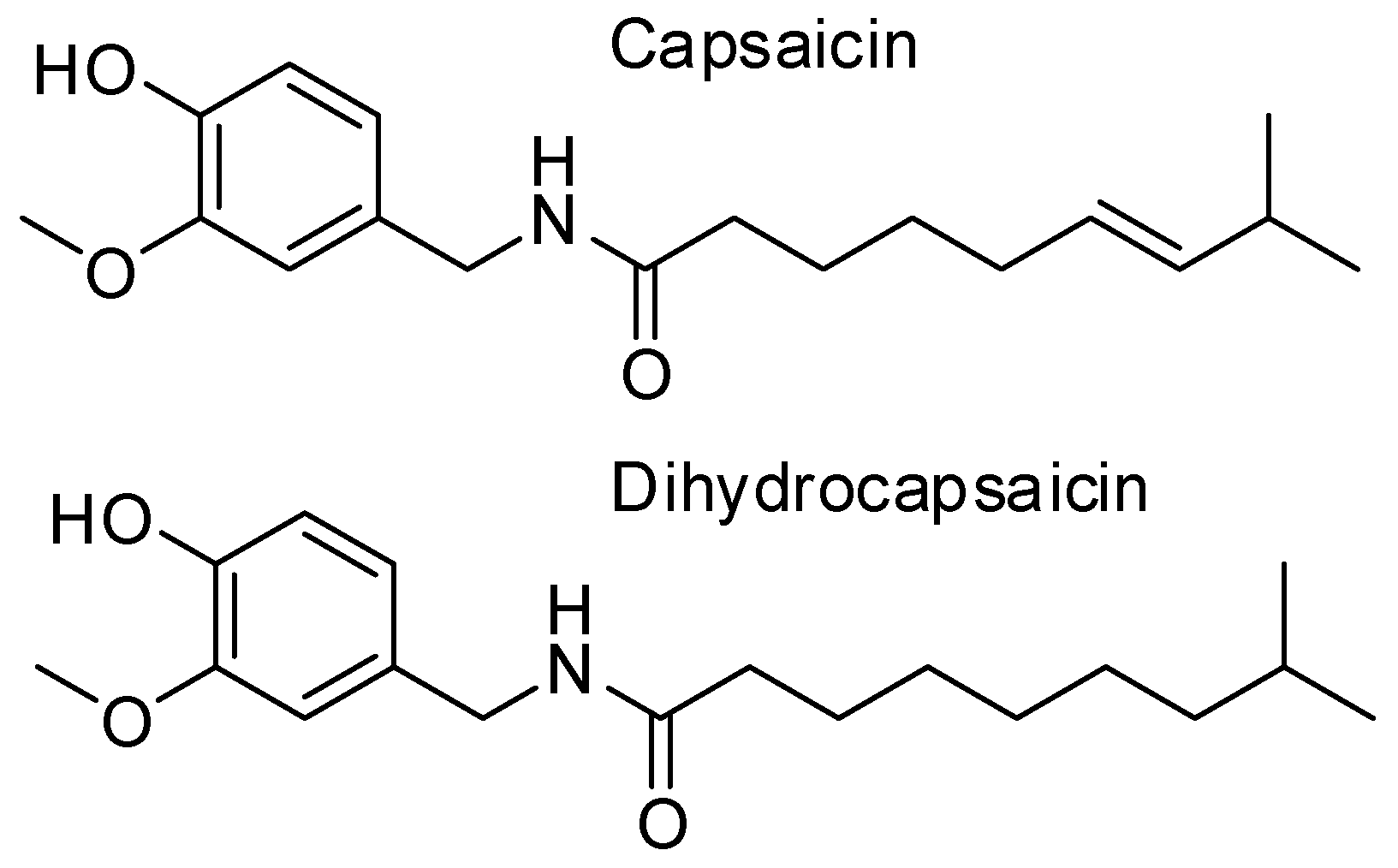
Capsaicin, a Powerful •OH-Inactivating Ligand
Abstract
:1. Introduction
2. Computational Methods
2.1. Electronic Calculations
2.2. Molecular Dynamics
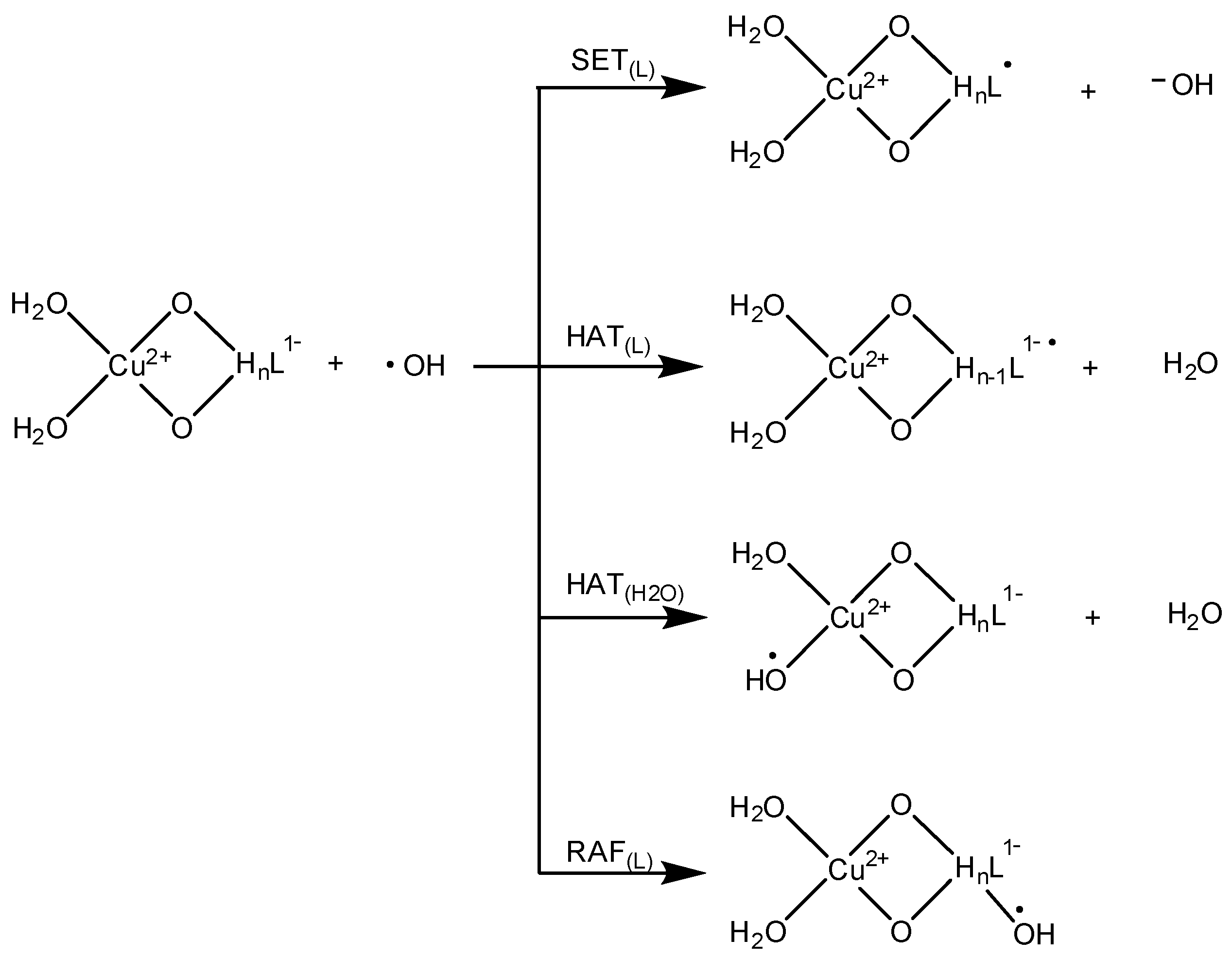
2.3. Reduction Reactions
3. Results and Discussion
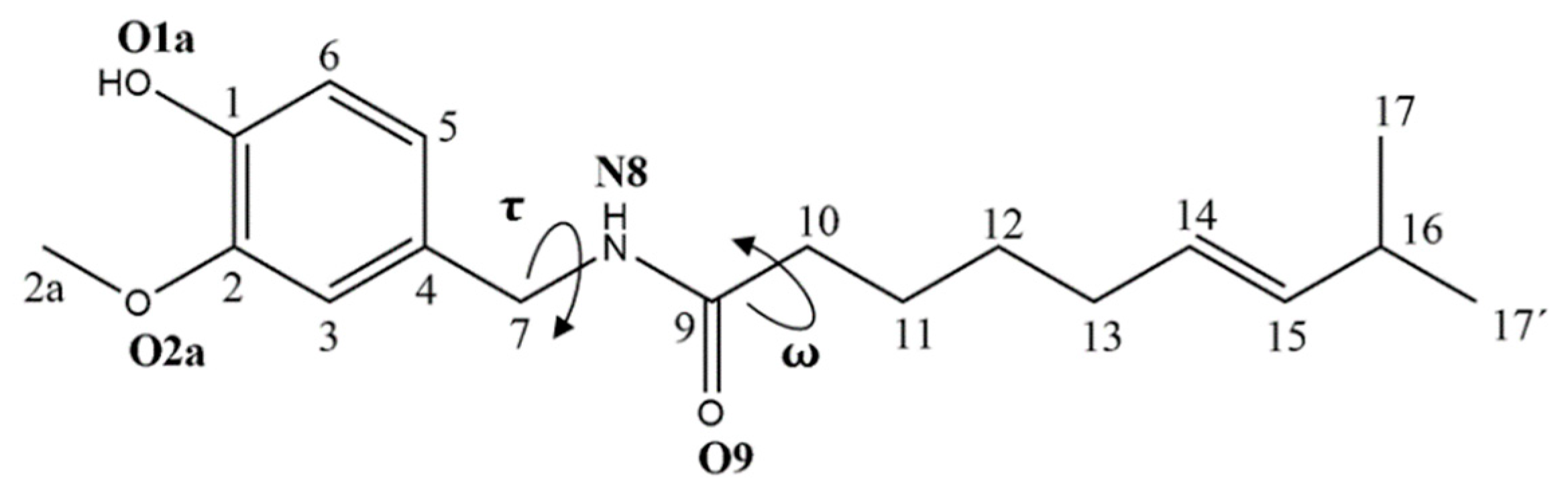
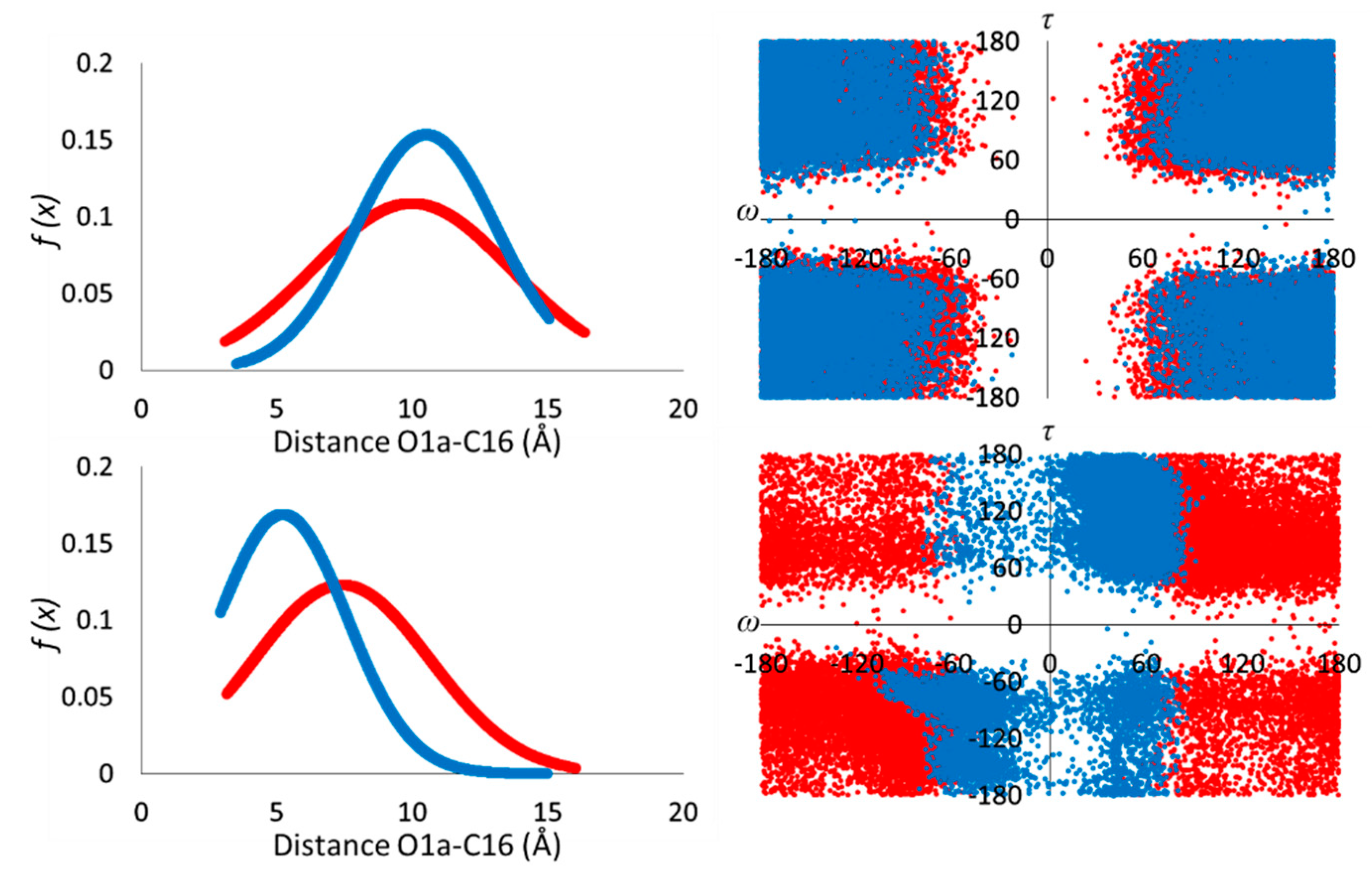
3.1. Conformational Analysis
3.2. Cu(II) Chelating Ability of CAP
3.3. OIL-1 (Inhibiting the Reduction in Metal Ions)
3.4. OIL-2 (Scavenging •OH Yielded through Fenton-Like Reactions)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: www.fao.org. (accessed on 3 July 2019).
- Iqbal, Q.; Amjad, M.; Asi, M.R.; Ariño, A. Characterization of Capsaicinoids and Antioxidants in Hot Peppers as Influenced by Hybrid and Harvesting Stage. Plant Foods Hum. Nutr. 2013, 68, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Hayman, M.; Kam, P.C.A. Capsaicin: A review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care 2008, 19, 338–343. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Antonious, G.F.; Kochhar, T.S. Mobility of heavy metals from soil into hot pepper fruits: A field study. Bull. Environ. Contam. Toxicol. 2009, 82, 59–63. [Google Scholar] [CrossRef]
- Inoue, K.; Kaneko, M.; Hino, T.; Oka, H. Simple and Novel Screening Assay of Natural Antioxidants for Cu(II) Ion/Adrenaline-Mediated Oxidation of N-Terminal Amyloid β by Liquid Chromatography/Mass Spectrometry. J. Agric. Food Chem. 2010, 58, 9413–9417. [Google Scholar] [CrossRef]
- Ahuja, K.D.K.; Kunde, D.A.; Ball, M.J.; Geraghty, D.P. Effects of Capsaicin, Dihydrocapsaicin, and Curcumin on Copper-Induced Oxidation of Human Serum Lipids. J. Agric. Food Chem. 2006, 54, 6436–6439. [Google Scholar] [CrossRef]
- Ahuja, K.D.K.; Ball, M.J. Effects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and women. Br. J. Nutr. 2006, 96, 239–242. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.A.; Syazwanie, N.F.; Mahmod, N.H.; Badaluddin, N.A.; Mustafa, K.; Alias, N.; Prodhan, M.A. Evaluation of antioxidant compounds, antioxidant activities and capsaicinoid compounds of Chili (Capsicum sp.) germplasms available in Malaysia. J. Appl. Res. Med. Aromat. Plants 2018, 9, 46–54. [Google Scholar] [CrossRef]
- Almaghrabi, S.Y.; Geraghty, D.P.; Ahuja, K.D.K.; Adams, M.J. Vanilloid-like agents inhibit aggregation of human platelets. Thromb. Res. 2014, 134, 412–417. [Google Scholar] [CrossRef]
- Careaga, M.O.; Fernández, E.; Dorantes, L.; Mota, L.; Jaramillo, M.E.; Hernandez-Sanchez, H. Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int J. Food Microbiol. 2003, 83, 331–335. [Google Scholar] [CrossRef]
- Prasch, S.; Duran, A.G.; Chinchilla, N.; Molinillo, J.M.G.; Macías, F.A.; Bucar, F. Resistance modulatory and efflux-inhibitory activities of capsaicinoids and capsinoids. Bioorg. Chem. 2019, 82, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Da Motta, O.V.; Machado, O.L.T.; Rodrigues, R.; Carvalho, A.O.; Teixeira-Ferreira, A.; Gomes, V.M. Thionin-like peptides from Capsicum annuum fruits with high activity against human pathogenic bacteria and yeasts. Biopolymers 2014, 102, 30–39. [Google Scholar] [CrossRef]
- Perucka, I.; Materska, M. Phenylalanine ammonia-lyase and antioxidant activities of lipophilic fraction of fresh pepper fruits Capsicum annum L. Innov. Food Sci. Emerg. Technol. 2001, 2, 189–192. [Google Scholar] [CrossRef]
- Galano, A.; Martínez, A. Capsaicin, a tasty free radical scavenger: Mechanism of action and kinetics. J. Phys. Chem. B 2012, 116, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Gaubert, S.; Bouchaut, M.; Brumas, V.; Berthon, G. Copper-ligand interactions and physiological free radical processes. Part 3. Influence of histidine, salicylic acid and anthranilic acid on copper-driven Fenton chemistry in vitro. Free Radic. Res. 2000, 32, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Miche, H.; Brumas, V.; Berthon, G. Copper(II) interactions with nonsteroidal antiinflammatory agents. II. Anthranilic acid as a potential OH-inactivating ligand. J. Inorg. Biochem. 1997, 68, 27–38. [Google Scholar] [CrossRef]
- Berthon, G. Is copper pro- or anti-inflammatory? A reconciling view and a novel approach for the use of copper in the control of inflammation. Agents Actions 1993, 39, 210–217. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Gajewski, E.; Dizdaroglu, M. Copper-iondependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem. J. 1991, 273, 601–604. [Google Scholar] [CrossRef] [Green Version]
- Letelier, M.E.; Sanchez-Jofre, S.; Peredo-Silva, L.; Cortés-Troncoso, J.; Aracena-Parks, P. Mechanisms underlying iron and copper ions toxicity in biological systems: Pro-oxidant activity and protein-binding effects. Chem. Biol. Interact. 2010, 188, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.V.; Hanna, P.M.; Mason, R.P. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic. Biol. Med. 1997, 22, 885–888. [Google Scholar] [CrossRef]
- Lloyd, D.R.; Phillips, D.H. Oxidative DNA damage mediated by copper(II), iron(II) and nickel(II) Fenton reactions: Evidence for site-specific mechanisms in the formation of double-strand breaks, 8-hydroxydeoxyguanosine and putative intrastrand cross-links. Mutat. Res. 1999, 424, 23–36. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Fox, D.J. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2006, 2, 364–382. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. A computational methodology for accurate predictions of rate constants in solution: Application to the assessment of primary antioxidant activity. J. Comput. Chem. 2013, 34, 2430–2445. [Google Scholar] [CrossRef]
- Galano, A.; Francisco Marquez, M.; Pérez-González, A. Ellagic acid: An unusually versatile protector against oxidative stress. Chem. Res. Toxicol. 2014, 27, 904–918. [Google Scholar] [CrossRef]
- Galano, A.; Muñoz-Rugeles, L.; Alvarez-Idaboy, J.R.; Bao, J.L.; Truhlar, D.G. Hydrogen Abstraction Reactions from Phenolic Compounds by Peroxyl Radicals: Multireference Character and Density Functional Theory Rate Constants. J. Phys. Chem. A 2016, 120, 4634–4642. [Google Scholar] [CrossRef]
- Li, R.; Peverati, R.; Isegawa, M.; Truhlar, D.G. Assessment and validation of density functional approximations for iron carbide and iron carbide cation. J. Phys. Chem. A 2013, 117, 169–173. [Google Scholar] [CrossRef]
- Shil, S.; Bhattacharya, D.; Sarkar, S.; Misra, A. Performance of the widely used Minnesota density functionals for the prediction of heat of formations, ionization potentials of some benchmarked first row transition metal complexes. J. Phys. Chem. A 2013, 117, 4945–4955. [Google Scholar] [CrossRef]
- Yu, H.; Truhlar, D.G. Components of the Bond Energy in Polar Diatomic Molecules, Radicals, and Ions Formed by Group-1 and Group-2 Metal Atoms. J. Chem. Theory Comput. 2015, 11, 2968–2983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. Density functional theory for reaction energies: Test of meta and hybrid meta functionals, range-separated functionals, and other high-performance functionals. J. Chem. Theory Comput. 2011, 7, 669–676. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Prejanò, M.; Marino, T.; Russo, N. On the inhibition mechanism of glutathione transferase P1 by piperlongumine. Insight from theory. Front. Chem. 2018, 6. [Google Scholar] [CrossRef]
- Prejanò, M.; Romeo, I.; Sgrizzi, L.; Russo, N.; Marino, T. Why hydroxy-proline improves the catalytic power of the peptidoglycan: N-deacetylase enzyme: Insight from theory. Phys. Chem. Chem. Phys. 2019, 21, 23338–23345. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general Amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Bayly, C.I.; Cieplak, P.; Cornell, W.D.; Kollman, P.A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J. Phys. Chem. 1993, 97, 10269–10280. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Ewald, P.P. Die Berechnung optischer und elektrostatischer Gitterpotentiale. Ann. Phys. 1921, 369, 253–287. [Google Scholar] [CrossRef] [Green Version]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Kollman, P.A. AMBER 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Evans, M.G.; Polanyi, M. Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans. Faraday Soc. 1935, 31, 875–894. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 63–71. [Google Scholar] [CrossRef]
- Truhlar, D.G.; Garrett, B.C.; Klippenstein, S.J. Current status of transition-state theory. J. Phys. Chem. 1996, 100, 12771–12800. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 1993, 65, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Marcus, R.A. Electron transfer reactions in chemistry: Theory and experiment (Nobel lecture). Angew. Chem. Int. Ed. Engl. 1993, 32, 1111–1121. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry. Theory and experiment. Pure Appl. Chem. 1997, 69, 13–29. [Google Scholar] [CrossRef]
- Marcus, R.A. Electron transfer reactions in chemistry Theory and experiment. J. Electroanal. Chem. 1997, 438, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Collins, F.C.; Kimball, G.E. Diffusion-controlled reaction rates. J. Colloid. Sci. 1949, 4, 425–437. [Google Scholar] [CrossRef]
- Smoluchowski, M. Mathematical theory of the kinetics of the coagulation of colloidal solutions. Z Phys. Chem. 1917, 92, 1839950. [Google Scholar]
- Stokes, G.G. Mathematical and Physical Papers; Cambridge University Press: Cambridge, UK, 1903; Volume III, pp. 1–55. [Google Scholar]
- Einstein, A. On the movement of small particles suspended in a stationary liquid demanded by the molecular-kinetic theory of heat. Ann. Phys. 1905, 17, 559–560. [Google Scholar]
- Galano, A. Free Radicals Induced Oxidative Stress at a Molecular Level: The Current Status, Challenges and Perspectives of Computational Chemistry Based Protocols. J. Mex. Chem. Soc. 2015, 59, 231–262. [Google Scholar] [CrossRef] [Green Version]
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum. Chem. 2019, 119. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Diduk, R.; Galano, A. Adrenaline and noradrenaline: Protectors against oxidative stress or molecular targets? J. Phys. Chem. B 2015, 119, 3479–3491. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Medina, M.E.; Tan, D.X.; Reiter, R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: A physicochemical analysis. J. Pineal. Res. 2015, 58, 107–116. [Google Scholar] [CrossRef] [PubMed]
- McLatchie, L.M.; Bevan, S. The effects of pH on the interaction between capsaicin and the vanilloid receptor in rat dorsal root ganglia neurons. Br. J. Pharmacol. 2001, 132, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O−2 Radicals in Aqueous Solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]


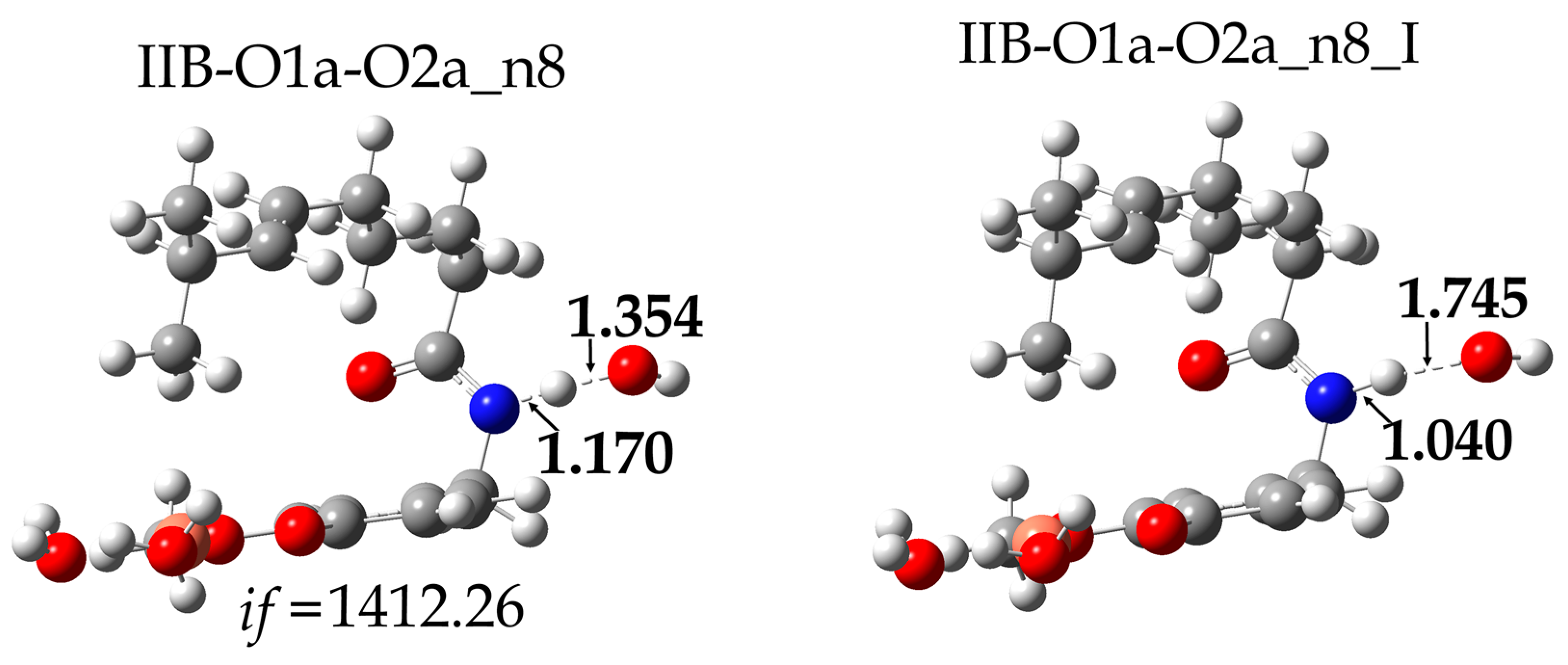
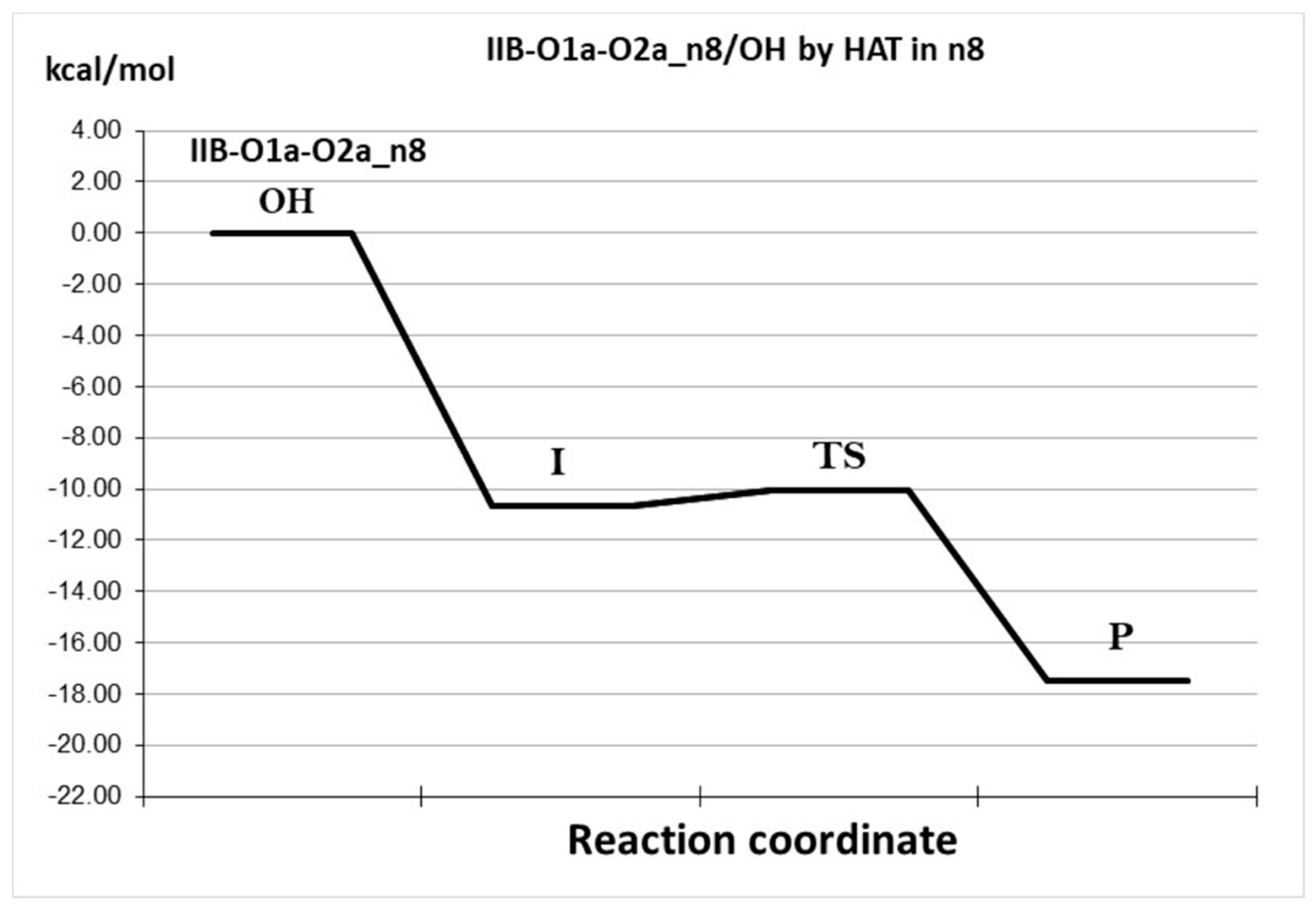
| Species * | ΔE (kcal/mol) | MB% |
|---|---|---|
| capN | 0.00 | 89.01 |
| capN_ext | 1.24 | 10.99 |
| capA | 0.00 | 67.66 |
| capA_ext | 0.44 | 32.34 |
| Complex | ∆G° | MB% |
|---|---|---|
| IA-O1a | 3.29 | - |
| IA-O1a_ext | 5.92 | - |
| IA-O2a | 5.91 | - |
| IA-O2a_ext | 7.84 | - |
| IA-O9 | 0.61 | - |
| IA-O9_ext | 2.74 | - |
| IB-O1a-O2a | 1.33 | - |
| IB-O1a-O2a_ext | 3.97 | - |
| IIA-O1a | −17.10 | 0.03 |
| IIA-O1a_ext | −17.07 | 0.03 |
| IIA-O2a_ext | −5.12 | - |
| IIA-O9_ext | 2.26 | - |
| IIB-O1a-O2a | −21.88 | 91.96 |
| IIB-O1a-O2a_ext | −20.43 | 7.98 |
| IIIA-O1a | −8.90 | - |
| IIIA-O1a_ext | −8.35 | - |
| IIIA-N8 | 3.25 | - |
| IIIA-N8_ext | 5.64 | - |
| IIIB-O1a-O2a | −13.68 | - |
| IIIB-O1a-O2a_ext | −11.71 | - |
| IIIB-N8-O9 | −3.00 | - |
| IVA-N8 | 2.79 | - |
| IVA-N8_ext | 5.24 | - |
| IVB-N8-O9 | −3.00 | - |
| Complex | ΔG° | Ki | |
|---|---|---|---|
| IIB-O1a-O2a | −21.88 | 1.09 × 1016 | 2.16 × 1013 |
| IIB-O1a-O2a_ext | −20.43 | 9.43 × 1014 | 1.88 × 1012 |
| Kapp | 2.35 × 1013 |
| O2•− | ΔG° | λ | ΔG≠ | kiapp | kCu(II)app/kiapp |
|---|---|---|---|---|---|
| O2•− | |||||
| Cu(II) | −24.01 | 51.87 | 3.74 | 4.46 × 109 | |
| IIB-O1a-O2a | −13.21 | 50.11 | 6.79 | 5.92 × 107 | 75.39 |
| IIB-O1a-O2a_ext | −13.96 | 50.09 | 6.52 | 8.14 × 106 | 548.32 |
| koverall | 6.73 × 107 | 66.28 | |||
| Asc− | |||||
| Cu(II) | −4.67 | 34.14 | 6.36 | 1.33 × 108 | |
| IIB-O1a-O2a | 6.12 | 32.38 | 11.45 | 2.31 × 104 | 5744.24 |
| IIB-O1a-O2a_ext | 5.38 | 32.37 | 11.00 | 4.25 × 103 | 31,316.16 |
| koverall | 2.74 × 104 | 4853.91 |
| IIB-O1a-O2a | IIB-O1a-O2a_ext | |
|---|---|---|
| SET | ||
| O1a-O2a_ct | −23.85 | −24.63 |
| HAT | ||
| c2a | −22.35 | −22.84 |
| c7 | −41.97 | −41.46 |
| n8 | −17.48 | −17.99 |
| c10 | −29.71 | −31.44 |
| c11 | −27.34 | −27.07 |
| c12 | −27.07 | −27.36 |
| c13 | −39.82 | −42.34 |
| c17 | −22.14 | −22.18 |
| c17p | −20.05 | −22.77 |
| o1a | −36.19 | −33.99 |
| o2a | −40.28 | −38.86 |
| RAF | ||
| c1 | −16.30 | −16.51 |
| c2 | −19.19 | −21.73 |
| c3 | −13.16 | −13.72 |
| c4 | −17.26 | −18.51 |
| c5 | −14.89 | −15.32 |
| c6 | −16.40 | −16.21 |
| c14 | −26.15 | −26.44 |
| c15 | −26.23 | −24.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-González, A.; Prejanò, M.; Russo, N.; Marino, T.; Galano, A. Capsaicin, a Powerful •OH-Inactivating Ligand. Antioxidants 2020, 9, 1247. https://doi.org/10.3390/antiox9121247
Pérez-González A, Prejanò M, Russo N, Marino T, Galano A. Capsaicin, a Powerful •OH-Inactivating Ligand. Antioxidants. 2020; 9(12):1247. https://doi.org/10.3390/antiox9121247
Chicago/Turabian StylePérez-González, Adriana, Mario Prejanò, Nino Russo, Tiziana Marino, and Annia Galano. 2020. "Capsaicin, a Powerful •OH-Inactivating Ligand" Antioxidants 9, no. 12: 1247. https://doi.org/10.3390/antiox9121247
APA StylePérez-González, A., Prejanò, M., Russo, N., Marino, T., & Galano, A. (2020). Capsaicin, a Powerful •OH-Inactivating Ligand. Antioxidants, 9(12), 1247. https://doi.org/10.3390/antiox9121247








