Synthetic Secoisolariciresinol Diglucoside Attenuates Established Pain, Oxidative Stress and Neuroinflammation in a Rodent Model of Painful Radiculopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedures and SDG Administration
2.2. Pharmacokinetic Evaluation of SDG in Rat Plasma
2.3. Behavioral Assessment
2.4. Tissue Harvest, Immunohistochemistry and Analyses
3. Results
3.1. Plasma SDG Levels Increased after Subcutaneous Administration
3.2. SDG Attenuates Established Behavioral Sensitivity
3.3. Oxidative Stress Markers in the DRG and Spinal Cord Decrease with SDG Treatment
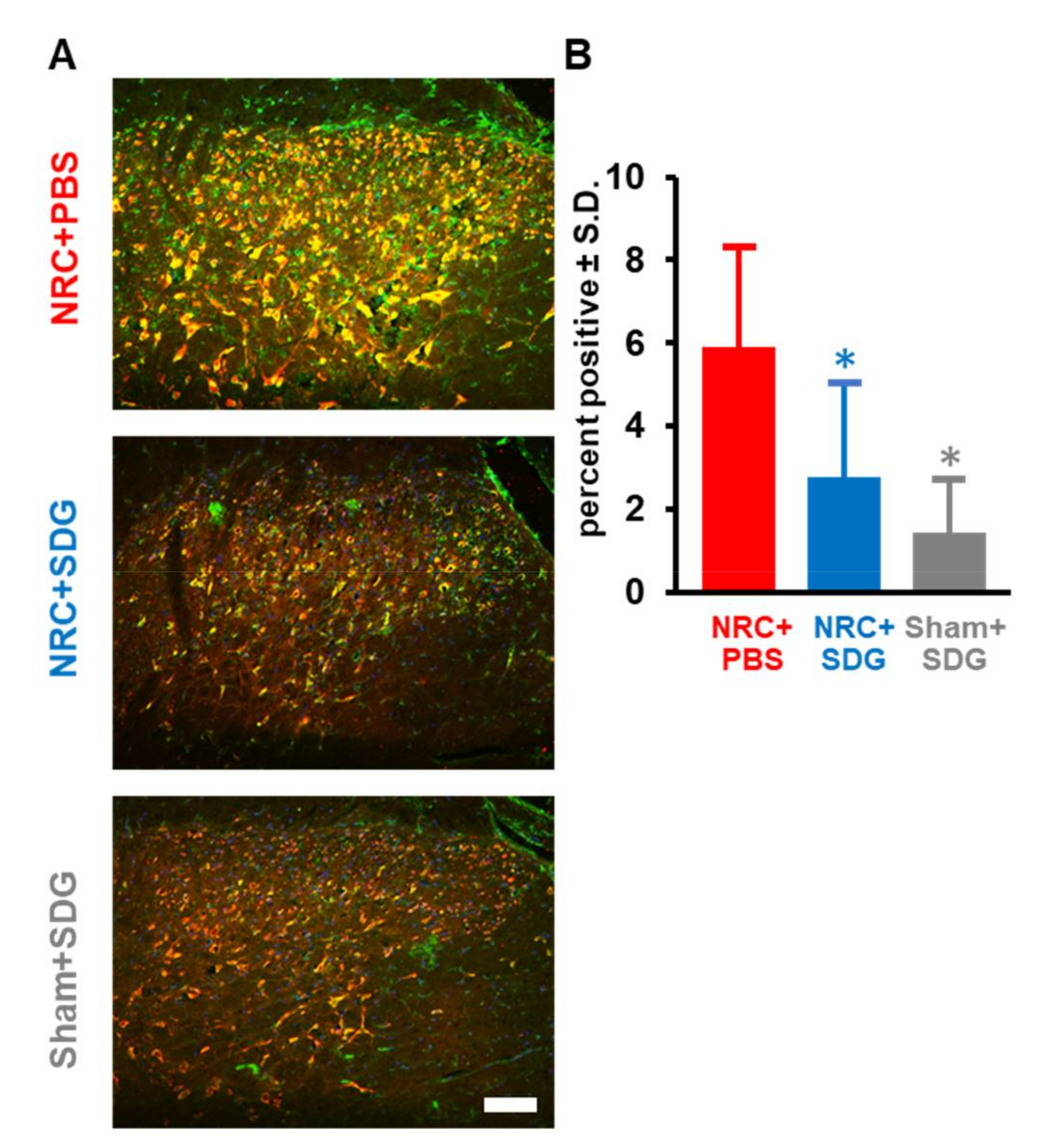
3.4. SDG Reduces Spinal Glial Activation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Patel, E.A.; Perloff, M.D. Radicular pain syndromes: Cervical, lumbar and spinal stenosis. Semin. Neurol. 2018, 38, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.J.; Zhang, S.; Gilliland, T.M.; Winkelstein, B.A. Riluzole effects on behavioral sensitivity and the development of axonal damage and spinal modifications that occur after painful nerve root compression. J. Neurosurg. Spine 2014, 20, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.D.; Chen, Z.; Winkelstein, B.A. Transient cervical nerve root compression modulates pain: Load thresholds for allodynia and sustained changes in spinal neuropeptide expression. J. Biomech. 2008, 41, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, R.D.; Quinn, K.P.; Martínez, J.J.; Winkelstein, B.A. The role of graded nerve root compression on axonal damage, neuropeptide changes, and pain-related behaviors. Stapp Car Crash J. 2008, 52, 33–58. [Google Scholar] [PubMed]
- DeLeo, J.A.; Yezierski, R.P. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 2001, 90, 1–6. [Google Scholar] [CrossRef]
- Winkelstein, B.A.; Rutkowski, M.D.; Sweitzer, S.M.; Pahl, J.L.; DeLeo, J.A. Nerve injury proximal or distal to the DRG induces similar spinal glial activation and selective cytokine expression but differential behavioral responses to pharmacologic treatment. J. Comp. Neurol. 2001, 439, 127–139. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F.; Goehler, L.E. Immune activation: The role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain 1995, 63, 289–302. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef]
- Kumar, H.; Koppula, S.; Kim, I.S.; Vasant More, S.; Kim, B.W.; Choi, D.K. Nuclear factor erythroid 2-related factor 2 signaling in Parkinson disease: A promising multi therapeutic target against oxidative stress, neuroinflammation and cell death. CNS Neurol. Disord. Drug Targets 2012, 11, 1015–1029. [Google Scholar] [CrossRef]
- Carrasco, C.; Naziroǧlu, M.; Rodríguez, A.B.; Pariente, J.A. Neuropathic pain: Delving into the oxidative origin and the possible implication of transient receptor potential channels. Front. Physiol. 2018, 9, 95. [Google Scholar] [CrossRef]
- Rivas-Arancibia, S.; Guevara-Guzmán, R.; López-Vidal, Y.; Rodríguez-Martínez, E.; Zanardo-Gomes, M.; Angoa-Pérez, M.; Raisman-Vozari, R. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol. Sci. 2010, 113, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Kartha, S.; Yan, L.; Weisshaar, C.L.; Ita, M.E.; Shuvaev, V.V.; Muzykantov, V.R.; Tsourkas, A.; Winkelstein, B.A.; Cheng, Z. Superoxide dismutase-loaded porous polymersomes as highly efficient antioxidants for treating neuropathic pain. Adv. Healthc. Mater. 2017, 6, 1700500. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of chronic oxidative stress on neuroinflammatory response mediated by CD4+T cells in neurodegenerative diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The role of mitochondria in reactive oxygen species generation and its implications for neurodegenerative diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Kartha, S.; Weisshaar, C.L.; Philips, B.H.; Winkelstein, B.A. Pre-treatment with meloxicam prevents the spinal inflammation and oxidative stress in DRG neurons that accompany painful cervical radiculopathy. Neuroscience 2018, 388, 393–404. [Google Scholar] [CrossRef]
- Silva, A.R.T.; Santos, A.C.F.; Farfel, J.M.; Grinberg, L.T.; Ferretti, R.E.L.; Campos, A.H.J.F.M.; Cunha, I.W.; Begnami, M.D.; Rocha, R.M.; Carraro, D.M.; et al. Repair of oxidative DNA damage, cell-cycle regulation and neuronal death may influence the clinical manifestation of Alzheimer’s disease. PLoS ONE 2014, 9, e99897. [Google Scholar] [CrossRef]
- Gwak, Y.S.; Hulsebosch, C.E.; Leem, J.W. Neuronal-glial interactions maintain chronic neuropathic pain after spinal cord injury. Neural Plast. 2017, 2017, 2480689. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Porreca, F.; Cuzzocrea, S.; Galen, K.; Lightfoot, R.; Masini, E.; Muscoli, C.; Mollace, V.; Ndengele, M.; Ischiropoulos, H.; et al. A newly identified role for superoxide in inflammatory pain. J. Pharmacol. Exp. Ther. 2004, 309, 869–878. [Google Scholar] [CrossRef]
- Salvemini, D.; Little, J.W.; Doyle, T.; Neumann, W.L. Roles of reactive oxygen and nitrogen species in pain. Free Radic. Biol. Med. 2011, 51, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Bakunina, N.; Pariante, C.M.; Zunszain, P.A. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 2015, 144, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Simmons, N.; Tyagi, S.; Pietrofesa, R.; Shuvaev, V.V.; Valiulin, R.A.; Heretsch, P.; Nicolaou, K.C.; Christofidou-Solomidou, M. Synthesis and antioxidant evaluation of (s,s)- and (r,r)-secoisolariciresinol diglucosides (sdgs). Bioorg. Med. Chem. Lett. 2013, 23, 5325–5328. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Tyagi, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Turowski, J.; Grieshaber, P.A.; Solomides, C.C.; Cengel, K.A. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (sdg). Radiat. Res. 2012, 178, 568–580. [Google Scholar] [CrossRef]
- Christofidou-Solomidou, M.; Tyagi, S.; Tan, K.S.; Hagan, S.; Pietrofesa, R.; Dukes, F.; Arguiri, E.; Heitjan, D.F.; Solomides, C.C.; Cengel, K.A. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer 2011, 11, 269. [Google Scholar] [CrossRef]
- Pietrofesa, R.; Turowski, J.; Tyagi, S.; Dukes, F.; Arguiri, E.; Busch, T.M.; Gallagher-Colombo, S.M.; Solomides, C.C.; Cengel, K.A.; Christofidou-Solomidou, M. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer 2013, 13, 179. [Google Scholar] [CrossRef]
- Pietrofesa, R.A.; Solomides, C.C.; Christofidou-Solomidou, M. Flaxseed mitigates acute oxidative lung damage in a mouse model of repeated radiation and hyperoxia exposure associated with space exploration. J. Pulm. Respir. Med. 2014, 4, 1000125. [Google Scholar] [CrossRef]
- Mishra, O.P.; Pietrofesa, R.; Christofidou-Solomidou, M. Novel synthetic (s,s) and (r,r)-secoisolariciresinol diglucosides (sdgs) protect naked plasmid and genomic DNA from gamma radiation damage. Radiat. Res. 2014, 182, 102–110. [Google Scholar] [CrossRef]
- Velalopoulou, A.; Tyagi, S.; Pietrofesa, R.A.; Arguiri, E.; Christofidou-Solomidou, M. The flaxseed-derived lignan phenolic secoisolariciresinol diglucoside (sdg) protects non-malignant lung cells from radiation damage. Int. J. Mol. Sci. 2015, 17, 7. [Google Scholar] [CrossRef]
- Rom, S.; Zuluaga-Ramirez, V.; Reichenbach, N.L.; Erickson, M.A.; Winfield, M.; Gajghate, S.; Christofidou-Solomidou, M.; Jordan-Sciutto, K.L.; Persidsky, Y. Secoisolariciresinol diglucoside is a blood-brain barrier protective and anti-inflammatory agent: Implications for neuroinflammation. J. Neuroinflamm. 2018, 15, 25. [Google Scholar] [CrossRef]
- Kokkinaki, D.; Hoffman, M.; Kalliora, C.; Kyriazis, I.D.; Maning, J.; Lucchese, A.M.; Shanmughapriya, S.; Tomar, D.; Park, J.Y.; Wang, H.; et al. Chemically synthesized secoisolariciresinol diglucoside (lgm2605) improves mitochondrial function in cardiac myocytes and alleviates septic cardiomyopathy. J. Mol. Cell. Cardiol. 2019, 127, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Flayer, C.H.; Larson, E.D.; Joseph, A.; Kao, S.; Qu, W.; Van Haren, A.; Royer, C.M.; Miller, L.A.; Capitanio, J.P.; Sielecki, T.; et al. Ozone-induced enhancement of airway hyperreactivity in rhesus macaques: Effects of antioxidant treatment. J. Allergy Clin. Immunol. 2020, 145, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Velalopoulou, A.; Chatterjee, S.; Pietrofesa, R.A.; Koziol-White, C.; Panettieri, R.A.; Lin, L.; Tuttle, S.; Berman, A.; Koumenis, C.; Christofidou-Solomidou, M. Synthetic secoisolariciresinol diglucoside (LGM2605) protects human lung in an ex vivo model of proton radiation damage. Int. J. Mol. Sci. 2017, 18, 2525. [Google Scholar] [CrossRef] [PubMed]
- Mishra, O.P.; Popov, A.V.; Pietrofesa, R.A.; Nakamaru-Ogiso, E.; Andrake, M.; Christofidou-Solomidou, M. Synthetic secoisolariciresinol diglucoside (lgm2605) inhibits myeloperoxidase activity in inflammatory cells. Biochimica Biophysica Acta Gen. Subj. 2018, 1862, 1364–1375. [Google Scholar] [CrossRef]
- Yamada, M.; Kurahashi, K. Regulation of myeloperoxidase gene expression during differentiation of human myeloid leukemia hl-60 cells. J. Biol. Chem. 1984, 259, 3021–3025. [Google Scholar]
- Koeffler, H.P.; Ranyard, J.; Pertcheck, M. Myeloperoxidase: Its structure and expression during myeloid differentiation. Blood 1985, 65, 484–491. [Google Scholar] [CrossRef]
- Nicholson, K.J.; Guarino, B.B.; Winkelstein, B.A. Transient nerve root compression load and duration differentially mediate behavioral sensitivity and associated spinal astrocyte activation and mGLuR5 expression. Neuroscience 2012, 209, 187–195. [Google Scholar] [CrossRef]
- Zimmerman, M. Committee for Research and Ethical Issues of the IASP, Ethical standards for investigations of experimental pain in animals. Pain 1983, 16, 109–110. [Google Scholar] [CrossRef]
- Pietrofesa, R.A.; Velalopoulou, A.; Arguiri, E.; Menges, C.W.; Testa, J.R.; Hwang, W.T.; Albelda, S.M.; Christofidou-Solomidou, M. Flaxseed lignans enriched in secoisolariciresinol diglucoside prevent acute asbestos-induced peritoneal inflammation in mice. Carcinogenesis 2016, 37, 177–187. [Google Scholar] [CrossRef]
- Kinniry, P.; Amrani, Y.; Vachani, A.; Solomides, C.C.; Arguiri, E.; Workman, A.; Carter, J.; Christofidou-Solomidou, M. Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J. Nutr. 2006, 136, 1545–1551. [Google Scholar] [CrossRef]
- Smith, J.R.; Lee, J.; Winkelstein, B.A. Nerve root compression increases spinal astrocytic vimentin in parallel with sustained pain and endothelial vimentin in association with spinal vascular reestablishment. Spine 2017, 42, 1434. [Google Scholar] [CrossRef] [PubMed]
- Crosby, N.D.; Goodman Keiser, M.D.; Smith, J.R.; Zeeman, M.E.; Winkelstein, B.A. Stimulation parameters define the effectiveness of burst spinal cord stimulation in a rat model of neuropathic pain. Neuromodul. Technol. Neural Interface 2015, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, M.E.; Kartha, S.; Winkelstein, B.A. Whole body vibration induces pain and lumbar spinal inflammation responses in the rat that vary with the vibration profile. J. Orthop. Res. 2016, 34, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Ba, X.; Boldogh, I. 8-Oxoguanine DNA glycosylase 1: Beyond repair of the oxidatively modified base lesions. Redox Biol. 2018, 14, 669–678. [Google Scholar] [CrossRef]
- Gradinaru, D.; Minn, A.L.; Artur, Y.; Minn, A.; Heydel, J.M. Effect of oxidative stress on UDP-glucuronosyltransferases in rat astrocytes. Toxicol. Lett. 2012, 213, 316–324. [Google Scholar] [CrossRef]
- Ji, X.T.; Qian, N.S.; Zhang, T.; Li, J.M.; Li, X.K.; Wang, P.; Zhao, D.S.; Huang, G.; Zhang, L.; Fei, Z.; et al. Spinal astrocytic activation contributes to mechanical allodynia in a rat chemotherapy-induced neuropathic pain model. PLoS ONE 2013, 8, e60733. [Google Scholar] [CrossRef]
- Hu, P.; Mei, Q.Y.; Ma, L.; Cui, W.G.; Zhou, W.H.; Zhou, D.S.; Zhao, Q.; Xu, D.Y.; Zhao, X.; Lu, Q.; et al. Secoisolariciresinol diglycoside, a flaxseed lignan, exerts analgesic effects in a mouse model of type 1 diabetes: Engagement of antioxidant mechanism. Eur. J. Pharmacol. 2015, 767, 183–192. [Google Scholar] [CrossRef]
- Riffel, A.P.K.; de Souza, J.A.; Maria do Carmo, Q.S.; Horst, A.; Scheid, T.; Kolberg, C.; Belló-Klein, A.; Partata, W.A. Systemic administration of vitamins C and E attenuates nociception induced by chronic constriction injury of the sciatic nerve in rats. Brain Res. Bull. 2016, 121, 169–177. [Google Scholar] [CrossRef]
- Muscoli, C.; Mollace, V.; Wheatley, J.; Masini, E.; Ndengele, M.; Wang, Z.Q.; Salvemini, D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: A novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain 2004, 111, 96–103. [Google Scholar] [CrossRef]
- Ndengele, M.M.; Cuzzocrea, S.; Masini, E.; Vinci, M.C.; Esposito, E.; Muscoli, C.; Petrusca, D.N.; Mollace, V.; Mazzon, E.; Li, D.; et al. Spinal ceramide modulates the development of morphine antinociceptive tolerance via peroxynitrite-mediated nitroxidative stress and neuroimmune activation. J. Pharmacol. Exp. Ther. 2009, 329, 64–75. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, L.; Westlund, K.N. Reactive oxygen species mediate TNFR1 increase after TRPV1 activation in mouse DRG neurons. Mol. Pain 2009, 5, 1744–8069. [Google Scholar] [CrossRef] [PubMed]
- Muscoli, C.; Cuzzocrea, S.; Ndengele, M.M.; Mollace, V.; Porreca, F.; Fabrizi, F.; Esposito, E.; Masini, E.; Matuschak, G.M.; Salvemini, D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J. Clin. Investig. 2007, 117, 3530–3539. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Ndengele, M.M.; Cuzzocrea, S.; Reboucas, J.S.; Spasojevic, I.; Salvemini, D. Lipophilicity is a critical parameter that dominates the efficacy of metalloporphyrins in blocking the development of morphine antinociceptive tolerance through peroxynitrite-mediated pathways. Free Radic. Biol. Med. 2009, 46, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kim, H.K.; Chung, J.M.; Chung, K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 2007, 131, 262–271. [Google Scholar] [CrossRef]
- Zanelli, S.A.; Ashraf, Q.M.; Mishra, O.P. Nitration is a mechanism of regulation of the NMDA receptor function during hypoxia. Neuroscience 2002, 112, 869–877. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Grace, P.M.; Gaudet, A.D.; Staikopoulos, V.; Maier, S.F.; Hutchinson, M.R.; Salvemini, D.; Watkins, L.R. Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci. 2016, 39, 862–879. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochimica Biophysica Acta Gen. Subj. 2014, 1840, 2709–2729. [Google Scholar] [CrossRef]
- Pietrofesa, R.A.; Velalopoulou, A.; Albelda, S.M.; Christofidou-Solomidou, M. Asbestos induces oxidative stress and activation of nrf2 signaling in murine macrophages: Chemopreventive role of the synthetic lignan secoisolariciresinol diglucoside (LGM2605). Int. J. Mol. Sci. 2016, 17, 322. [Google Scholar] [CrossRef]
- Moree, S.S.; Rajesha, J. Investigation of in vitro and in vivo antioxidant potential of secoisolariciresinol diglucoside. Mol. Cell. Biochem. 2013, 373, 179. [Google Scholar] [CrossRef]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell. Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Puukila, S.; Fernandes, R.O.; Turck, P.; Carraro, C.C.; Bonetto, J.H.P.; de Lima-Seolin, B.G.; da Rosa Araujo, A.S.; Bello-Klein, A.; Boreham, D.; Khaper, N. Secoisolariciresinol diglucoside attenuates cardiac hypertrophy and oxidative stress in monocrotaline-induced right heart dysfunction. Mol. Cell. Biochem. 2017, 432, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.A.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Traber, M.; Ursini, F. Antioxidants: GRABbing new headlines. Free Radic. Biol. Med. 2014, 66, 1–2. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Pietrofesa, R.A.; Woodruff, P.; Hwang, W.T.; Patel, P.; Chatterjee, S.; Albelda, S.M.; Christofidou-Solomidou, M. The synthetic lignan secoisolariciresinol diglucoside prevents asbestos-induced nlrp3 inflammasome activation in murine macrophages. Oxid. Med. Cell. Longev. 2017, 2017, 7395238. [Google Scholar] [CrossRef]
- Zhu, B.; Suzuki, Y.; DiSanto, T.; Rubin, S.; Penfil, Z.; Pietrofesa, R.A.; Chatterjee, S.; Christofidou-Solomidou, M.; Cantu, E. Applications of out of body lung perfusion. Acad. Radiol. 2019, 26, 404–411. [Google Scholar] [CrossRef]
- Pietrofesa, R.A.; Chatterjee, S.; Park, K.; Arguiri, E.; Albelda, S.M.; Christofidou-Solomidou, M. Synthetic lignan secoisolariciresinol diglucoside (LGM2605) reduces asbestos-induced cytotoxicity in an nrf2-dependent and -independent manner. Antioxidants 2018, 17, 322339. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. A J. Virtual Libr. 2008, 13, 453. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, W.; Li, B.S.; Alkon, D.L.; Barker, J.L.; Chang, Y.H.; Wu, M.; Rubinow, D.R. TNF-α induced over-expression of GFAP is associated with MAPKs. Neuroreport 2000, 11, 409–412. [Google Scholar] [CrossRef]
- Forman, J.H.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalyanaraman, B.; Ischiropoulos, H. Even free radicals should follow some rules: A Guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015, 78, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Galie, P.A.; Slochower, D.R.; Weisshaar, C.L.; Janmey, P.A.; Winkelstein, B.A. Salmon-derived thrombin inhibits development of chronic pain through an endothelial barrier protective mechanism dependent on APC. Biomaterials 2016, 80, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Rickard, S.E.; Thompson, L.U. Urinary composition and postprandial blood changes in Hsecoisolariciresinol diglycoside (SDG) metabolites in rats do not differ between acute and chronic SDG treatments. J. Nutr. 2000, 130, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Saarinen, N.M.; Thompson, L.U. Prolonged administration of secoisolariciresinol diglycoside increases lignan excretion and alters lignan tissue distribution in adult male and female rats. Br. J. Nutr. 2010, 104, 833–841. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.R.; Christensen, F.M.; Aschberger, K.; Stone, V. The biological mechanisms and physicochemical characteristics responsible for driving fullerene toxicity. Toxicol. Sci. 2010, 114, 162–182. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartha, S.; Weisshaar, C.L.; Pietrofesa, R.A.; Christofidou-Solomidou, M.; Winkelstein, B.A. Synthetic Secoisolariciresinol Diglucoside Attenuates Established Pain, Oxidative Stress and Neuroinflammation in a Rodent Model of Painful Radiculopathy. Antioxidants 2020, 9, 1209. https://doi.org/10.3390/antiox9121209
Kartha S, Weisshaar CL, Pietrofesa RA, Christofidou-Solomidou M, Winkelstein BA. Synthetic Secoisolariciresinol Diglucoside Attenuates Established Pain, Oxidative Stress and Neuroinflammation in a Rodent Model of Painful Radiculopathy. Antioxidants. 2020; 9(12):1209. https://doi.org/10.3390/antiox9121209
Chicago/Turabian StyleKartha, Sonia, Christine L. Weisshaar, Ralph A. Pietrofesa, Melpo Christofidou-Solomidou, and Beth A. Winkelstein. 2020. "Synthetic Secoisolariciresinol Diglucoside Attenuates Established Pain, Oxidative Stress and Neuroinflammation in a Rodent Model of Painful Radiculopathy" Antioxidants 9, no. 12: 1209. https://doi.org/10.3390/antiox9121209
APA StyleKartha, S., Weisshaar, C. L., Pietrofesa, R. A., Christofidou-Solomidou, M., & Winkelstein, B. A. (2020). Synthetic Secoisolariciresinol Diglucoside Attenuates Established Pain, Oxidative Stress and Neuroinflammation in a Rodent Model of Painful Radiculopathy. Antioxidants, 9(12), 1209. https://doi.org/10.3390/antiox9121209




