ti,kw OR rozerem:ab,ti,kw OR hetlioz:ab,ti,kw OR valdoxan:ab,ti,kw OR thymanax:ab,ti,kw OR melitor:ab,ti,kw OR melovine:ab,ti,kw OR ‘5 methoxy n acetyltryptamine’:ab,ti,kw OR ‘n acetyl 5 methoxytryptamine’:ab,ti,kw OR ‘vec 162′:ab,ti,kw OR vec162:ab,ti,kw OR ‘tak 375′:ab,ti,kw OR tak375:ab,ti,kw OR mt:ab,ti,kw OR mlt:ab,ti,kw).
2.2. Inclusion and Exclusion Criteria
The following inclusion criteria were applied to study selection (Supplementary Table S1): (1) animal models of burn wounds; (2) any type of MT treatment compared with a placebo control; (3) a control intervention of saline, ethyl alcohol, or other vehicle; and (4) anti-oxidative and anti-inflammatory effects of MT in an animal model with severe burn-induced DOI. The following exclusion criteria were applied to the study selection (Supplementary Table S2): (1) clinical case reports, ex vivo, and studies solely in vitro, (2) non-original studies (e.g., editorials or literature reviews), (3) studies not freely available, and (4) conference abstracts.
2.3. Study Selection
After the removal of duplicates, all unique trials were imported into the Rayyan-a web application to allocate the references randomly [55]. Next, two of the authors independently screened the titles and abstracts to select relevant studies from the randomly allocated references. It should be noted that we did not screen for the presence or absence of specific outcome measures during this phase because, often, not all outcome measures were described in the abstract. Finally, the full-texts of the selected articles were evaluated to identify those that fulfilled the inclusion criteria. Any disagreement concerning study selection was settled by consultation with the third author.
2.4. Data Extraction
Two authors independently extracted from each of the included studies information on the authors, publication year, species, weight, sample size, animal model, intervention (dose and administration time), outcome measures, and mean and SD of each oxidative and/or inflammatory marker (for both MT-treated and controls). In studies with multiple interventions, only data from the control and MT experimental groups were considered in the analysis. If the published outcome data were incomplete, we attempted to contact the authors to obtain the original data. A reminder was sent by email to those who had not responded within 2 weeks. If efforts to achieve the original data failed, the article was eliminated from the meta-analysis. If the data were presented graphically, GetData Graph Digitizer (http://getdata-graph-digitizer.com/) was employed to extract numerical data from graphs or figures.
2.5. Assessment of Methodological Quality
The risk of bias (RoB) in the included articles was evaluated by two independent reviewers using the SYRCLE RoB tool [56]. Based on the Cochrane RoB tool [57], the RoB tool was developed to evaluate the aspects of bias specific to animal intervention studies. The tool contains 10 items related to six types of bias (selection, performance, detection, attrition, reporting, and other). Scores of ‘yes’, ‘no’, and ‘unsure’ indicate a low, high, and unclear RoB, respectively.
2.6. Data Analysis
The experimental and control group data from the included studies were extracted and input into Review Manager Software (ver. 5.3, The Nordic Cochrane Centre, Copenhagen, Denmark). The meta-analysis was executed when a minimum of two studies were analogous in terms of the population, intervention, comparison, outcomes, and design, and provided relevant data. In the effect-size analysis, the mean difference (MD) was used when the outcome measure of all studies employed the same scale, whereas the standardized mean difference (SMD) was used when the studies assessed the same outcome but measured it in different ways. For both strategies, 95% confidence intervals (CIs) were calculated. The I2 test was utilized to assess heterogeneity among the studies. The fixed-effects model was used for the meta-analysis when I2 was ≤50% and the random-effects model when I2 was >50% (indicative of substantial heterogeneity) [58]. Subgroup analyses were performed only if the subgroups contained a minimum of two independent comparisons. To determine the influence of each study on the overall effect size, a sensitivity analysis was conducted using the leave-one-out approach [59]. To generate the leave-one-out forest plot, we used OpenMeta [Analyst] software (http://www.cebm.brown.edu/openmeta/).
2.7. Publication Bias
Based on the Cochrane recommendations, we analyzed funnel plot asymmetry when one outcome variable was associated with at least 10 studies in the meta-analysis, since with <10 studies the power of the tests is too low [60]. Potential publication bias was assessed by visual inspection of funnel plot asymmetry and Egger’s test of asymmetry [61]. Whenever asymmetry was detected in the funnel plot, the trim and fill method was used to calculate the effect size by estimating the number of missing studies [62]. All statistical analyses related to publication bias were carried out in JASP, an open-source statistical program developed by the University of Amsterdam (https://jasp-stats.org/).
3. Results
3.1. Study Selection
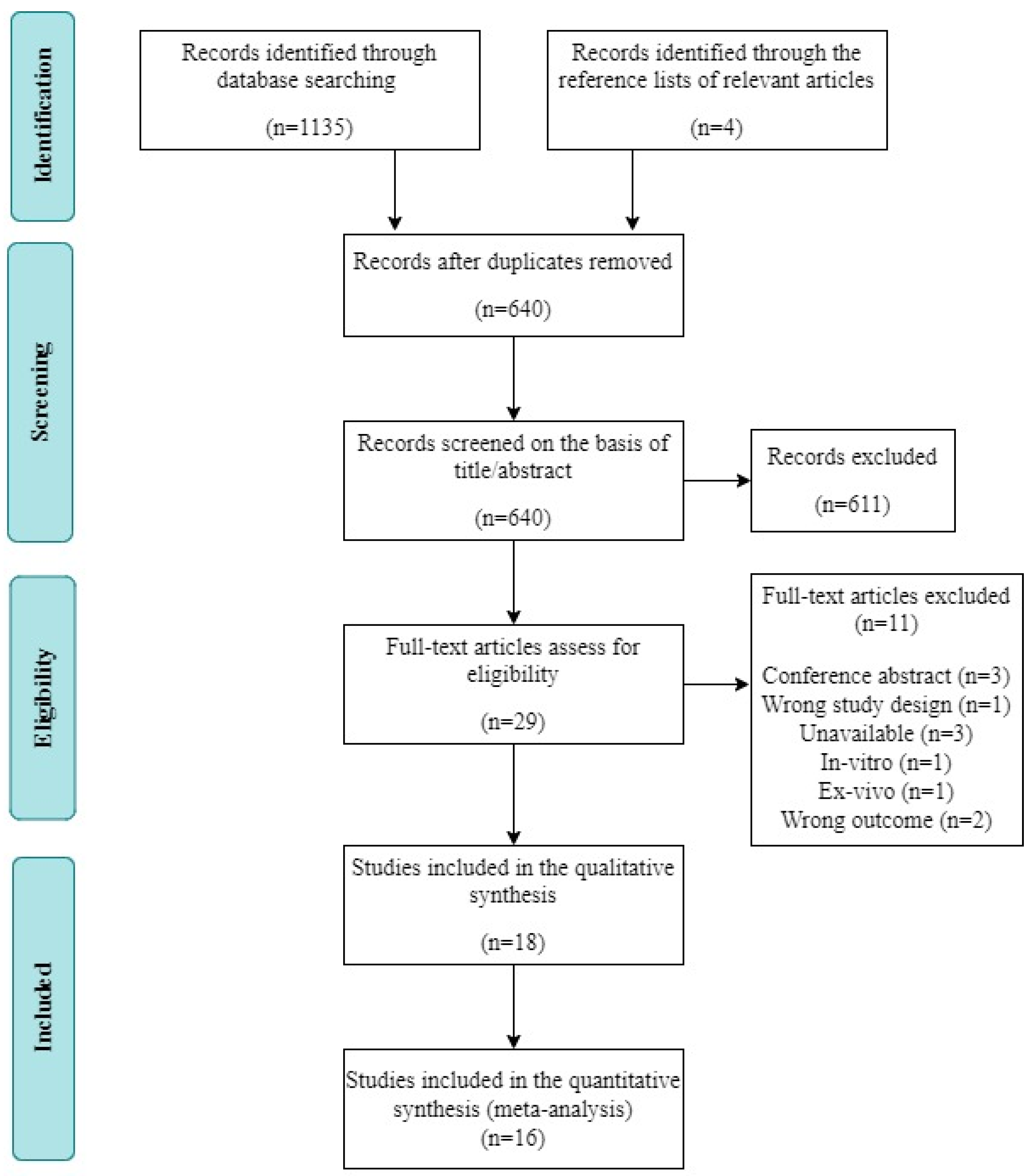
A total of 1135 studies were identified via electronic database searches. After filtering out duplicate studies, the titles and abstracts of 640 potentially relevant articles were screened. Of these articles, 611 were excluded. The remaining 29 studies were subjected to full-text screening. Among them, 11 studies were excluded for the following reasons: conference abstract (n = 3), inappropriate study design (n = 1), unavailable data (n = 3), in vitro design (n = 1), ex vivo design (n = 1), and inappropriate outcome measure (n = 2). Ultimately, 18 studies fulfilled the inclusion criteria and were included in the systematic review; data could not be extracted from two of those studies, leaving 16 records available for our meta-analysis (Figure 1).
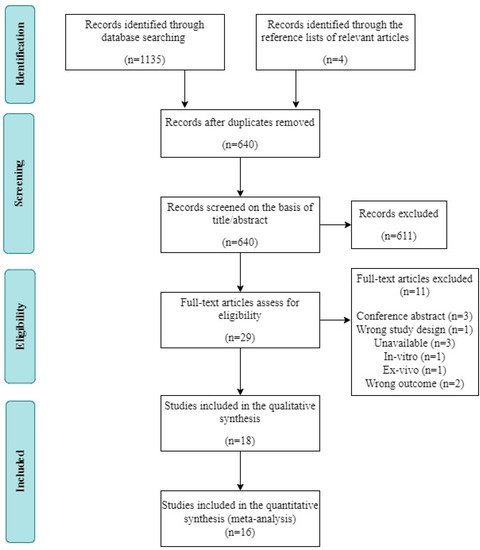
Figure 1.
Flow diagram of the systematic review and literature search results.
3.2. Study Characteristics
The main characteristics of the 18 included studies are listed in Table 1. Among these studies, the sample size per group ranged from 3 to 19. Eight studies focused on hepatic injury [63,64,65,66,67,68,69,70], four on gastric mucosal damage [45,71,72,73], two on kidney injury [74,75], two on plasma [76,77], one on intestine injury [78], and one on lung injury [79]. All of the studies used hot water (90–100 °C) to induce burn wounds in rats. Most of the studies involved burns to 30% of the total body surface area (TBSA), although one study [69] involved burns to 20% of the TBSA, and another to 40% of the TBSA [75]. The exposure time ranged from 10–15 s.

Table 1.
Characteristics of the included studies.
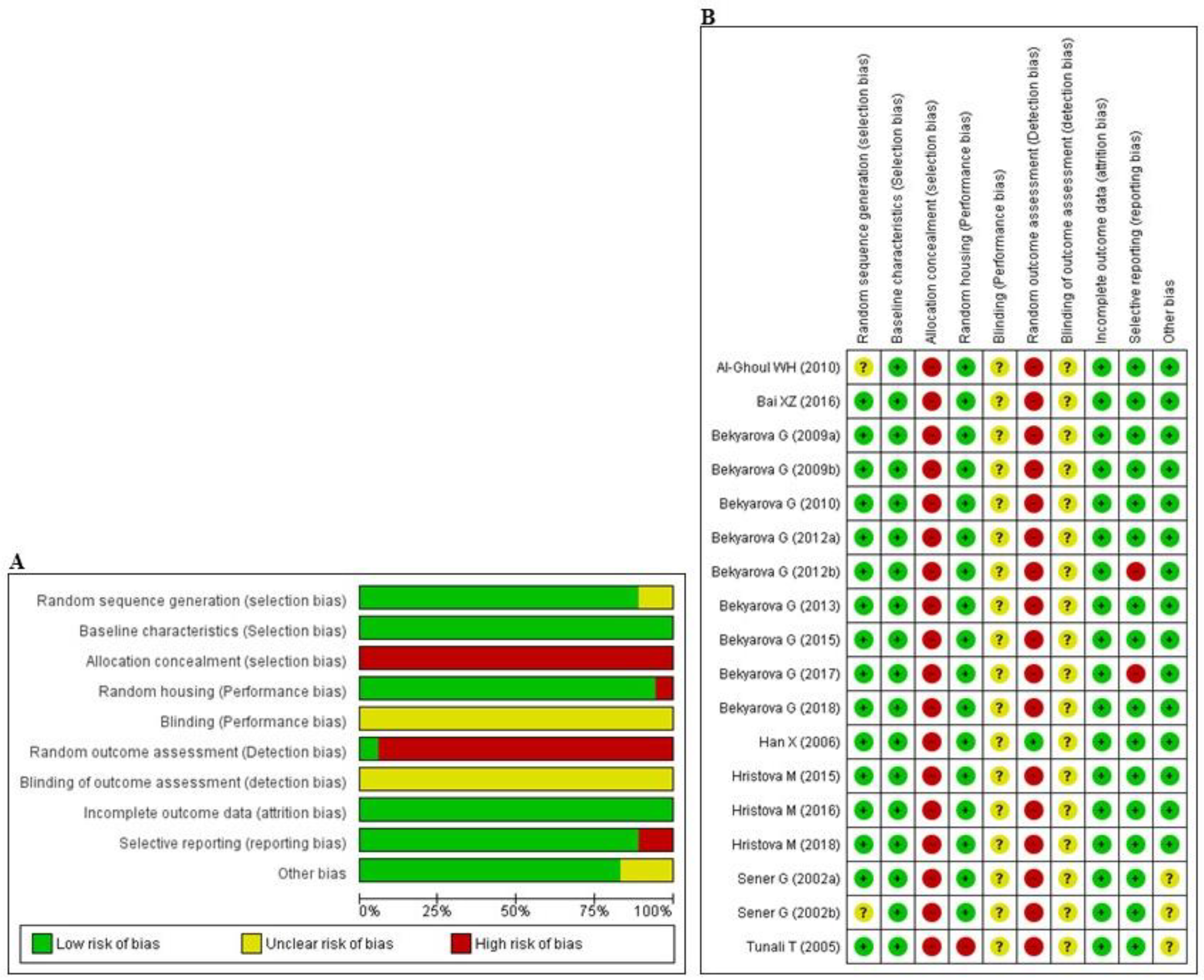
3.3. Risk of Bias and Quality of Reporting
The abridged risk of bias (RoB) assessment is presented in Figure 2A, and the individual RoB scores of each study are shown in Figure 2B. Experimental animals in most of the studies had randomly allocated, and maintained under unified room temperature, humidity, and food supply. However, allocation concealment and random outcome assessment were not described, resulting in a high risk of selection and detection bias, respectively, in all studies. In addition, the issue of selective reporting was not discussed in two studies [64,68], suggesting that they may have had a high level of reporting bias. All of the studies reported that the animals were of similar weight and underwent the same experimental design at baseline. Inadequate reporting of the measures used to decrease bias was captured by our RoB assessment; in numerous cases, the measures used were unclear.
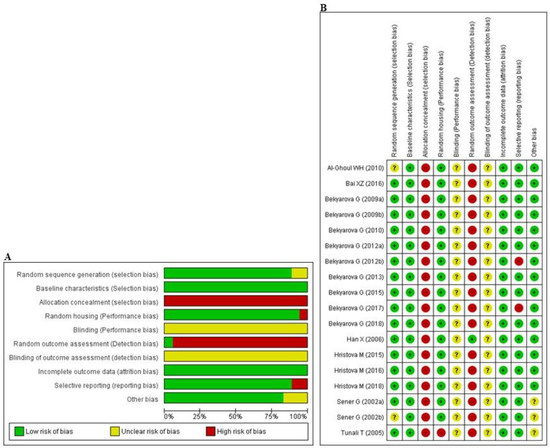
Figure 2.
Risk of bias. (A) Overall risk of bias for each item in the SYRCLE tool for all included studies. Each risk of bias item is presented as a percentage based on all included studies. (B) Individual risk of bias for each of the included animal studies. Each item in the SYRCLE tool was scored as ‘yes’, ‘no’, or ‘unclear’.
3.4. Data Analysis
3.4.1. Effect of MT on Oxidative Stress Markers
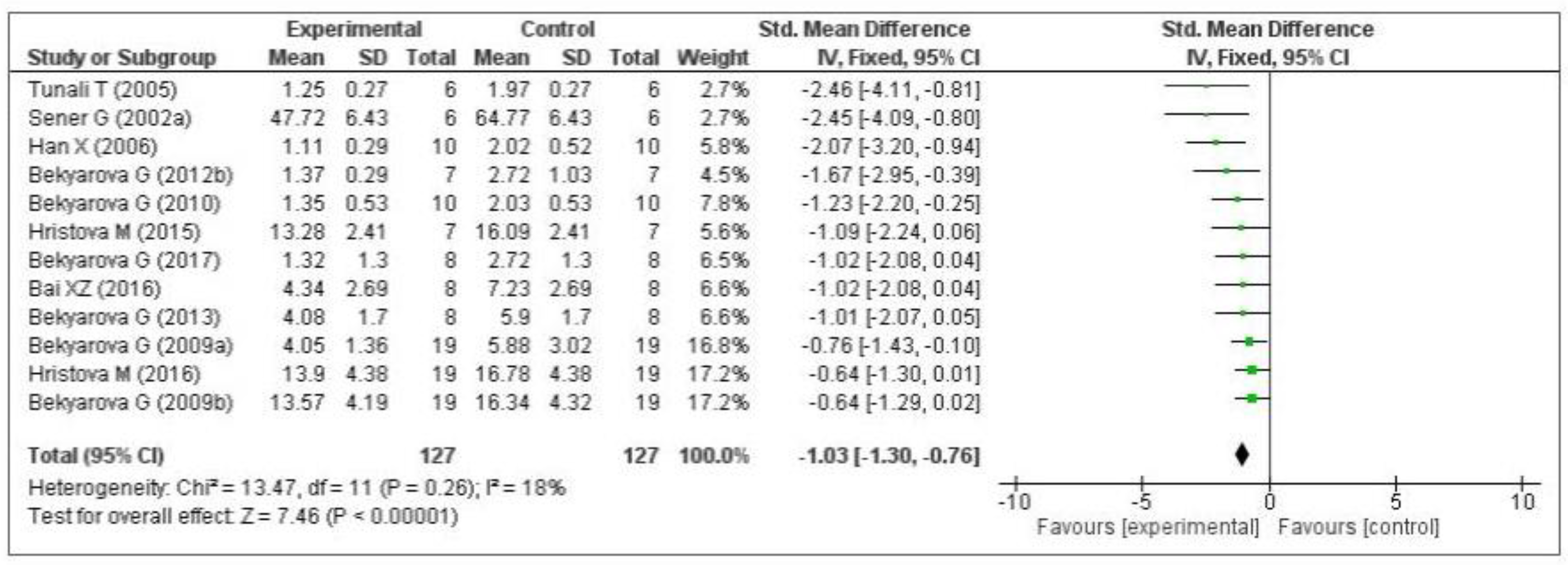
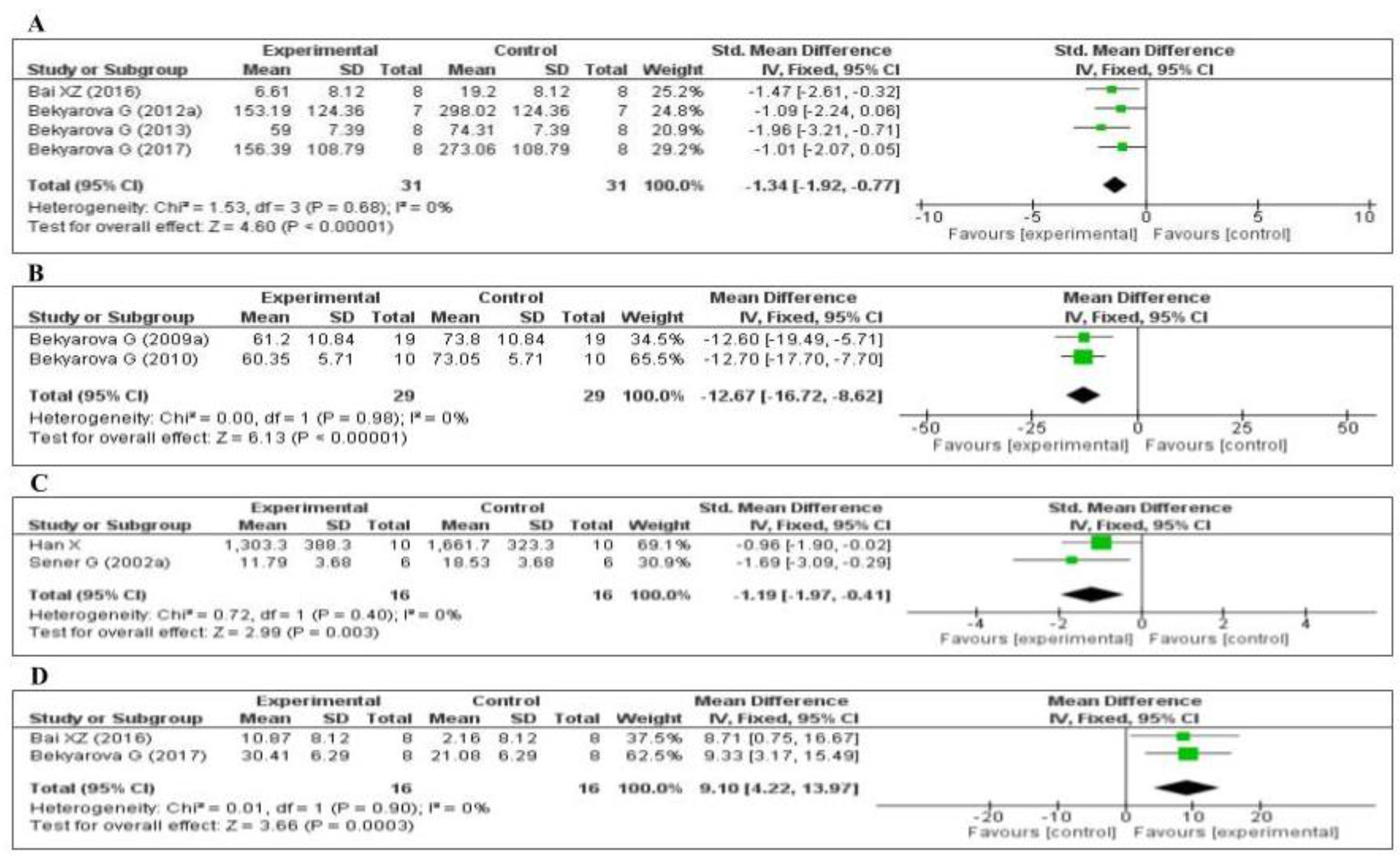
Thirteen of the included studies measured the malondialdehyde (MDA) level. Of these 13 studies, 1 [74] did not provide the number of samples per group and was therefore eliminated from the meta-analysis. Of the remaining 12 studies, five measured the MDA level in the liver [64,66,68,69,70], three in the gastric mucosa [71,72,73], one in the kidneys [75], two in the plasma [76,77], and one in the lungs [79]. The data of the 12 eligible studies are shown in Figure 3. Compared with the control group, MT significantly decreased the level of malondialdehyde (standardized mean difference [SMD], −1.03; 95% confidence interval [CI], −1.30, −0.76, p < 0.00001). This effect size was robust according to the sensitivity analysis, i.e., the omission of individual studies did not abolish the significance (Supplementary Figure S1).
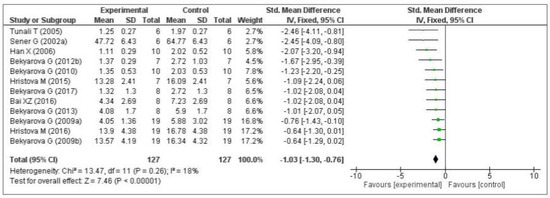
Figure 3.
Forest plot showing the impact of MT on MDA levels. MDA, malondialdehyde; CI, confidence interval; IV, independent variable.
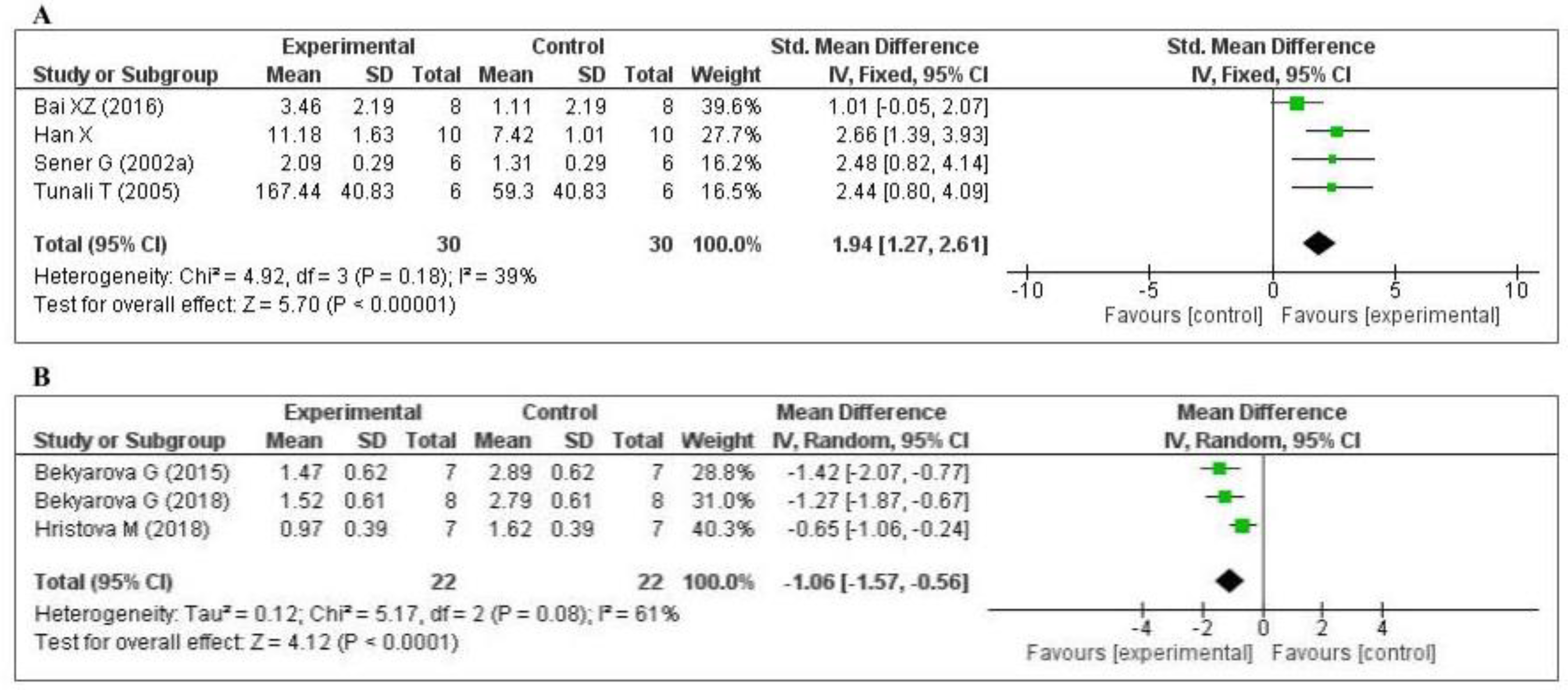
Five studies [70,74,75,77,79] measured the glutathione (GSH) level: one in the liver, two in the kidney, one in the lung, and one in the plasma. Of the five studies, one [74] did not provide the number of samples per group and was eliminated from the meta-analysis. The data of the four studies are shown in Figure 4A. MT increased the level of glutathione (SMD, 1.94; 95% CI, 1.27, 2.61, p < 0.00001). Three studies [45,63,65] measured the tissue 4-hydroxynonenal (4-HNE) level: two in the liver and one in the gastric mucosa. The data of the three studies are shown in Figure 4B. MT significantly decreased the level of 4-HNE (mean difference [MD], −1.06; 95% CI, −1.57, −0.56, p < 0.0001). The effect sizes were robust according to the sensitivity analysis, i.e., the omission of individual studies did not abolish the significance (Supplementary Figures S2 and S3). Thus, when compared with the control group, MT-treatment was significantly increased GSH level and decreased 4-HNE level.
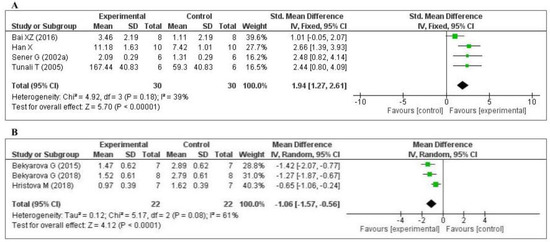
Figure 4.
Forest plot showing the impact of MT on GSH and 4-HNE levels ((A) GSH; (B) 4-HNE). GSH, glutathione; 4-HNE, 4-hydroxynonena; CI, confidence interval; IV, independent variable.
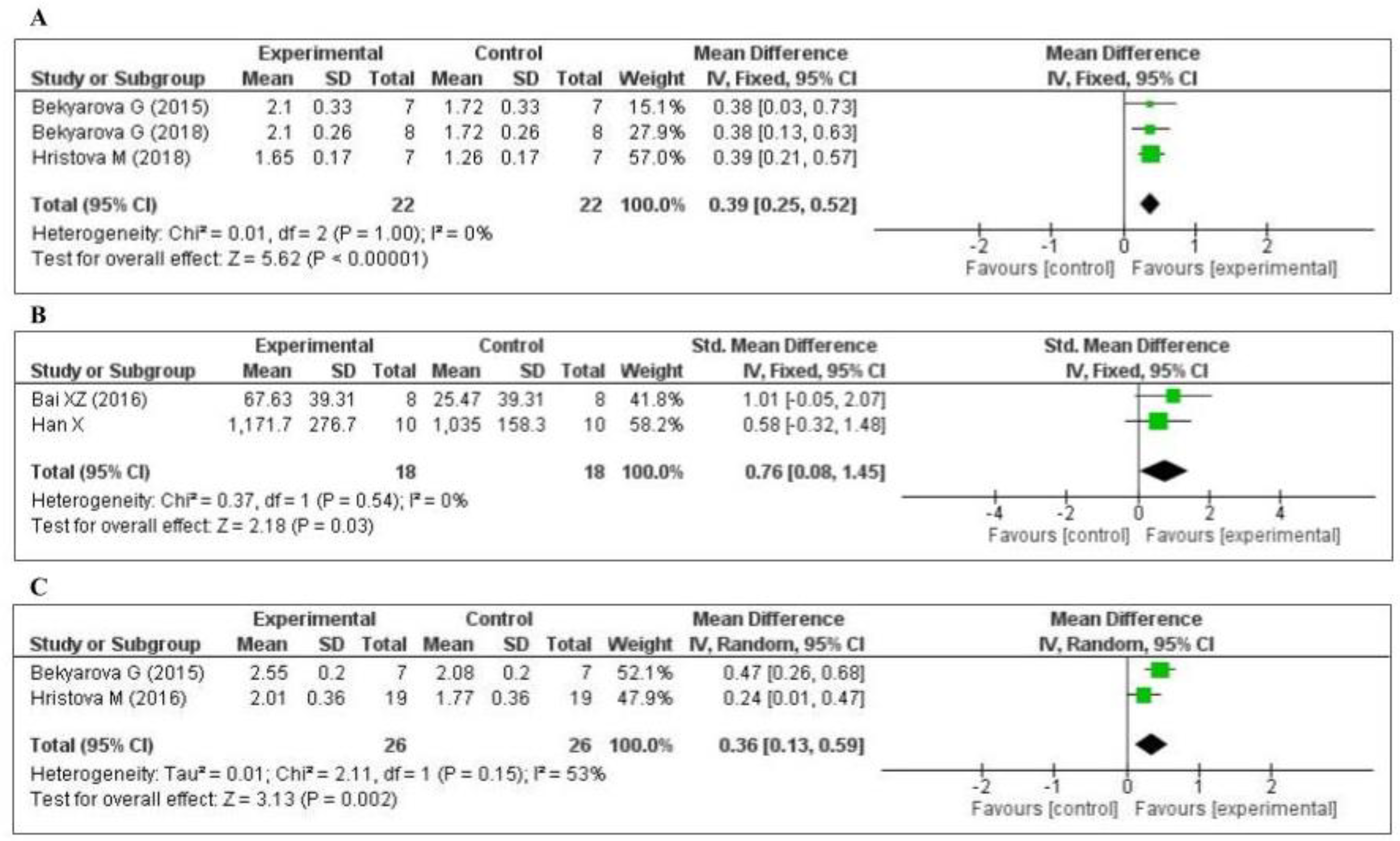
Three studies [45,63,65] measured the nuclear factor erythroid 2-related factor 2 (Nrf2) level: two in the liver and one in the gastric mucosa. The data of the three studies are shown in Figure 5A. MT increased the level of Nrf2 (MD, 0.39; 95% CI, 0.25, 0.52, p < 0.00001). Two studies [75,79] measured the tissue superoxide dismutase (SOD) level: one in the kidney and one in the lung. The data of the two studies are shown in Figure 5B. MT significantly decreased the level of SOD (SMD, 0.76; 95% CI, 0.08, 1.45, p = 0.03). Two studies [65,71] measured the tissue heme oxygenase-1 (HO-1) level: one in the liver and one in the gastric mucosa. The data of the two studies are shown in Figure 5C. MT increased the level of HO-1 (MD, 0.36; 95% CI, 0.13, 0.59, p = 0.002). The effect sizes were robust according to the sensitivity analysis, i.e., the omission of individual studies did not abolish the significance (Supplementary Figures S4–S6).
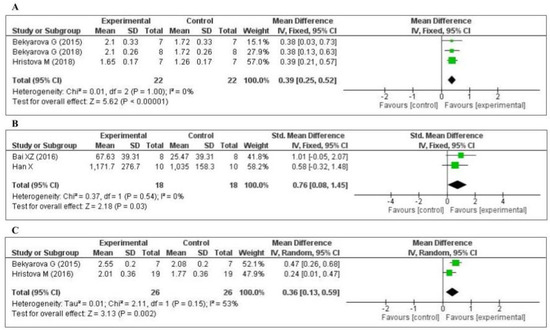
Figure 5.
Forest plot showing the impact of MT on Nrf2, SOD, and HO-1 levels ((A) Nrf2, (B) SOD, (C) HO-1). Nrf2, nuclear factor erythroid 2-related factor 2; SOD, superoxide dismutase; HO-1, heme oxygenase-1, CI, confidence interval; IV, independent variable.
3.4.2. Effect of MT on Inflammatory Markers
Four of the included studies measured the tumor necrosis factor-α (TNF-α) level: three [64,66,67] in the liver and one [75] in the kidney. The data of the four studies are shown in Figure 6A. MT significantly decreased the level of TNF-α (SMD, −1.34; 95% CI, −1.92, −0.77, p < 0.00001). Two of the studies measured the plasma C-reactive protein (CRP) level [69,76]. The data of the two studies are shown in Figure 6B. MT significantly decreased the level of CRP (MD, −12.67; 95% CI, −16.72, −8.62, p < 0.00001). Two of the studies measured the myeloperoxidase (MPO) level [70,79]. The data of the two studies are shown in Figure 6C. MT significantly decreased the level of MPO (SMD, −1.19; 95% CI, −1.97, −0.41, p = 0.003). Finally, two of the studies measured the tissue interleukin-10 (IL-10) level [64,75]: one in the liver and one in the kidney. The data of the two studies are shown in Figure 6D. MT significantly decreased the tissue level of IL-10 (MD, 9.10; 95% CI, 4.22, 13.97, p = 0.0003). The effect sizes were robust according to the sensitivity analysis, i.e., the omission of individual studies did not abolish the significant effect (Supplementary Figures S7–S10).
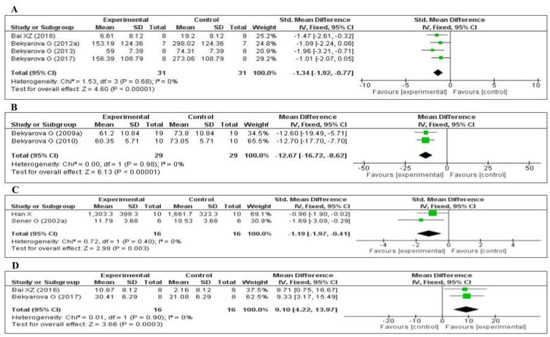
Figure 6.
Forest plot showing the impact of MT on various inflammatory markers ((A) TNF-α, (B) CRP, (C) MPO, (D) IL-10). TNF-α, tumor necrosis factor-α; CRP, C-reactive protein; MPO, myeloperoxidase, IL-10, Interleukin-10, CI, confidence interval; IV, independent variable.
3.4.3. Effect of MT on Other Inflammatory Markers
Nuclear factor κB (NF-κB), a transcriptional factor, is activated immediately after severe burn injury and is thought to regulate the expression of several inflammatory mediators, including TNF-α [80]. It has previously been observed that an increase the expression of TNF-α further stimulates for the production of several secondary cytokines, which is considered a crucial pathophysiological mechanism of liver injury following severe burns [81,82]. Interestingly, the liver is one of the most prone organs to inflammatory damage in thermal injury [83]. It is hypothesized that MT decrease severe burn-induced liver damage by inhibiting NF-κB activation. In this systematic review, we found one study that assessed the effects of MT on NF-κB expression in the liver of third-degree burn rats [67]. The findings of this study disclosed that the TNF-α level increased with hepatic NF-κB expression in the burned group. However, MT treatment significantly decreased not only in the hepatic TNF-α level but also reduced NF-κB expression in the liver. These results indicating elevated NF-κB expression triggers hepatic TNF-α production, which further stimulates liver NF-κB activation.
Following heat insult, the inducible nitric oxide synthase (iNOS) acts as a downstream mediator of inflammation in various organs, including the stomach [45,84]. Previous research has established that a significant reduction of iNOS expression is associated with decreased systemic inflammation and oxidative stress in burn injury [19]. Despite the comprehensive search strategy, we identified only one study, which examined the effects of MT on iNOS expression in gastric mucosa [71]. In this study, iNOS expression was markedly elevated in gastric mucosa after severe thermal injury. In contrast, MT restricted the increased iNOS (p < 0.05) levels in gastric mucosa [71].
Most recently, Guo et al. [85] reported that compared with iNOS deficient burned mice, interleukin-1β (IL-1β) mRNA expression gradually increased with elevated iNOS levels in wild-type burned mice. This finding is supported by Yuan and peers study who disclosed that pro-inflammatory cytokines such as IL-1β began to increase following thermal injury, which responsible for various organ damage, including kidney [86]. According to our inclusion criteria, we included one study that evaluated the effects of MT on IL-1β levels in post-burn induced acute kindly injury [75]. This study demonstrated that severe burns could increase IL-1β levels in renal tissues, which could be decreased by MT treatment.
Disruption of the intestinal barrier plays a pivotal role in the pathophysiology of severe burn injury [87]. Although molecular mechanisms of severe burn-induced intestinal barrier disruption are not well-understood yet, extensive research is ongoing to elucidate its pathophysiology. However, recent evidence suggests that intestinal inflammation may contribute to intestinal barrier disruption [88,89]. Nitrotyrosine, a biomarker of inflammation [90,91,92], can increase with iNOS expression in full-thickness third-degree burn injury (40% TBSA burn) [93]. On the other hand, the nitrotyrosine contents are markedly reduced by the iNOS inhibitor [93]. Interestingly, we found one study that shows nitrotyrosine levels was peak in ileum tissue of post-burn rats, whereas this marker was apparent significantly decreased in MT treated rats [78].
3.4.4. Assessment of Publication Bias
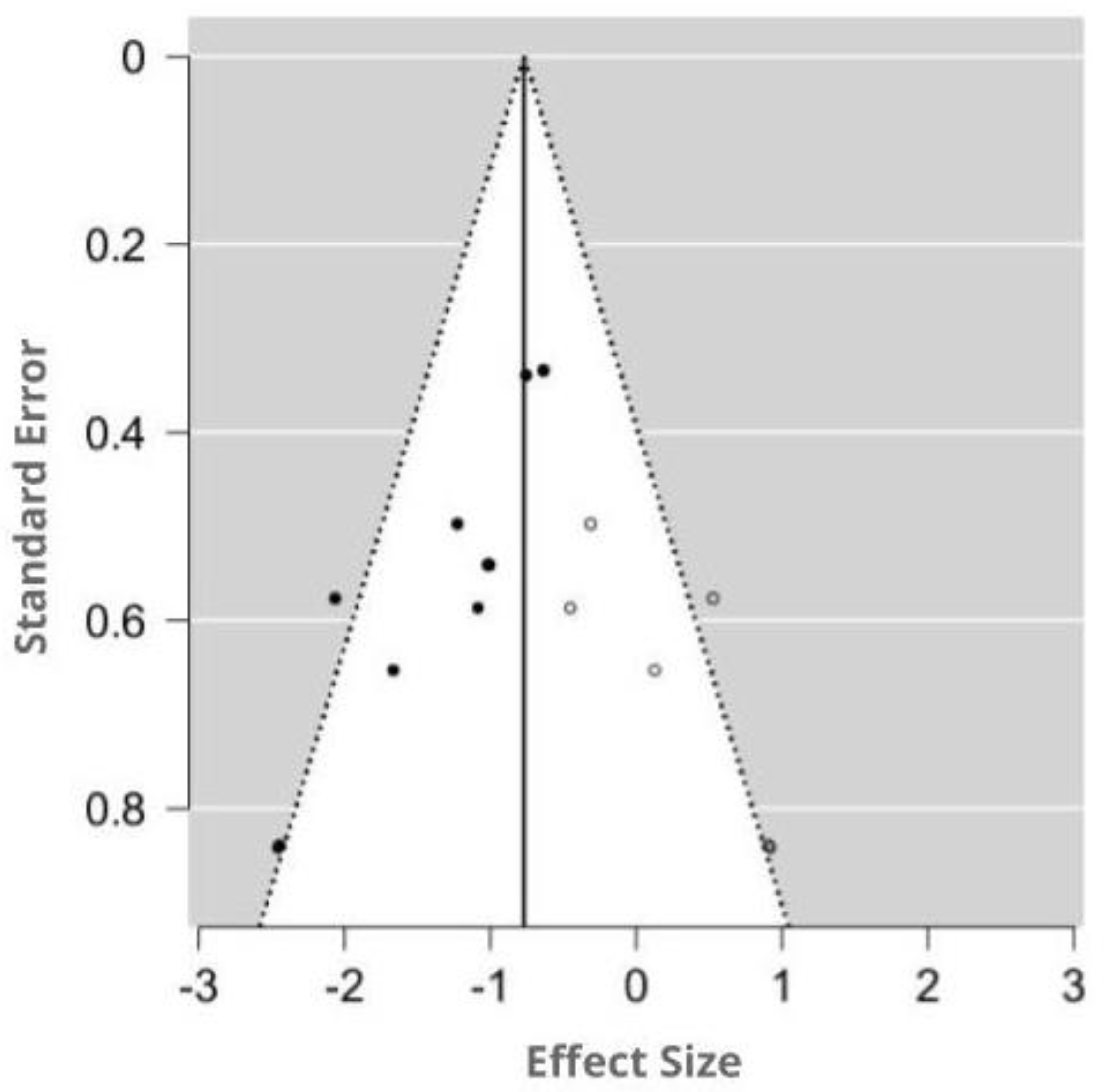
The funnel plot was asymmetry by visual inspection, and the significant publication bias was detected by Egger’s test (p = 0.001). Using the trim-and-fill method, six potentially missing studies were imputed for the analysis of MDA. The imputed effect size of MDA was SMD −0.77 (95% CI −1.01, −0.53). A funnel plot of the impact of MT on MDA activity is shown in Figure 7.
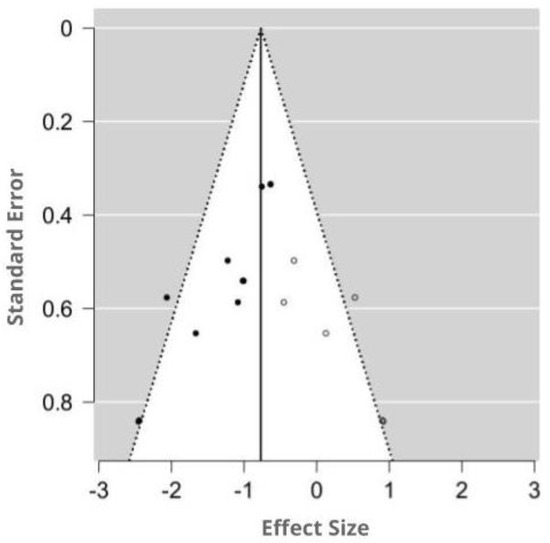
Figure 7.
Funnel plot showing the publication bias in studies reporting the impact of MT on MDA levels. Open circles represent published studies; closed circles represent unpublished studies.
4. Discussion
4.1. Main Findings
The results of this systematic review and meta-analysis demonstrated that exogenous MT effectively regulated oxidative stress and inflammatory markers in different distant organs including, liver, gastric mucosa, lung, and kidney in animal models. Sixteen studies were eligible for meta-analysis, which showed that MT significantly reduced the MDA and 4-HNE levels and increased the GSH, Nrf2, SOD, and HO-1 levels. Therefore, MT decreased the levels of lipid peroxidation products and increased those of antioxidant enzymes and of the transcription factors that regulate the synthesis of antioxidant proteins. Furthermore, MT significantly decreased the TNF-α, CRP, and MPO levels and increased the IL-10 level. These findings highlight the therapeutic potential of MT for burn-induced injury to distant organs.
4.2. Strengths and Limitations
To our knowledge, this is the first systematic review of the effects of MT on burn wound-induced damage to distant organs. We applied a comprehensive search strategy to multiple databases, had access to the full texts of all identified studies, used the SYRCLE RoB tool to assess the methodological quality of the studies, and extracted data pertaining to the levels of a wide range of oxidative stress and inflammatory markers. Furthermore, we performed a sensitivity analysis to validate our findings. Whenever asymmetry was detected in the funnel plot, the trim and fill method were used to adjust the effect size according to the number of missing studies.
This study also had several limitations. First, we assessed the effects of MT on antioxidant and anti-inflammatory activities 24 h after a burn injury; the early effects were not assessed. Second, during full-text screening, we had to exclude many studies that were not freely available. Third, quality assessment using the SYRCLE RoB tool indicated that all of the included studies had significant methodological limitations and a high risk of selection and detection bias. Finally, although we contacted the study authors to obtain missing data, the data were not provided.
4.3. Clinical Importance
The limitations of the data notwithstanding, the results indicate that MT prevents injury to distant organs by ameliorating oxidative stress and inflammation. This finding is consistent with previous reports [70,73,75]. Multiple organ dysfunction syndromes (MODS) can develop in patients with extensive burns [94,95]. Huang [96] showed that the levels of all inflammatory mediators markedly increased in animals and patients who sustained organ damage or MODS. Our finding is also in agreement with the report by Huang [96] that severe burns increased the levels of inflammatory mediators in distant organs and that MT significantly decreased the TNF-α, CRP, and MPO levels and increased those of anti-inflammatory cytokines such as IL-10. Recently, Lv et al. [97] demonstrated that hepatic NF-κB and TNF-α expression were upregulated after 30% of full-thickness burns, suggesting elevated NF-κB expression associated with activation of inflammatory response. In line with these findings, our results also indicate that MT treatment reduced the hepatic TNF-α level by downregulation of NF-κB expression following thermal injury. We also narratively presented that the iNOS expression was upregulated following the post-burn, on the other hand, MT intervention significantly reduced the iNOS levels. This finding is contrary to previous studies which have reported that there is no significant difference between control and burn injury groups [98]. A possible explanation for this discrepancy might be that the iNOS expression may be time-dependent in burn injury. More clearly, iNOS related inflammation obviously appears in the initial stage of thermal injury [99], not later stages. Furthermore, oxidative stress is one of the main causes of pathophysiological alterations during burn injury. Exogenous antioxidant therapy reportedly prevents oxidative injury experimentally and clinically [100,101]. Administration of an antioxidant to rats markedly inhibited the increase in the levels of lipid peroxides in the plasma, lung, and kidney and decreased the plasma protein level, particularly at the early stage of burn injury [100]. Indeed, we found that MT not only significantly reduced the levels of lipid peroxidation products (including MDA and 4-HNE) but also increased the levels of antioxidant enzymes such as GSH, SOD and HO-1. Furthermore, the activation of Nrf2 protects cells from oxidative stress and inhibits the redox-mediated inflammatory response [102]. For example, Braun et al. reported that Nrf2 modulates gene expression and the inflammatory response during healing of skin wounds [103]. In recent several studies further reported that Nrf2 and antioxidant enzymes (SOD, HO-1) were upregulated by MT treatment, suggesting MT promotes the translocation of Nrf2 from the cytoplasm to the nucleus and increased Nrf2 mediated antioxidant enzymatic activities [104,105]. In the same vein, our meta-analysis also demonstrated that MT significantly increased the Nrf2 level in severely burn-induced experimental rats.
4.4. Implications for Future Research
In reviewing the existing literature, it is assumed that oxidative stress not only directly damages the tissue following the thermal injury of the skin but also it activates an inflammatory response that combinedly contributes to MOF. These findings support the idea that antioxidant therapy might be useful in post-burn injury to protect against DOI, especially when TBSA greater than 20%. To date, several antioxidant molecules have been proposed for restoring the antioxidant defenses and reduce mortality after thermal injury. Even some of them have already shown beneficial effects in both animal [106] as well as human studies [107]. However, the clinical application of antioxidants in burn injury is limited. For example, although MT, widely known as an antioxidant, shows salutary effects in experimental burn injury, the clinical use of MT is only restricted to sleep-related outcomes (NCT01598259). Last but not least, along with antioxidative effects, MT also reduces the inflammatory response in 30% TBSA burned rats. Although these combined effects of MT would make it comparatively highly attractive as an add-on therapy for the management of progressive organ failure after burn injury, the pharmacokinetics of MT has not been reported in burns. Maybe due to the lacking of pharmacokinetics data, the applicability of MT in burn injury is only restricted to sleep-related outcomes. Further studies, which take these variables into account, will need to be undertaken.
Another critical issue that is still unclear is whether a single antioxidant intervention is enough for optimal outcomes in burn injury or a combination of antioxidants is necessary. Preliminary, ascorbic acid (vitamin-C) has shown the beneficial effects regarding the improvement of wound healing and reduction of ventilation requirements in severe burns patients [108]. However, to achieve these goals, a high dose of ascorbic acid (66 mg/kg and maintenance dose 33 mg/kg/h) administration is required; unless otherwise, half of the dose is practically ineffective [109,110]. In a randomized controlled trial, high-dose of ascorbic acid causes oxalate crystallization, stone formation, and nephropathy in susceptible patients [111]. In addition, gender is considered one of the risk factors for ascorbic acid associated renal insufficiency. For example, >1000 mg/day is not related to renal stone formation in females, surprisingly, 700 mg/day of the ascorbic acid dose is enough to form stones in males [112]. To overcome this problem, a combination of ascorbic acid with other antioxidants like MT might be an attractive alternative strategy for the management of burn care. Because of the unique strength of MT to improve sleep parameters against severe burns, together with its low toxicity and its capability to reduce inflammation and oxidative stress, are all significant to its efficiency for protecting severe burn mediated distant organ damage. It has already proven that high-dose and prolonged MT treatment did not associate with any adverse effects in different human studies [113,114]. Recently, numerous studies evidence that MT and ascorbic acid combinedly express synergistic effects in several oxidative stress-induced disease conditions [115,116,117]. Interestingly, one recent study reported that ascorbic acid is less effective than MT to improve stress-induced gastric mucosal damage [118]. Thereby, we may assume that the combination of MT and ascorbic acid or other antioxidants, together not only enhances the burn injury recovery process, but also MT may cut-off the higher dose of ascorbic acid. There is abundant room for further progress in determining the efficacy of this combination in thermal injury. Further work is also required when MT is given in combination with other drugs for the treatment of burn injury. Taken together, MT may reduce the adverse effects of antioxidants and/or other drugs that currently used to treat burn patients, consequently will increase their efficacy.
Last but not least, in recent years, population-based studies reported that both severe and mild thermal injury leading to long-term health effects, including acute kidney injury [119], respiratory infection [120], circulatory system diseases [121], hepatic dysfunction [122]. Although the clinical management of burns has improved significantly, resulting in gradually increase in survival rates, there is growing evidence of longtime impacts of burn injury. Recent precedents suggest burn injury is linked to continuous changes to immune function [123], which contribute to multiple organ damage. In this context, MT would be an ideal candidate to stimulate immune function for long-term burn care because it has inherent immunomodulatory effects [124,125,126]. In our review, MT has been highlighted as a potential therapeutic candidate for short-term burns care, however; the data of long-term effects of this pineal hormone for the management of burn care are absent. Future studies are required to assess the impact of MT on the long-term burden of burn injury.
5. Conclusions
In conclusion, we found that MT treatment significantly reduced lipid peroxidation products (MDA and 4-HNE) and inflammatory molecules (TNF-α, CRP, and MPO) in burn-induced DOI. Additionally, our findings demonstrate that MT may exert a protective effect by increasing the levels of GSH, SOD, and HO-1 via Nrf2 activation. Moreover, MT treatment notably increased the level of anti-inflammatory molecules (e.g., IL-10) in damaged tissues. Combining the attributes of an antioxidant and an anti-inflammatory agent, MT could ameliorate DOI following thermal skin injury. However, all in vivo studies to date have major methodological limitations, including a small sample size, and detection and selection biases. Therefore, future studies should aim to enhance quality through precise experimental design by improving the sample size and reducing the bias in animal trials.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/9/12/1196/s1, Supplementary Table S1. Study inclusion criteria. Supplementary Table S2. Explanations for the exclusions of full-text articles. Supplementary Figure S1. Forest plot: leave-one-out sensitivity analysis of the impact of MT on the level of MDA. Supplementary Figure S2. Forest plot: leave-one-out sensitivity analysis of the impact of MT on the level of GSH. Supplementary Figure S3. Forest plot: leave-one-out sensitivity analysis of the impact of MT on the level of 4-HNE. Supplementary Figure S4. Forest plot: leave-one-out sensitivity analysis of the impact of MT on the level of Nrf2. Supplementary Figure S5. Forest plot: leave-one-out sensitivity analysis of the impact of MT on the level of SOD. Supplementary Figure S6. Forest plot: leave-one-out sensitivity analysis of the impact of MT on the level of HO-1. Supplementary Figure S7. Forest plot: leave-one-out sensitivity analysis of the impact of MT on the level of TNF-α. Supplementary Figure S8. Forest plot: leave-one-out sensitivity analysis of the impact of MT on the level of CRP. Supplementary Figure S9. Forest plot: leave-one-out sensitivity analysis of the impact of MT on the level of MPO. Supplementary Figure S10. Forest plot: leave-one-out sensitivity analysis of the impact of MT on the level of IL-10. Supplementary Figure S11. Forest plot: trim and fill analysis.
Author Contributions
Conceptualization, Y.H.; methodology, D.M.S., J.C. and Z.A.K.; software, D.M.S.; validation, Y.H.; formal analysis, D.M.S., J.C. and Z.A.K.; investigation, D.M.S. and J.C.; data curation, D.M.S., J.C. and Z.A.K.; writing-original draft preparation, Y.H. and D.M.S.; writing-review and editing, Y.H. and D.M.S.; visualization, D.M.S., J.C. and Z.A.K.; supervision, Y.H.; project administration, J.C.; funding acquisition, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by grants from the National Research Foundation (2017R1D1A1B0302956514, 2020R1A2C201215511 to Y.H.), Korea. J.C. is supported by a post-doctoral fellowship from National Research Foundation (NRF-2019R1A6A3A01091422), Korea.
Acknowledgments
The authors would like to acknowledge the invaluable support and critical comments of members in ‘Biological Clock & Aging Control’ laboratory. This study was completed as part of the doctoral dissertation by D.M.S.
Conflicts of Interest
The authors declare no conflict of interest.
References
- American Burn Association. National Burn Repository 2019 Update, Report of Data from 2009–2018; American Burn Association: Chicago, IL, USA, 2019. [Google Scholar]
- Peck, M.D. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 2011, 37, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Hop, M.J.; Polinder, S.; van der Vlies, C.H.; Middelkoop, E.; van Baar, M.E. Costs of burn care: A systematic review. Wound Repair Regen. 2014, 22, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.A.R.; Johnson, W.D. Burns in the Third World: An unmet need. Ann. Burns Fire Disasters 2017, 30, 243–246. [Google Scholar] [PubMed]
- Smolle, C.; Cambiaso-Daniel, J.; Forbes, A.A.; Wurzer, P.; Hundeshagen, G.; Branski, L.K.; Huss, F.; Kamolz, L.-P. Recent trends in burn epidemiology worldwide: A systematic review. Burns 2017, 43, 249–257. [Google Scholar] [CrossRef]
- Brusselaers, N.; Monstrey, S.; Vogelaers, D.; Hoste, E.; Blot, S. Severe burn injury in Europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit. Care 2010, 14, R188. [Google Scholar] [CrossRef]
- Seada, A.; Younis, G. Identification of predisposing factors to multiple organ dysfunctions syndrome among burned patients in intensive care unit at Mansoura University. Int. Acad. J. Health Med. Nurs. 2020, 2, 12–25. [Google Scholar]
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Prim. 2020, 6, 11. [Google Scholar] [CrossRef]
- Kallinen, O.; Maisniemi, K.; Böhling, T.; Tukiainen, E.; Koljonen, V. Multiple organ failure as a cause of death in patients with severe burns. J. Burn Care Res. 2012, 33, 206–211. [Google Scholar] [CrossRef]
- AbuBakr, H.O.; Aljuaydi, S.H.; Abou-Zeid, S.M.; El-Bahrawy, A. Burn-Induced Multiple Organ Injury and Protective Effect of Lutein in Rats. Inflammation 2018, 41, 760–772. [Google Scholar] [CrossRef]
- Stanojcic, M.; Abdullahi, A.; Rehou, S.; Parousis, A.; Jeschke, M.G. Pathophysiological Response to Burn Injury in Adults. Ann. Surg. 2018, 267, 576–584. [Google Scholar] [CrossRef]
- Beiraghi-Toosi, A.; Askarian, R.; Sadrabadi Haghighi, F.; Safarian, M.; Kalantari, F.; Hashemy, S.I. Burn-induced Oxidative Stress and Serum Glutathione Depletion; a Cross Sectional Study. Emergency 2018, 6, e54. [Google Scholar] [PubMed]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns. J. Burn Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef] [PubMed]
- Marsden, N.J.; Van, M.; Dean, S.; Azzopardi, E.A.; Hemington-Gorse, S.; Evans, P.A.; Whitaker, I.S. Measuring coagulation in burns: An evidence-based systematic review. Scars Burn. Health 2017, 3, 205951311772820. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Parihar, M.S.; Milner, S.; Bhat, S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 2008, 34, 6–17. [Google Scholar] [CrossRef]
- Bekyarova, G.; Yankova, T.; Galunska, B. Increased antioxidant capacity, suppression of free radical damage and erythrocyte aggrerability after combined application of alpha-tocopherol and FC-43 perfluorocarbon emulsion in early postburn period in rats. Artif. Cells Blood Substit. Immobil. Biotechnol. 1996, 24, 629–641. [Google Scholar] [CrossRef]
- Ding, H.Q.; Zhou, B.J.; Liu, L.; Cheng, S. Oxidative stress and metallothionein expression in the liver of rats with severe thermal injury. Burns 2002, 28, 215–221. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Naito, Y.; Ueda, S.; Oyamada, H.; Takemura, T.; Yoshida, N.; Sugino, S.; Kondo, M. Role of Oxygen-Derived Free Radicals in the Pathogenesis of Gastric Mucosal Lesions in Rats. J. Clin. Gastroenterol. 1990, 12, S65–S71. [Google Scholar] [CrossRef]
- Gong, Z.-Y.; Yuan, Z.-Q.; Dong, Z.-W.; Peng, Y.-Z. Glutamine with probiotics attenuates intestinal inflammation and oxidative stress in a rat burn injury model through altered iNOS gene aberrant methylation. Am. J. Transl. Res. 2017, 9, 2535–2547. [Google Scholar]
- Alican, İ.; Ünlüer, E.E.; Yeğen, C.; Yeğen, B.Ç. Bombesin improves burn-induced intestinal injury in the rat. Peptides 2000, 21, 1265–1269. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, W.; Deng, J.; Lan, L.; Xue, X.; Zhang, C.; Cai, G.; Luo, X.; Liu, J. Polydatin protects cardiac function against burn injury by inhibiting sarcoplasmic reticulum Ca2+ leak by reducing oxidative modification of ryanodine receptors. Free Radic. Biol. Med. 2013, 60, 292–299. [Google Scholar] [CrossRef]
- Deng, J.; Wang, G.; Huang, Q.; Yan, Y.; Li, K.; Tan, W.; Jin, C.; Wang, Y.; Liu, J. Oxidative stress-induced leaky sarcoplasmic reticulum underlying acute heart failure in severe burn trauma. Free Radic. Biol. Med. 2008, 44, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Li, T.; Sun, Q.; Xin, Q.; Xu, T.; Yu, J.; Wang, Y.; Wei, L. Protective effect of baicalin against severe burn-induced remote acute lung injury in rats. Mol. Med. Rep. 2017, 17, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Till, G.O.; Beauchamp, C.H.A.R.L.E.S.; Menapace, D.A.V.I.D.; Tourtellotte, W., Jr.; Kunkel, R.; Johnson, K.J.; Ward, P.A. Oxygen Radical Dependent Lung Damage following Thermal Injury of Rat Skin. J. Trauma Inj. Infect. Crit. Care 1983, 23, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.W. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology 2003, 189, 75–88. [Google Scholar] [CrossRef]
- Demling, R.H.; Lalonde, C. Systemic Lipid Peroxidation and Inflammation Induced by Thermal Injury Persists into the Post-resuscitation Period. J. Trauma Inj. Infect. Crit. Care 1990, 30, 69–74. [Google Scholar] [CrossRef]
- Youn, Y.-K.; Suh, G.-J.; Jung, S.-E.; Oh, S.-K.; Demling, R. Recombinant Human Growth Hormone Decreases Lung and Liver Tissue Lipid Peroxidation and Increases Antioxidant Activity after Thermal Injury in Rats. J. Burn Care Rehabil. 1998, 19, 542. [Google Scholar] [CrossRef]
- Hansbrough, J.F.; Wikström, T.; Braide, M.; Tenenhaus, M.; Rennekampff, O.H.; Kiessig, V.; Bjursten, L.M. Neutrophil Activation and Tissue Neutrophil Sequestration in a Rat Model of Thermal Injury. J. Surg. Res. 1996, 61, 17–22. [Google Scholar] [CrossRef][Green Version]
- Cetinkale, O.; Senel, O.; Bulan, R. The effect of antioxidant therapy on cell-mediated immunity following burn injury in an animal model. Burns 1999, 25, 113–118. [Google Scholar] [CrossRef]
- Rizzo, J.A.; Rowan, M.P.; Driscoll, I.R.; Chung, K.K.; Friedman, B.C. Vitamin C in Burn Resuscitation. Crit. Care Clin. 2016, 32, 539–546. [Google Scholar] [CrossRef]
- Omran AL-Watify, D.G.; Abdul-Khaleq Abd-Zaid, W. Effecieny of Antioxidant defenses System in Burned Patients of Both Sexes with Second and Third Degrees of Burn. J. Pure Sci. 2017, 21, 81–92. [Google Scholar]
- Youn, Y.K.; Lalonde, C.; Demling, R. Oxidants and the pathophysiology of burn and smoke inhalation injury. Free Radic. Biol. Med. 1992, 12, 409–415. [Google Scholar] [CrossRef]
- Klein, G.L. Why so little effort to study anti-oxidant therapy in burns? Burn. Trauma 2016, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Adjepong, M.; Agbenorku, P.; Brown, P.; Oduro, I. The role of antioxidant micronutrients in the rate of recovery of burn patients: A systematic review. Burn. Trauma 2016, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.L.; Shahrokhi, S.; Jeschke, M.G. Enteral nutrition support in burn care: A review of current recommendations as instituted in the Ross Tilley Burn Centre. Nutrients 2012, 4, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Hong, Y.; Choi, J.; Lee, Y.; Jin, Y.; Hong, Y. Melatonin: A Potent Therapeutic Candidate in Degenerative Neural Damages. Chronobiol. Med. 2020, 2, 85–95. [Google Scholar] [CrossRef]
- Mistraletti, G.; Paroni, R.; Umbrello, M.; D’Amato, L.; Sabbatini, G.; Taverna, M.; Formenti, P.; Finati, E.; Favero, G.; Bonomini, F.; et al. Melatonin Pharmacological Blood Levels Increase Total Antioxidant Capacity in Critically Ill Patients. Int. J. Mol. Sci. 2017, 18, 759. [Google Scholar] [CrossRef]
- Agabiti-Rosei, C.; Favero, G.; De Ciuceis, C.; Rossini, C.; Porteri, E.; Rodella, L.F.; Franceschetti, L.; Maria Sarkar, A.; Agabiti-Rosei, E.; Rizzoni, D.; et al. Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens. Res. 2017, 40, 41–50. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.-X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.; Manchester, L.C.; Qi, W. Biochemical Reactivity of Melatonin with Reactive Oxygen and Nitrogen Species: A Review of the Evidence. Cell Biochem. Biophys. 2001, 34, 237–256. [Google Scholar] [CrossRef]
- Leon, J.; Acuna-Castroviejo, D.; Escames, G.; Tan, D.-X.; Reiter, R.J. Melatonin mitigates mitochondrial malfunction. J. Pineal Res. 2005, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tiong, Y.L.; Ng, K.Y.; Koh, R.Y.; Ponnudurai, G.; Chye, S.M. Melatonin Prevents Oxidative Stress-Induced Mitochondrial Dysfunction and Apoptosis in High Glucose-Treated Schwann Cells via Upregulation of Bcl2, NF-κB, mTOR, Wnt Signalling Pathways. Antioxidants 2019, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Han, X.N.; Wen, G.Q.; Xu, L. Protective effect of melatonin against remal dysfunction following severe burn in rats. Chin. Crit. Care Med. 2007, 12, 721–723. [Google Scholar]
- Hristova, M.; Tzaneva, M.; Bekyarova, G.; Chivchibashi, D.; Stefanova, N.; Kiselova-Kaneva, Y. Molecular Mechanisms of Melatonin Protection from Gastric Mucosal Apoptotic Injury in Experimental Burns. Molecules 2018, 23, 749. [Google Scholar] [CrossRef]
- Wiggins-Dohlvik, K.; Han, M.S.; Stagg, H.W.; Alluri, H.; Shaji, C.A.; Oakley, R.P.; Davis, M.L.; Tharakan, B. Melatonin inhibits thermal injury–induced hyperpermeability in microvascular endothelial cells. J. Trauma Acute Care Surg. 2014, 77, 899–905. [Google Scholar] [CrossRef]
- Kayapinar, M. Saving the zone of stasis in burns with melatonin: An experimental study in rats. Turk. J. Trauma Emerg. Surg. 2015, 21, 419–424. [Google Scholar] [CrossRef][Green Version]
- Bekyarova, G.; Tzaneva, M.; Hristova, M.; Hristov, K. Melatonin protection against burn-induced liver injury. A review. Open Med. 2014, 9, 148–158. [Google Scholar] [CrossRef]
- Maldonado, M.-D.; Murillo-Cabezas, F.; Calvo, J.-R.; Lardone, P.-J.; Tan, D.-X.; Guerrero, J.-M.; Reiter, R.J. Melatonin as pharmacologic support in burn patients: A proposed solution to thermal injury-related lymphocytopenia and oxidative damage. Crit. Care Med. 2007, 35, 1177–1185. [Google Scholar] [CrossRef]
- Halpern, B.; Mancini, M.C.; Bueno, C.; Barcelos, I.P.; de Melo, M.E.; Lima, M.S.; Carneiro, C.G.; Sapienza, M.T.; Buchpiguel, C.A.; do Amaral, F.G.; et al. Melatonin Increases Brown Adipose Tissue Volume and Activity in Patients with Melatonin Deficiency: A Proof-of-Concept Study. Diabetes 2019, 68, 947–952. [Google Scholar] [CrossRef]
- Genario, R.; Cipolla-Neto, J.; Bueno, A.A.; Santos, H.O. Melatonin supplementation in the management of obesity and obesity-associated disorders: A review of physiological mechanisms and clinical applications. Pharmacol. Res. 2020. [Google Scholar] [CrossRef]
- Permuy, M.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Melatonin: A Review of Its Potential Functions and Effects on Dental Diseases. Int. J. Mol. Sci. 2017, 18, 865. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H.; Gholinezhad, H.; Moradi, L.; Salehi, P.; Abadi, F.; Ravanbakhsh, M.; Zare Javid, A. The effects of melatonin supplementation in adjunct with non-surgical periodontal therapy on periodontal status, serum melatonin and inflammatory markers in type 2 diabetes mellitus patients with chronic periodontitis: A double-blind, placebo-controlled t. Inflammopharmacology 2019, 27, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Tabrizi, R.; Vakili, S.; Akbari, M.; Mirhosseini, N.; Lankarani, K.B.; Rahimi, M.; Mobini, M.; Jafarnejad, S.; Vahedpoor, Z.; Asemi, Z. The effects of curcumin-containing supplements on biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 253–262. [Google Scholar] [CrossRef] [PubMed]
- 10.4.3.1 Recommendations on Testing for Funnel Plot Asymmetry. Available online: https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm (accessed on 11 July 2020).
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, G.; Hristova, M.; Tzaneva, M.; Kotzev, A. Hepatoprotective effect of melatonin via activation of Nrf2 and anti-apoptotic proteins in burn rats. Oxid. Antioxid. Med. Sci. 2018, 8, 11. [Google Scholar] [CrossRef]
- Bekyarova, G. Melatonin modulates inflammatory response and suppresses burn-induced apoptotic injury. J. Mind Med. Sci. 2017, 4, 59–66. [Google Scholar] [CrossRef]
- Bekyarova, G.; Tzaneva, M.; Hristova, M. Melatonin protects against burn-induced hepatic oxidative injury by inducing HO-1 via the Nrf2 pathway. Vet. Med. 2016, 60, 621–628. [Google Scholar] [CrossRef]
- Bekyarova, G.; Tzaneva, M.; Hristova, M. Melatonin modulates the expression of Bcl-2 family proteins in liver after thermal injury in rats. Adv. Biosci. Biotechnol. 2013, 4, 41–47. [Google Scholar] [CrossRef]
- Bekyarova, G.; Apostolova, M.; Kotzev, I. Melatonin protection against burn-induced hepatic injury by down-regulation of nuclear factor kappa B activation. Int. J. Immunopathol. Pharmacol. 2012, 25, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, G.; Bratchkova, Y.; Tancheva, S.; Hristova, M. Effective melatonin protection of burn-induced hepatic disorders in rats. Open Med. 2012, 7, 533–538. [Google Scholar] [CrossRef]
- Bekyarova, G.; Tancheva, S.; Hristova, M. Protective effect of melatonin against oxidative hepatic injury after experimental thermal trauma. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 11–14. [Google Scholar] [CrossRef]
- Sener, G.; Sehirli, A.O.; Satiroğlu, H.; Keyer-Uysal, M.; Yeğen, B.C. Melatonin improves oxidative organ damage in a rat model of thermal injury. Burns 2002, 28, 419–425. [Google Scholar] [CrossRef]
- Hristova, M.; Bekyarova, G.; Tzaneva, M. Heme oxygenase-1 expression and oxidative stress—Related markers in gastric mucosa in skin burns and protection with melatonin. Trakia J. Sci. 2016, 14, 307–313. [Google Scholar] [CrossRef]
- Hristova, M.; Bekyarova, G.; Tzaneva, M. Heme oxygenase-1 upregulated by melatonin: Potential protection against burn-induced oxidative gastric mucosal injury. J. IMAB Annu. Proc. Sci. Pap. 2015, 21, 779–783. [Google Scholar] [CrossRef][Green Version]
- Bekyarova, G.; Galunska, B.; Ivanova, D.; Yankova, T. Effect of melatonin on burn-induced gastric mucosal injury in rats. Burns 2009, 35, 863–868. [Google Scholar]
- Sener, G.; Sehirli, A.O.; Satiroğlu, H.; Keyer-Uysal, M.; Yeğen, B.C. Melatonin prevents oxidative kidney damage in a rat model of thermal injury. Life Sci. 2002, 70, 2977–2985. [Google Scholar] [CrossRef]
- Bai, X.-Z.; He, T.; Gao, J.-X.; Liu, Y.; Liu, J.-Q.; Han, S.-C.; Li, Y.; Shi, J.-H.; Han, J.-T.; Tao, K.; et al. Melatonin prevents acute kidney injury in severely burned rats via the activation of SIRT1. Sci. Rep. 2016, 6, 32199. [Google Scholar] [CrossRef] [PubMed]
- Bekyarova, G.; Tancheva, S.; Hristova, M. The effects of melatonin on burn-induced inflammatory responses and coagulation disorders in rats. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Tunali, T.; Sener, G.; Yarat, A.; Emekli, N. Melatonin reduces oxidative damage to skin and normalizes blood coagulation in a rat model of thermal injury. Life Sci. 2005, 76, 1259–1265. [Google Scholar] [CrossRef]
- Al-Ghoul, W.M.; Abu-Shaqra, S.; Park, B.G.; Fazal, N. Melatonin plays a protective role in postburn rodent gut pathophysiology. Int. J. Biol. Sci. 2010, 6, 282–293. [Google Scholar] [CrossRef]
- Han, X.; Xu, L. The protective effect of melatonin on acute lung injury in severely burned rats. Chin. J. Clin. Rehabil. 2006, 10, 70–72. [Google Scholar]
- Vaughn, L.; Beckel, N. Severe burn injury, burn shock, and smoke inhalation injury in small animals. Part 1: Burn classification and pathophysiology. J. Vet. Emerg. Crit. Care 2012, 22, 179–186. [Google Scholar] [CrossRef]
- Nishiura, T.; Nishimura, T.; DeSerres, S.; Godfrey, V.; Bradham, C.A.; Nakagawa, T.; Brenner, D.A.; Meyer, A.A. Gene expression and cytokine and enzyme activation in the liver after a burn injury. J. Burn Care Rehabil. 2000, 21, 135–141. [Google Scholar] [CrossRef]
- Rao, R.; Orman, M.A.; Berthiaume, F.; Androulakis, I.P. Dynamics of hepatic gene expression and serum cytokine profiles in single and double-hit burn and sepsis animal models. Data Br. 2015, 3, 229–233. [Google Scholar] [CrossRef][Green Version]
- Fang, W.-H.; Yao, Y.-M.; Shi, Z.-G.; Yu, Y.; Wu, Y.; Lu, L.-R.; Sheng, Z.-Y. The mRNA expression patterns of tumor necrosis factor-alpha and TNFR-I in some vital organs after thermal injury. World J. Gastroenterol. 2003, 9, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Rawlingson, A. Nitric oxide, inflammation and acute burn injury. Burns 2003, 29, 631–640. [Google Scholar] [CrossRef]
- Guo, Y.; You, Y.; Lv, D.; Yan, J.; Shang, F.-F.; Wang, X.; Zhang, C.; Fan, Q.; Luo, S. Inducible nitric oxide synthase contributes to insulin resistance and cardiac dysfunction after burn injury in mice. Life Sci. 2019, 239, 116912. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.-Y.; Wang, Q.-C.; Chen, X.-L.; Wang, Q.; Sun, C.-S.; Sun, Y.-X.; Wang, C.-H.; Su, M.-X.; Wang, H.-Y.; Wu, X.-S. Hypertonic saline resuscitation protects against kidney injury induced by severe burns in rats. Burns 2019, 45, 641–648. [Google Scholar] [CrossRef]
- He, W.; Wang, Y.; Wang, P.; Wang, F. Intestinal barrier dysfunction in severe burn injury. Burn. Trauma 2019, 7, 24. [Google Scholar] [CrossRef]
- Morris, N.L.; Li, X.; Earley, Z.M.; Choudhry, M.A. Regional variation in expression of pro-inflammatory mediators in the intestine following a combined insult of alcohol and burn injury. Alcohol 2015, 49, 507–511. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Xiao, Y.; Hu, W.; Wang, X.; Wang, P.; Zhang, X.; Yang, J.; Huang, Y.; He, W.; et al. Neutralization of interleukin-17A alleviates burn-induced intestinal barrier disruption via reducing pro-inflammatory cytokines in a mouse model. Burn. Trauma 2019, 7, 37. [Google Scholar] [CrossRef]
- Banerjee, S.; Shah, S.K.; Melnyk, S.B.; Pathak, R.; Hauer-Jensen, M.; Pawar, S.A. Cebpd Is Essential for Gamma-Tocotrienol Mediated Protection against Radiation-Induced Hematopoietic and Intestinal Injury. Antioxidants 2018, 7, 55. [Google Scholar] [CrossRef]
- Pepe, G.; Rapa, S.F.; Salviati, E.; Bertamino, A.; Auriemma, G.; Cascioferro, S.; Autore, G.; Quaroni, A.; Campiglia, P.; Marzocco, S. Bioactive Polyphenols from Pomegranate Juice Reduce 5-Fluorouracil-Induced Intestinal Mucositis in Intestinal Epithelial Cells. Antioxidants 2020, 9, 699. [Google Scholar] [CrossRef]
- Murata, M.; Kawanishi, S. Oxidative DNA damage induced by nitrotyrosine, a biomarker of inflammation. Biochem. Biophys. Res. Commun. 2004, 316, 123–128. [Google Scholar] [CrossRef]
- Kaneki, M.; Fukushima, Y.; Shinozaki, S.; Fukaya, M.; Habiro, M.; Shimizu, N.; Chang, K.; Yasuhara, S.; Martyn, J.A.J. iNOS inhibitor, L-NIL, reverses burn-induced glycogen synthase kinase-3β activation in skeletal muscle of rats. Metabolism 2013, 62, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z. Prevention of multiple organ dysfunction syndrome in patients with extensive deep burns. Chin. J. Traumatol. = Zhonghua Chuang Shang Za Zhi 2002, 5, 195–199. [Google Scholar] [PubMed]
- Nguyen, L.N.; Nguyen, T.G. Characteristics and outcomes of multiple organ dysfunction syndrome among severe-burn patients. Burns 2009, 35, 937–941. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Yang, Z.C.; Liu, X.S.; Chen, F.M.; He, B.B.; Li, A.; Crowther, R.S. Serial experimental and clinical studies on the pathogenesis of multiple organ dysfunction syndrome (MODS) in severe burns. Burns 1998, 24, 706–716. [Google Scholar] [CrossRef]
- Lv, Q.; Gu, Y.; Qi, Y.; Liu, Z.; Ma, G.-E. Effects of lentinan on NF-κB activity in the liver of burn rats with sepsis. Exp. Ther. Med. 2020, 20, 2279–2283. [Google Scholar]
- Bortolin, J.A.; Quintana, H.T.; de Tomé, T.C.; Ribeiro, F.A.P.; Ribeiro, D.A.; de Oliveira, F. Burn injury induces histopathological changes and cell proliferation in liver of rats. World J. Hepatol. 2016, 8, 322–330. [Google Scholar] [CrossRef]
- Luo, G.; Peng, D.; Zheng, J.; Chen, X.; Wu, J.; Elster, E.; Tadaki, D. The role of NO in macrophage dysfunction at early stage after burn injury. Burns 2005, 31, 138–144. [Google Scholar] [CrossRef]
- Saitoh, D.; Okada, Y.; Ookawara, T.; Yamashita, H.; Takahara, T.; Ishihara, S.; Ohno, H.; Mimura, K. Prevention of ongoing lipid peroxidation by wound excision and superoxide dismutase treatment in the burned rat. Am. J. Emerg. Med. 1994, 12, 142–146. [Google Scholar] [CrossRef]
- Thomson, P.D.; Till, G.O.; Woolliscroft, J.O.; Smith, D.J.; Prasad, J.K. Superoxide dismutase prevents lipid peroxidation in burned patients. Burns 1990, 16, 406–408. [Google Scholar] [CrossRef]
- Sussan, T.E.; Jun, J.; Thimmulappa, R.; Bedja, D.; Antero, M.; Gabrielson, K.L.; Polotsky, V.Y.; Biswal, S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS ONE 2008, 3, e3791. [Google Scholar] [CrossRef]
- Braun, S.; Hanselmann, C.; Gassmann, M.G.; auf dem Keller, U.; Born-Berclaz, C.; Chan, K.; Kan, Y.W.; Werner, S. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol. Cell. Biol. 2002, 22, 5492–5505. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Wang, H.; Xu, J.; Li, T.; Zhang, L.; Ding, Y.; Zhu, L.; He, J.; Zhou, M. Melatonin stimulates antioxidant enzymes and reduces oxidative stress in experimental traumatic brain injury: The Nrf2-ARE signaling pathway as a potential mechanism. Free Radic. Biol. Med. 2014, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, C.; Zhang, K.; Lan, X.; Chen, X.; Zang, W.; Wang, Z.; Guan, F.; Zhu, C.; Yang, X.; et al. Melatonin receptor activation provides cerebral protection after traumatic brain injury by mitigating oxidative stress and inflammation via the Nrf2 signaling pathway. Free Radic. Biol. Med. 2019, 131, 345–355. [Google Scholar] [CrossRef] [PubMed]
- LaLonde, C.; Nayak, U.; Hennigan, J.; Demling, R.H. Excessive liver oxidant stress causes mortality in response to burn injury combined with endotoxin and is prevented with antioxidants. J. Burn Care Rehabil. 1997, 18, 187–192. [Google Scholar] [CrossRef]
- Sahib, A.S.; Al-Jawad, F.H.; Alkaisy, A.A. Effect of antioxidants on the incidence of wound infection in burn patients. Ann. Burns Fire Disasters 2010, 23, 199–205. [Google Scholar] [PubMed]
- Ghanayem, H. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. BET 3: Vitamin C in severe burns. Emerg. Med. J. 2012, 29, 1017–1018. [Google Scholar]
- Kremer, T.; Harenberg, P.; Hernekamp, F.; Riedel, K.; Gebhardt, M.M.; Germann, G.; Heitmann, C.; Walther, A. High-dose vitamin C treatment reduces capillary leakage after burn plasma transfer in rats. J. Burn Care Res. 2010, 31, 470–479. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsuda, T.; Miyagantani, Y.; Yukioka, T.; Matsuda, H.; Shimazaki, S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: A randomized, prospective study. Arch. Surg. 2000, 135, 326–331. [Google Scholar] [CrossRef]
- De Grooth, H.-J.; Manubulu-Choo, W.-P.; Zandvliet, A.S.; Spoelstra-de Man, A.M.E.; Girbes, A.R.; Swart, E.L.; Oudemans-van Straaten, H.M. Vitamin C Pharmacokinetics in Critically Ill Patients: A Randomized Trial of Four IV Regimens. Chest 2018, 153, 1368–1377. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Total, Dietary, and Supplemental Vitamin C Intake and Risk of Incident Kidney Stones. Am. J. Kidney Dis. 2016, 67, 400–407. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Werner, M.U.; Rosenkilde, M.M.; Fenger, A.Q.; Petersen, M.C.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of high-dose intravenous melatonin in humans. J. Clin. Pharmacol. 2016, 56, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Maras, A.; Schroder, C.M.; Malow, B.A.; Findling, R.L.; Breddy, J.; Nir, T.; Shahmoon, S.; Zisapel, N.; Gringras, P. Long-term efficacy and safety of pediatric prolonged-release melatonin for insomnia in children with autism spectrum disorder. J. Child Adolesc. Psychopharmacol. 2018, 28, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Alagbonsi, I.A.; Olayaki, L.A. Role of oxidative stress in Cannabis sativa-associated spermatotoxicity: Evidence for ameliorative effect of combined but not separate melatonin and vitamin C. Middle East Fertil. Soc. J. 2017, 22, 136–144. [Google Scholar] [CrossRef]
- Gitto, E.; Tan, D.X.; Reiter, R.J.; Karbownik, M.; Manchester, L.C.; Cuzzocrea, S.; Fulia, F.; Barberi, I. Individual and synergistic antioxidative actions of melatonin: Studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J. Pharm. Pharmacol. 2001, 53, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Chitsazi, M.; Faramarzie, M.; Sadighi, M.; Shirmohammadi, A.; Hashemzadeh, A. Effects of adjective use of melatonin and vitamin C in the treatment of chronic periodontitis: A randomized clinical trial. J. Dent. Res. Dent. Clin. Dent. Prospect. 2017, 11, 236–240. [Google Scholar]
- Akinci, A.; Esrefoglu, M.; Cetin, A.; Ates, B. Melatonin is more effective than ascorbic acid and β-carotene in improvement of gastric mucosal damage induced by intensive stress. Arch. Med. Sci. 2015, 11, 1129–1136. [Google Scholar]
- Thalji, S.Z.; Kothari, A.N.; Kuo, P.C.; Mosier, M.J. Acute Kidney Injury in Burn Patients: Clinically Significant Over the Initial Hospitalization and 1 Year After Injury: An Original Retrospective Cohort Study. Ann. Surg. 2017, 266, 376–382. [Google Scholar] [CrossRef]
- Fear, V.S.; Boyd, J.H.; Rea, S.; Wood, F.M.; Duke, J.M.; Fear, M.W. Burn Injury Leads to Increased Long-Term Susceptibility to Respiratory Infection in both Mouse Models and Population Studies. PLoS ONE 2017, 12, e0169302. [Google Scholar] [CrossRef]
- Duke, J.M.; Randall, S.M.; Fear, M.W.; Boyd, J.H.; Rea, S.; Wood, F.M. Understanding the long-term impacts of burn on the cardiovascular system. Burns 2016, 42, 366–374. [Google Scholar] [CrossRef]
- Kraft, R.; Herndon, D.N.; Finnerty, C.C.; Shahrokhi, S.; Jeschke, M.G. Occurrence of multiorgan dysfunction in pediatric burn patients: Incidence and clinical outcome. Ann. Surg. 2014, 259, 381–387. [Google Scholar] [CrossRef]
- Barrett, L.W.; Fear, V.S.; Waithman, J.C.; Wood, F.M.; Fear, M.W. Understanding acute burn injury as a chronic disease. Burn. Trauma 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Esquifino, A.I.; Perumal, S.R.P.; Miller, S.C. Melatonin, immune function and aging. Immun. Ageing 2005, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- Calvo, J.R.; González-Yanes, C.; Maldonado, M.D. The role of melatonin in the cells of the innate immunity: A review. J. Pineal Res. 2013, 55, 103–120. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).