Post-Translational Modification Analysis of VDAC1 in ALS-SOD1 Model Cells Reveals Specific Asparagine and Glutamine Deamidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. NSC34 Cell Lines
2.3. Extraction of Mitochondrial Proteins from NSC34 Cells under Reducing Condition
2.4. Liquid Chromatography and Tandem Mass Spectrometry (LC–MS/MS) Analysis
2.5. Database Search
2.5.1. Identification of Deamidation Sites on VDAC1
2.5.2. Semi-Quantitative Analysis
2.6. Modelling and Bioinformatics Analysis
3. Results
3.1. Mass Spectrometry Analysis of VDAC1 from NSC34 Cell Line
3.2. Mass Spectrometry Analysis of VDAC1 from NSC34-SOD1WT Cell Line
3.3. Mass Spectrometry Analysis of VDAC1 from NSC34-SOD1G93A Cell Line
3.4. Post-Translational Modifications Found in VDAC1 HTP-Prepared from Various NSC34 Cell Lines
3.4.1. Phosphorylation, Succination, Ubiquitin and Ubiquitination, and N-Terminal Acetylation
3.4.2. Identification of VDAC1 Deamidation Sites from NSC34-SOD1G93A Cell Line
3.5. Semi-Quantitative Analysis of VDAC1 in NSC34 Cell Lines
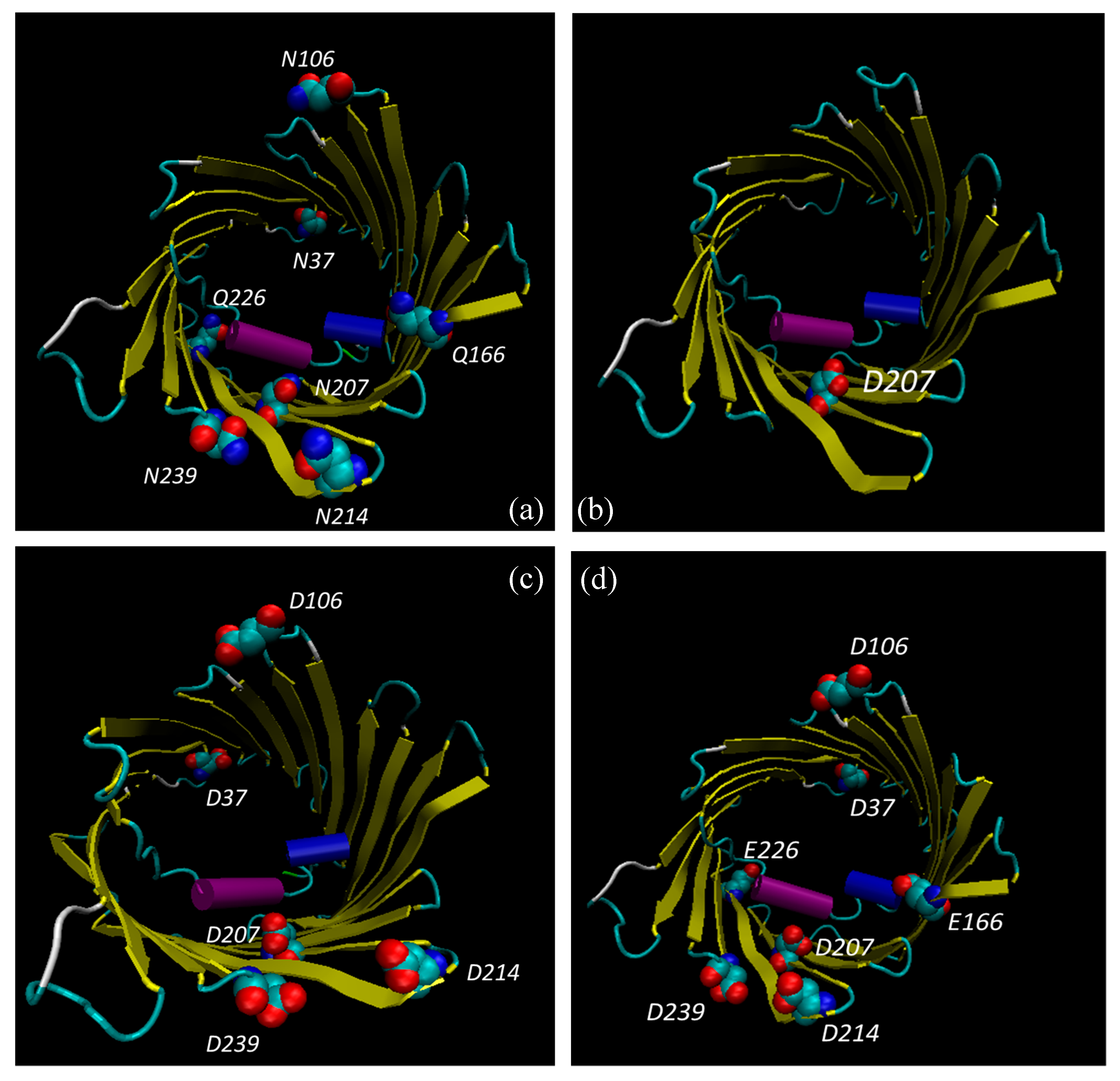
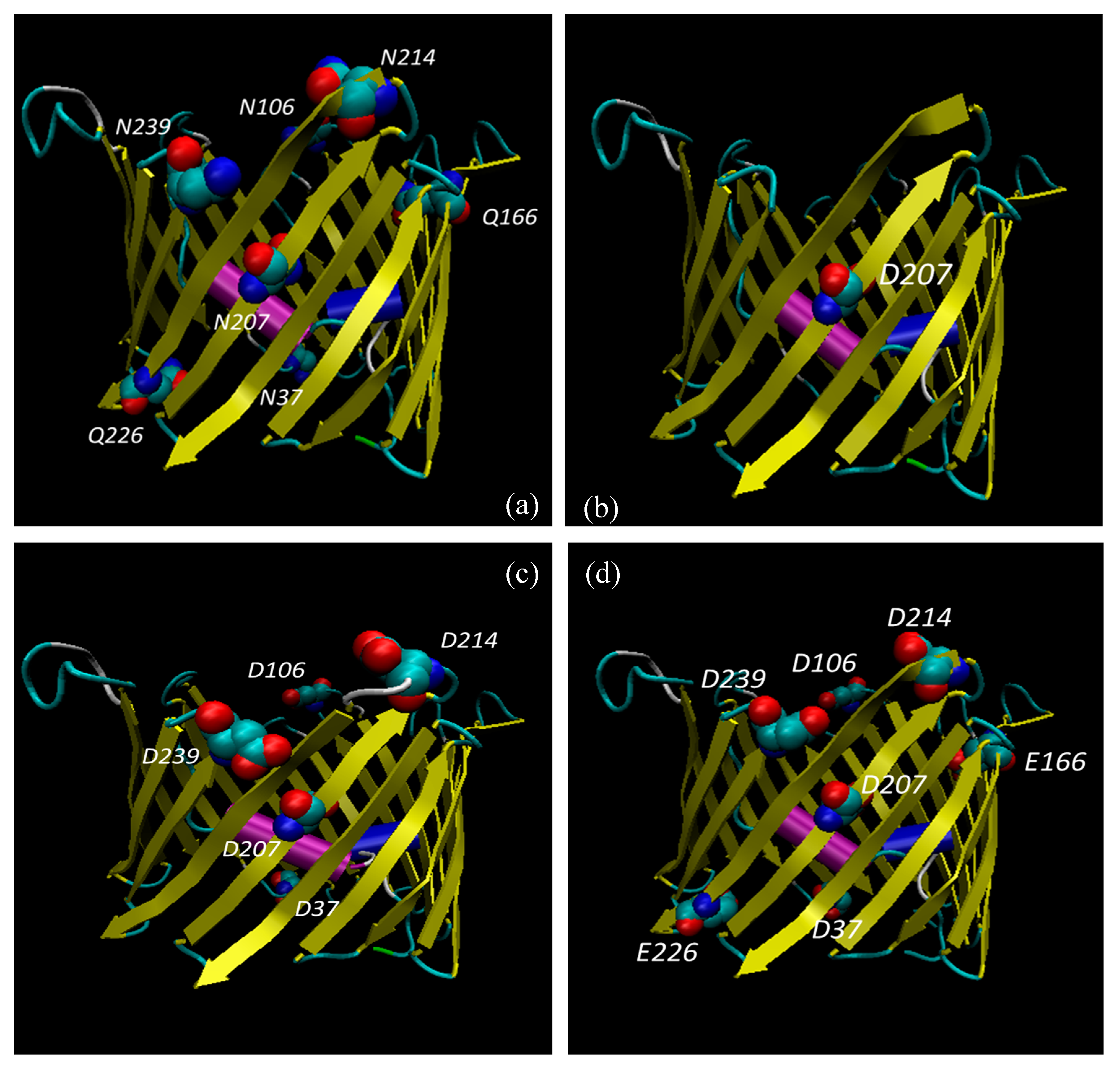
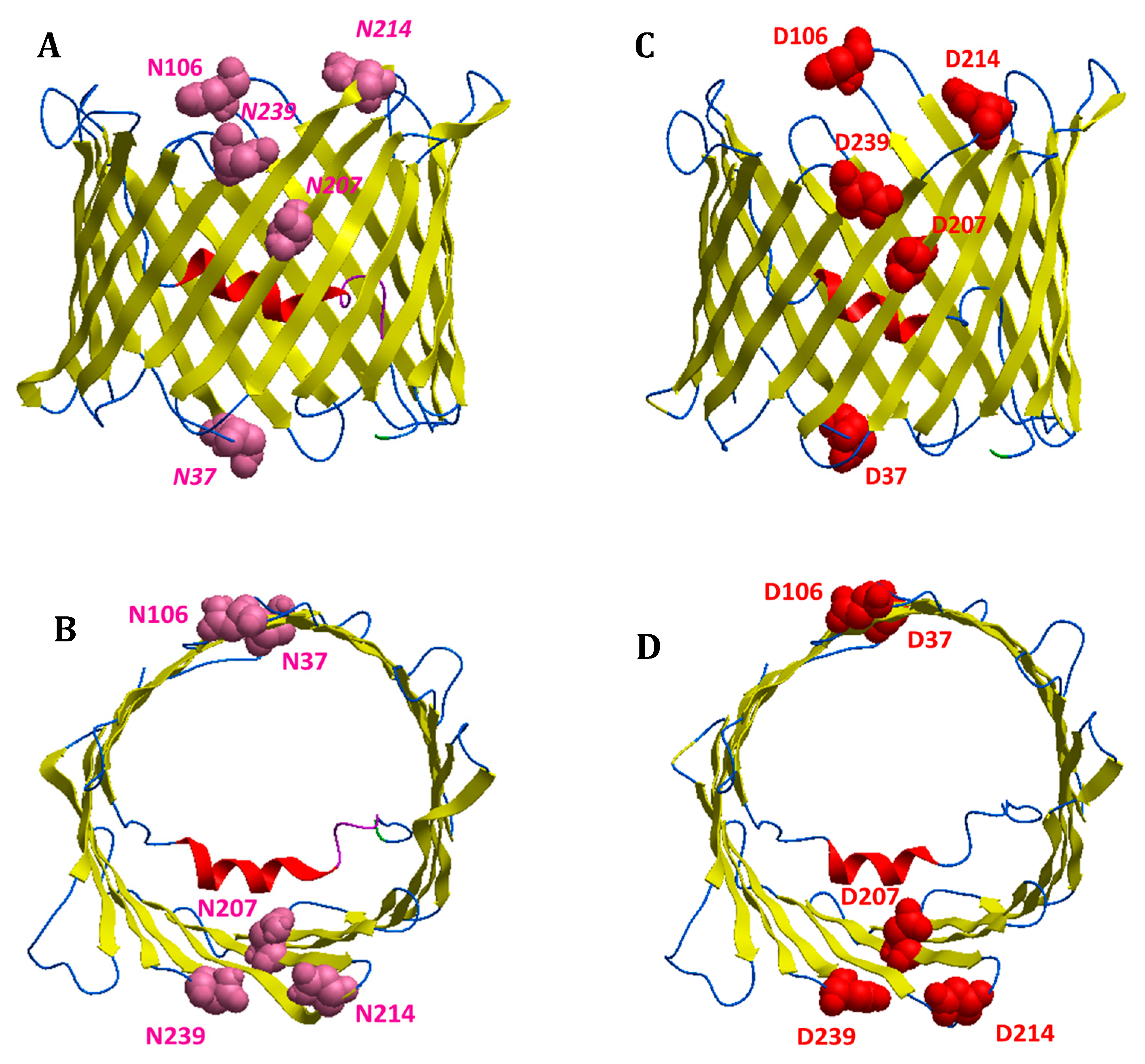
3.6. Bioinformatic Analysis Reveals an Increased Instability for Mutant Structures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pasinelli, P.; Brown, R.H. Molecular biology of amyotrophic lateral sclerosis: Insights from genetics. Nat. Rev. Neurosci. 2006, 7, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, S.C.; Mead, R.J.; Shaw, P.L. Oxidative stress in ALS: A mechanism of neurodegeneration and a therapeutic target. Biochim. Biophys. Acta 2006, 1762, 1051–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massaad, C.A.; Klann, E. Reactive Oxygen Species in the Regulation of Synaptic Plasticity and Memory. Antioxid. Redox Signal. 2011, 14, 2013–2054. [Google Scholar] [CrossRef] [Green Version]
- Marden, J.J.; Harraz, M.M.; Williams, A.J.; Nelson, K.; Luo, M.; Paulson, H.; Engelhardt, J.F. Redox modifier genes in amyotrophic lateral sclerosis in mice. J. Clin. Investig. 2007, 117, 2913–2919. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, T.; Pannel, B.K.; Ziegler, A.C.; Best, T.M. Biological and physiological role of reactive oxygen species--the good, the bad and the ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Kaur, S.J.; McKeown, S.R.; Rashid, S. Mutant Sod1 mediated pathogenesis of amyotrophic lateral sclerosis. Gene 2016, 577, 109–118. [Google Scholar] [CrossRef]
- Vande Velde, C.; Miller, T.M.; Cashman, N.R.; Cleveland, D.W. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc. Natl. Acad. Sci. USA 2008, 105, 4022–4027. [Google Scholar] [CrossRef] [Green Version]
- Ferri, A.; Cozzolino, M.; Crosio, C.; Nencini, M.; Casciati, A.; Gralla, E.B.; Rotilio, G.; Valentine, J.S.; Carrì, M.T. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc. Natl. Acad. Sci. USA 2006, 103, 13860–13865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magrì, A.; Belfiore, R.; Reina, S.; Tomasello, M.F.; Di Rosa, M.C.; Guarino, F.; Leggio, L.; De Pinto, V.; Messina, A. Hexokinase I N-terminal based peptide prevents the VDAC1-SOD1 G93A interaction and re-establishes ALS cell viability. Sci. Rep. 2016, 6, 34802. [Google Scholar] [CrossRef] [PubMed]
- Israelson, A.; Arbel, N.; Da Cruz, S.; Ilieva, H.; Yamanaka, K.; Shoshan-Barmatz, V.; Cleveland, D.W. Misfolded Mutant SOD1 Directly Inhibits VDAC1 Conductance in a Mouse Model of Inherited ALS. Neuron 2010, 67, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Magrì, A.; Di Rosa, M.C.; Orlandi, I.; Guarino, F.; Reina, S.; Guarnaccia, M.; Morello, G.; Spampinato, A.; Cavallaro, S.; Messina, A.; et al. Deletion of Voltage-Dependent Anion Channel 1 knocks mitochondria down triggering metabolic rewiring in yeast. Cell. Mol. Life Sci. 2020, 77, 3195–3213. [Google Scholar] [CrossRef]
- Leggio, L.; Guarino, F.; Accardi-Gheit, R.; Reina, S.; Specchia, V.; Damiano, F.; Tomasello, M.F.; Tommasino, M.; Messina, A. Mechanism of translation control of the alternative Drosophila melanogaster Voltage Dependent Anion-selective Channel 1 mRNAs. Sci. Rep. 2018, 8, 5347. [Google Scholar] [CrossRef] [Green Version]
- Guarino, F.; Zinghirino, F.; Mela, L.; Pappalardo, X.G.; Ichas, F.; De Pinto, V.; Messina, A. NRF-1 and HIF-1α contribute to modulation of human VDAC1 gene promoter during starvation and hypoxia in HeLa cells. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148289. [Google Scholar] [CrossRef] [PubMed]
- Keinan, N.; Tyomikin, D.; Shoshan-Barmatz, V. Oligomerization of the Mitochondrial Protein Voltage-Dependent Anion Channel Is Coupled to the Induction of Apoptosis. Mol. Cell. Biol. 2010, 30, 5698–5709. [Google Scholar] [CrossRef] [Green Version]
- Reina, S.; De Pinto, V. Anti-cancer compounds targeted to VDAC: Potential and perspectives. Curr. Med. Chem. 2017, 24, 4447–4469. [Google Scholar] [CrossRef]
- Magrì, A.; Reina, S.; De Pinto, V. VDAC1 as pharmacological target in cancer and neurodegeneration: Focus on its role in apoptosis. Front. Chem. 2018, 6, 108. [Google Scholar] [CrossRef] [Green Version]
- Zilocchi, M.; Moutaoufik, M.T.; Jessulat, M.; Phanse, S.; Aly, K.A.; Babu, M. Misconnecting the dots: Altered mitochondrial protein-protein interactions and their role in neurodegenerative disorders. Expert Rev. Proteom. 2020, 17, 119–136. [Google Scholar] [CrossRef]
- Martel, C.; Wang, Z.; Brenner, C. VDAC phosphorylation, a lipid sensor influencing the cell fate. Mitochondrion 2014, 19, 69–77. [Google Scholar] [CrossRef]
- Yu, H.; Diao, H.; Wang, C.; Lin, Y.; Yu, F.; Lu, H.; Xu, W.; Li, Z.; Shi, H.; Zhao, S.; et al. Acetylproteomic analysis reveals functional implications of lysine acetylation in human spermatozoa (sperm). Mol. Cell. Proteom. 2015, 14, 1009–1023. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Camara, A.K.; Wakim, B.T.; Zhou, Y.; Gadicherla, A.K.; Kwok, W.M.; Stowe, D.F. Tyrosine nitration of voltage-dependent anion channels in cardiacischemia-reperfusion: Reduction by peroxynitrite scavenging. Biochim. Biophys. Acta 2012, 1817, 2049–2059. [Google Scholar] [CrossRef] [Green Version]
- Saletti, R.; Reina, S.; Pittalà, M.G.G.; Magrì, A.; Cunsolo, V.; Foti, S.; De Pinto, V. Post-translational modifications of VDAC1 and VDAC2 cysteines from rat liver mitochondria. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 806–816. [Google Scholar] [CrossRef]
- Reina, S.; Pittalà, M.G.G.; Guarino, F.; Messina, A.; De Pinto, V.; Foti, S.; Saletti, R. Cysteine Oxidations in Mitochondrial Membrane Proteins: The Case of VDAC Isoforms in Mammals. Front. Cell. Dev. Biol. 2020, 8, 397. [Google Scholar] [CrossRef]
- Reina, S.; Checchetto, V.; Saletti, R.; Gupta, A.; Chaturvedi, D.; Guardiani, C.; Guarino, F.; Scorciapino, M.A.; Magrì, A.; Foti, S.; et al. VDAC3 as a sensor of oxidative state of the intermembrane space of mitochondria: The putative role of cysteine residue modifications. Oncotarget 2016, 7, 2249–2268. [Google Scholar] [CrossRef] [Green Version]
- Sarraf, S.A.; Raman, M.; Guarani-Pereira, V.; Sowa, M.E.; Huttlin, E.L.; Gygi, S.P.; Harper, J.W. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 2013, 496, 372–376. [Google Scholar] [CrossRef]
- Herst, P.M.; Rowe, M.R.; Carson, G.M.; Berridge, M.V. Functional Mitochondria in Health and Disease. Front. Endocrinol. 2017, 8, 296. [Google Scholar]
- Hoogerheide, D.P.; Gurnev, P.A.; Rostovtseva, T.K.; Bezrukov, S.M. Effect of a post-translational modification mimic on protein translocation through a nanopore. Nanoscale 2020, 12, 11070–11078. [Google Scholar] [CrossRef]
- Pittalà, M.G.G.; Saletti, R.; Reina, S.; Cunsolo, V.; De Pinto, V.; Foti, S. A High Resolution Mass Spectrometry Study Reveals the Potential of Disulfide Formation in Human Mitochondrial Voltage-Dependent Anion Selective Channel Isoforms (hVDACs). Int. J. Mol. Sci. 2020, 21, 1468. [Google Scholar] [CrossRef] [Green Version]
- De Pinto, V.; Benz, R.; Caggese, C.; Palmieri, F. Characterization of the mitochondrial porin from Drosophila melanogaster. Biochim. Biophys. Acta 1989, 905, 499–502. [Google Scholar] [CrossRef]
- Mackie, M.; Rüther, P.; Samodova, D.; Di Gianvincenzo, F.; Granzotto, C.; Lyon, D.; Peggie, D.A.; Howard, H.; Harrison, L.; Jensen, L.J.; et al. Palaeoproteomic Profiling of Conservation Layers on a 14th Century Italian Wall Painting. Angew. Chem. Int. Ed. 2018, 57, 7369–7374. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate Proteome-wide Label-free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [Green Version]
- Guan, Z.Q.; Yates, N.A.; Bakhtiar, R. Detection and characterization of methionine oxidation in peptides by collision-induced dissociation and electron capture dissociation. J. Am. Soc. Mass Spectrom. 2003, 14, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Galliano, F.; Saletti, R.; Cunsolo, V.; Foti, S.; Marletta, D.; Bordonaro, S.; D’Urso, G. Identification and characterization of a new β-casein variant in goat milk by high-performance liquid chromatography with electrospray ionization mass spectrometry and matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1972–1982. [Google Scholar] [CrossRef]
- Cunsolo, V.; Muccilli, V.; Saletti, R.; Foti, S. Applications of mass spectrometry techniques in the investigation of milk proteome. Eur. J. Mass Spectrom. 2011, 17, 305–320. [Google Scholar] [CrossRef]
- Ujwal, R.; Cascio, D.; Colletierc, J.P.; Fahama, S.; Zhanga, J.; Torod, L.; Ping, P.; Abramson, J. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc. Natl. Acad. Sci. USA 2008, 105, 17742–17747. [Google Scholar] [CrossRef] [Green Version]
- Didonna, A.; Benetti, F. Post-translational modifications in neurodegeneration. AIMS Biophys. 2016, 3, 27–49. [Google Scholar] [CrossRef]
- Michalska, P.; When, R.L. It Comes to an End: Oxidative Stress Crosstalk with Protein Aggregation and Neuro inflammation Induce Neurodegeneration. Antioxidants 2020, 9, 740. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS Signalingin Organismal Homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [Green Version]
- Andrus, P.K.; Fleck, T.J.; Gurney, M.E.; Hall, E.D. Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis. J. Neurochem. 1998, 71, 2041–2048. [Google Scholar] [CrossRef]
- Niebroj-Dobosz, I.; Dziewulska, D.; Kwiecinski, H. Oxidative damage to proteins in the spinal cord in amyotrophic lateral sclerosis (ALS). Folia Neuropathol. 2004, 42, 151–156. [Google Scholar]
- Wright, G.S.A.; Antonyuk, S.V.; Hasnain, S.S. The biophysics of superoxide dismutase-1 and amyotrophic lateral sclerosis. Q. Rev. Biophys. 2019, 52, e12. [Google Scholar] [CrossRef]
- Martins, D.; English, A.M. SOD1 oxidation and formation of soluble aggregates in yeast: Relevance to sporadic ALS development. Redox Biol. 2014, 26, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Banks, C.J.; Andersen, J.L. Mechanisms of SOD1 regulation by post-translational modifications. Redox Biol. 2019, 26, 101270. [Google Scholar] [CrossRef]
- Schmitt, N.D.; Agar, J.N. Parsing Disease-relevant Protein Modifications from Epiphenomena: Perspective on the Structural Basis of SOD1-Mediated ALS. J. Mass Spectrom. 2017, 52, 480–491. [Google Scholar] [CrossRef]
- D’Angelo, S.; Trojsi, F.; Salvatore, A.; Daniele, L.; Raimo, M.; Galletti, P.; Monsurrò, M.R. Accumulation of altered aspartyl residues in erythrocyte membrane proteins from patients with sporadic amyotrophic lateral sclerosis. Neurochem. Int. 2013, 63, 626–634. [Google Scholar] [CrossRef]
- Kametani, F.; Obi, T.; Shishido, T.; Akatsu, H.; Murayama, S.; Saito, Y.; Yoshida, M.; Hasegawa, M. Mass spectrometric analysis of accumulated TDP-43 in amyotrophic lateral sclerosis brains. Sci Rep. 2016, 6, 23281. [Google Scholar] [CrossRef]
- Bayrhuber, M.; Meins, T.; Habeck, M.; Becker, S.; Giller, K.; Villinger, S.; Vonrhein, C.; Griesinger, C.; Zweckstetter, M.; Zeth, K. Structure of the human voltage-dependent anion channel. Proc. Natl. Acad. Sci. USA 2008, 105, 15370–15375. [Google Scholar] [CrossRef] [Green Version]
- Hiller, S.; Garces, R.G.; Malia, T.J.; Orekhov, V.Y.; Colombini, M.; Wagner, G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science 2008, 321, 1206–1210. [Google Scholar] [CrossRef] [Green Version]
- De Pinto, V.; Al Jamal, J.A.; Benz, R.; Genchi, G.; Palmieri, F. Characterization of SH groups in porin of bovine heart mitochondria. Porin cysteines are localized in the channel walls. J. Biochem. 1991, 202, 903–911. [Google Scholar] [CrossRef]
- Aram, L.; Geula, S.; Arbel, N.; Shoshan-Barmatz, V. VDAC1 cysteine residues: Topology and function in channel activity and apoptosis. Biochem. J. 2010, 427, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Ravera, S.; Bonifacino, T.; Bartolucci, M.; Milanese, M.; Gallia, E.; Cortese, K.; Pandolfi, I.; Bonanno, G. Characterization of the Mitochondrial Aerobic Metabolismin the Pre- and Perisynaptic Districts of the SOD1G93A Mouse Model of Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2018, 55, 9220–9233. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.T. Nonenzymatic deamidation of asparaginyl and glutaminyl residues in proteins. Crit. Rev. Biochem. Mol. Biol. 1991, 26, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.E.; Robinson, Z.W.; Robinson, B.R.; Robinson, A.L.; Robinson, J.A.; Robinson, M.L.; Robinson, A.B. Structure-dependent nonenzymatic deamidation of glutaminyl and asparaginyl pentapeptides. J. Pept. Res. 2004, 63, 426–436. [Google Scholar] [CrossRef]
- Mikkat, S.; Kischstein, T.; Kreutzer, M.; Glocker, M.O. Mass spectrometric peptide analysis of 2DE-separated mouse spinal cord and rat hippocampus proteins suggests an NGxG motif of importance for in vivo deamidation. Electrophoresis 2013, 34, 1610–1618. [Google Scholar] [CrossRef]
- Robinson, N.E.; Robinson, M.L.; Schulze, E.S.; Lai, B.T.; Gray, H.B. Deamidation of alpha-synuclein. Protein Sci. 2009, 18, 1766–1773. [Google Scholar] [CrossRef]
- Bastrup, J.; Kastaniegaard, K.; Asuni, A.A.; Volbracht, C.; Stensballe, A. Proteomic and Unbiased Post-Translational Modification Profiling of Amyloid Plaques and Surrounding Tissue in a Transgenic Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 73, 393–411. [Google Scholar] [CrossRef]
- Sadakane, Y.; Kawahara, M. Implications of Metal Binding and Asparagine Deamidation for Amyloid Formation. Int. J. Mol. Sci. 2018, 19, 2449. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Watanabe, A.; Ogawara, M.; Mori, H.; Shirasawa, T. Isoaspartate formation and neurodegeneration in Alzheimer’s disease. Arch. Biochem. Biophys. 2000, 381, 225–234. [Google Scholar] [CrossRef]
- Ayers, J.I.; Fromholt, S.E.; O’Neal, V.O.; Diamond, J.H.; Borchelt, D.R. Prion-like propagation of mutant SOD1 misfolding and motor neuron disease spread along neuroanatomical pathways. Acta Neuropathol. 2016, 131, 103–114. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]

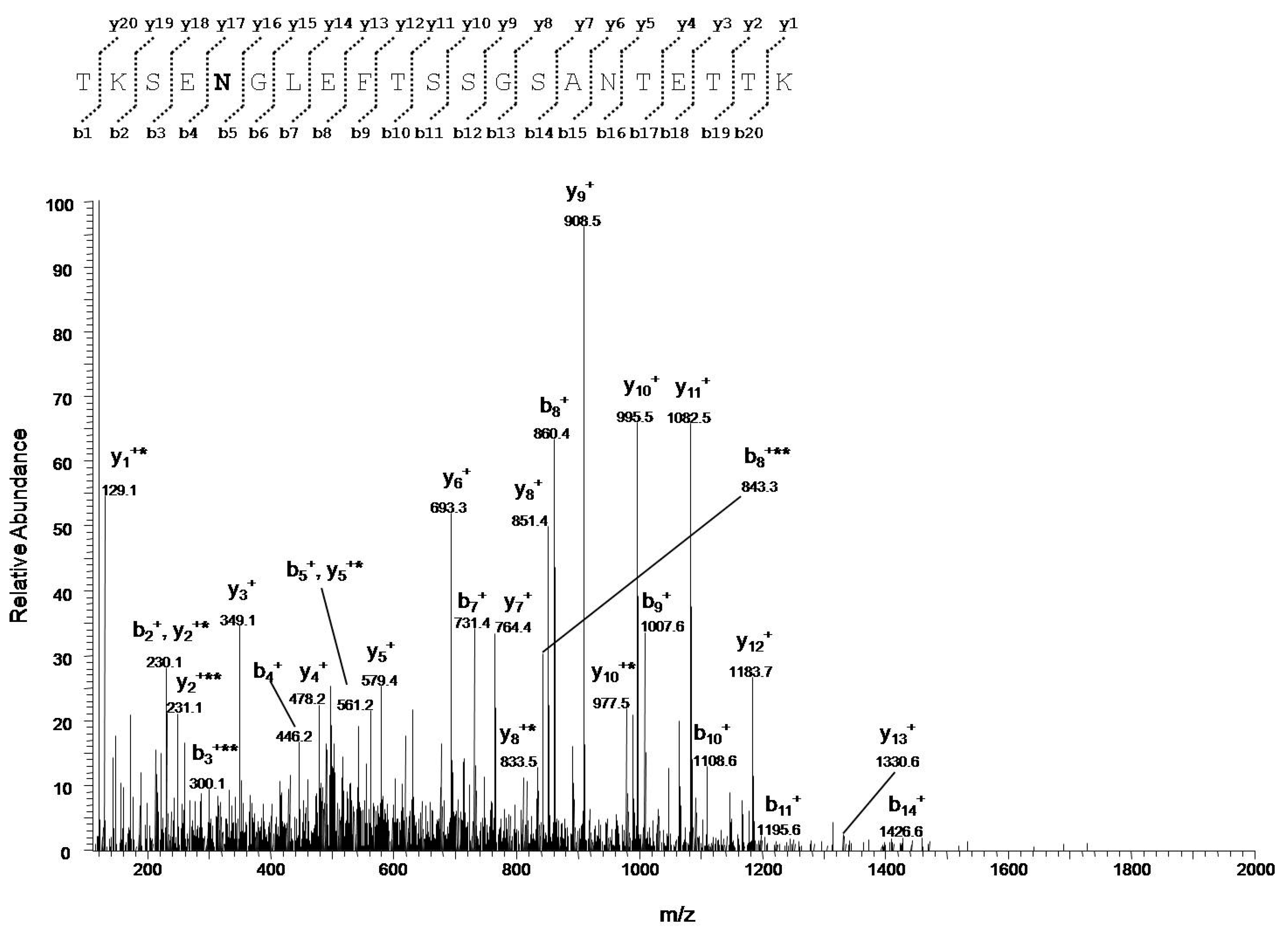

| Technical Replicate | Peptide | Position in the Sequence | Measured Monoisotopic m/z | Absolute Intensity | Ratio Ox/Red |
|---|---|---|---|---|---|
| I | EHINLGCDVDFDIAGPSIR | 121–139 | 1059.9921 (+2) | 8.2 × 105 | 29.3 |
| I | EHINLGCDVDFDIAGPSIR | 710.0155 (+3) | 2.8 × 104 | ||
| II | EHINLGCDVDFDIAGPSIR | 1059.9915 (+2) | 6.9 × 105 | 19.2 | |
| II | EHINLGCDVDFDIAGPSIR | 710.0140 (+3) | 3.6 × 104 | ||
| III | EHINLGCDVDFDIAGPSIR | 1059.9928 (+2) | 7.8 × 105 | 23.6 | |
| III | EHINLGCDVDFDIAGPSIR | 710.0140 (+3) | 3.3 × 104 | ||
| I | GALVLGYEGWLAGYQMNFETSK | 140–161 | 1225.5892 (+2) | 4.5 × 106 | 60.0 |
| I | GALVLGYEGWLAGYQMNFETSK | 1217.5931 (+2) | 7.5 × 104 | ||
| II | GALVLGYEGWLAGYQMNFETSK | 1225.5897 (+2) | 4.9 × 106 | 71.0 | |
| II | GALVLGYEGWLAGYQMNFETSK | 1217.5913 (+2) | 6.9 × 104 | ||
| III | GALVLGYEGWLAGYQMNFETSK | 817.3946 (+3) | 2.8 × 106 | 51.9 | |
| III | GALVLGYEGWLAGYQMNFETSK | 812.0656 (+3) | 5.4 × 104 | ||
| I | GALVLGYEGWLAGYQMNFETSK | 140–161 | 822.7261 (+3) | 2.2 × 105 | 2.9 |
| I | GALVLGYEGWLAGYQMNFETSK | 1217.5931 (+2) | 7.5 × 104 | ||
| II | GALVLGYEGWLAGYQMNFETSK | 822.7269 (+3) | 4.5 × 105 | 6.5 | |
| II | GALVLGYEGWLAGYQMNFETSK | 1217.5913 (+2) | 6.9 × 104 | ||
| III | GALVLGYEGWLAGYQMNFETSK | 822.7277 (+3) | 2.6 × 105 | 4.8 | |
| III | GALVLGYEGWLAGYQMNFETSK | 812.0656 (+3) | 5.4 × 104 |
| Peptide | Position in the Sequence | Measured Monoisotopic m/z | Absolute Intensity | Ratio Deam/Norm |
|---|---|---|---|---|
| TKSENGLEFTSSGSANTETTK | 33–53 | 730.6730 (+3) | 6.3 × 106 | 0.02 |
| TKSENGLEFTSSGSANTETTK | 730.3450(+3) | 3.3 × 108 | ||
| SENGLEFTSSGSANTETTK | 35–53 | 654.2921 (+3) | 6.3 × 105 | 0.01 |
| SENGLEFTSSGSANTETTK | 980.4425(+2) | 1.4 × 108 | ||
| LTFDSSFSPNTGKK | 97–110 | 510.5876 (+3) | 1.3 × 106 | 0.01 |
| LTFDSSFSPNTGKK | 764.8857 (+2) | 9.9 × 107 | ||
| VTQSNFAVGYK | 164–174 | 607.8062 (+2) | 1.4 × 106 | 0.002 |
| VTQSNFAVGYK | 607.3142 (+2) | 6.2 × 108 | ||
| KLETAVNLAWTAGNSNTR | 201–218 | 649.6692 (+3) | 9.6 × 106 | 0.6 |
| KLETAVNLAWTAGNSNTR | 649.3412 (+3) | 1.6 × 107 | ||
| LETAVNLAWTAGNSNTR | 202–218 | 909.9527(+2) | 6.8 × 105 | 0.04 |
| LETAVNLAWTAGNSNTR | 909.4607 (+2) | 1.9 × 107 | ||
| YQVDPDACFSAK | 225–236 | 701.3032(+2) | 1.6 × 106 | 0.002 |
| YQVDPDACFSAK | 700.8112(+2) | 7.1 × 108 | ||
| VNNSSLIGLGYTQTLKPGIK | 237–256 | 702.0598 (+3) | 2.4 × 106 | 0.03 |
| VNNSSLIGLGYTQTLKPGIK | 701.7318 (+3) | 7.4 × 107 |
| Cell Lines | Residue | Mean | Std | CI_Low | CI_Up | N. of Scansions |
|---|---|---|---|---|---|---|
| NSC34 | N | 0 | 0 | 0 | 0 | 51 |
| Q | 0 | 0 | 0 | 0 | 30 | |
| NSC34-SOD1WT | N | 0 | 0 | 0 | 0 | 61 |
| Q | 0 | 0 | 0 | 0 | 41 | |
| NSC34-SOD1G93A | N | 0.98 | 0.81 | 0.04 | 2.76 | 87 |
| Q | 0.02 | 0.02 | 0.00 | 0.06 | 47 |
| Protein | NSC34 | NSC34-SOD1WT | NSC34-SOD1G93A | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LFQ Intensity | SD% | % | LFQ Intensity | SD % | % | LFQ Intensity | SD % | % | |
| VDAC1 | 1.10 × 109 | 1 | 66.71 | 1.19 × 109 | 27 | 65.67 | 1.10 × 109 | 7 | 64.08 |
| VDAC2 | 2.40 × 108 | 4 | 14.55 | 2.46 × 108 | 20 | 13.59 | 3.51 × 108 | 11 | 20.43 |
| VDAC3 | 3.09 × 108 | 15 | 18.74 | 3.75 × 108 | 16 | 20.74 | 2.66 × 108 | 13 | 15.50 |
| Outliers | β-Strand | α-Helix | |||||
|---|---|---|---|---|---|---|---|
| Total | Allowed | Favored | Total | Allowed | Favored | Total | |
| VDAC1 WT | 12.4 | 11.3 | 67.6 | 78.9 | 6.3 | 2.4 | 8.7 |
| VDAC1 N37D | 10.6 | 13.5 | 63.2 | 76.7 | 7.8 | 4.9 | 12.7 |
| VDAC1 N106D | 8.8 | 12.8 | 65.4 | 78.2 | 7.0 | 6.0 | 13.0 |
| VDAC1 N207D | 9.1 | 13.6 | 64.6 | 78.2 | 7.4 | 5.3 | 12.7 |
| VDAC1N214D | 7.7 | 12.3 | 66.1 | 78.4 | 6.7 | 7.1 | 13.8 |
| VDAC1N239D | 8.1 | 12.3 | 66.4 | 78.7 | 7.1 | 6.1 | 13.2 |
| VDAC1 5 Asn/Asp | 7.3 | 16.6 | 60.7 | 77.3 | 8.4 | 7.0 | 15.4 |
| VDAC1 5 Asn/Asp + 2 Gln/Glu | 7.7 | 14.4 | 60.7 | 75.1 | 8.4 | 8.4 | 16.8 |
| Modified Residues in VDAC1 | |||
|---|---|---|---|
| NSC34 | NSC34-SOD1WT | NSC34-SOD1G93A | |
| ALA 2 | Acetylated | Acetylated | Acetylated |
| ASN 37 | - | - | Deamidated (0.01:1) |
| SER 104 | Phosphorylated (0.01:1) | Phosphorylated (0.01:1) | Phosphorylated (0.01:1) |
| ASN 106 | - | - | Deamidated (0.01:1) |
| CYS 127 | Sulfonic acid | Sulfonic acid | Sulfonic acid (24:1) |
| MET 155 | Met sulfoxide (5:1) Met sulfone (0.1:1) | Met sulfoxide (3:1) Met sulfone (0.1:1) | Met sulfoxide (61:1) Met sulfone (4.7:1) |
| GLN 166 | - | - | Deamidated (0.002:1) |
| ASN 207 | - | - | Deamidated (0.6) |
| ASN 214 | - | - | Deamidated (0.04:1) |
| GLN 226 | - | - | Deamidated (0.002:1) |
| CYS 232 | * | * | * |
| ASN 239 | - | - | Deamidated (0.03) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pittalà, M.G.G.; Reina, S.; Cubisino, S.A.M.; Cucina, A.; Formicola, B.; Cunsolo, V.; Foti, S.; Saletti, R.; Messina, A. Post-Translational Modification Analysis of VDAC1 in ALS-SOD1 Model Cells Reveals Specific Asparagine and Glutamine Deamidation. Antioxidants 2020, 9, 1218. https://doi.org/10.3390/antiox9121218
Pittalà MGG, Reina S, Cubisino SAM, Cucina A, Formicola B, Cunsolo V, Foti S, Saletti R, Messina A. Post-Translational Modification Analysis of VDAC1 in ALS-SOD1 Model Cells Reveals Specific Asparagine and Glutamine Deamidation. Antioxidants. 2020; 9(12):1218. https://doi.org/10.3390/antiox9121218
Chicago/Turabian StylePittalà, Maria Gaetana Giovanna, Simona Reina, Salvatore Antonio Maria Cubisino, Annamaria Cucina, Beatrice Formicola, Vincenzo Cunsolo, Salvatore Foti, Rosaria Saletti, and Angela Messina. 2020. "Post-Translational Modification Analysis of VDAC1 in ALS-SOD1 Model Cells Reveals Specific Asparagine and Glutamine Deamidation" Antioxidants 9, no. 12: 1218. https://doi.org/10.3390/antiox9121218
APA StylePittalà, M. G. G., Reina, S., Cubisino, S. A. M., Cucina, A., Formicola, B., Cunsolo, V., Foti, S., Saletti, R., & Messina, A. (2020). Post-Translational Modification Analysis of VDAC1 in ALS-SOD1 Model Cells Reveals Specific Asparagine and Glutamine Deamidation. Antioxidants, 9(12), 1218. https://doi.org/10.3390/antiox9121218







